Ultrasound Evaluation of Extracranial Cerebral Circulation (The Common, External and Internal Carotid Artery) in Different Breeds of Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Method
2.1. Study Population
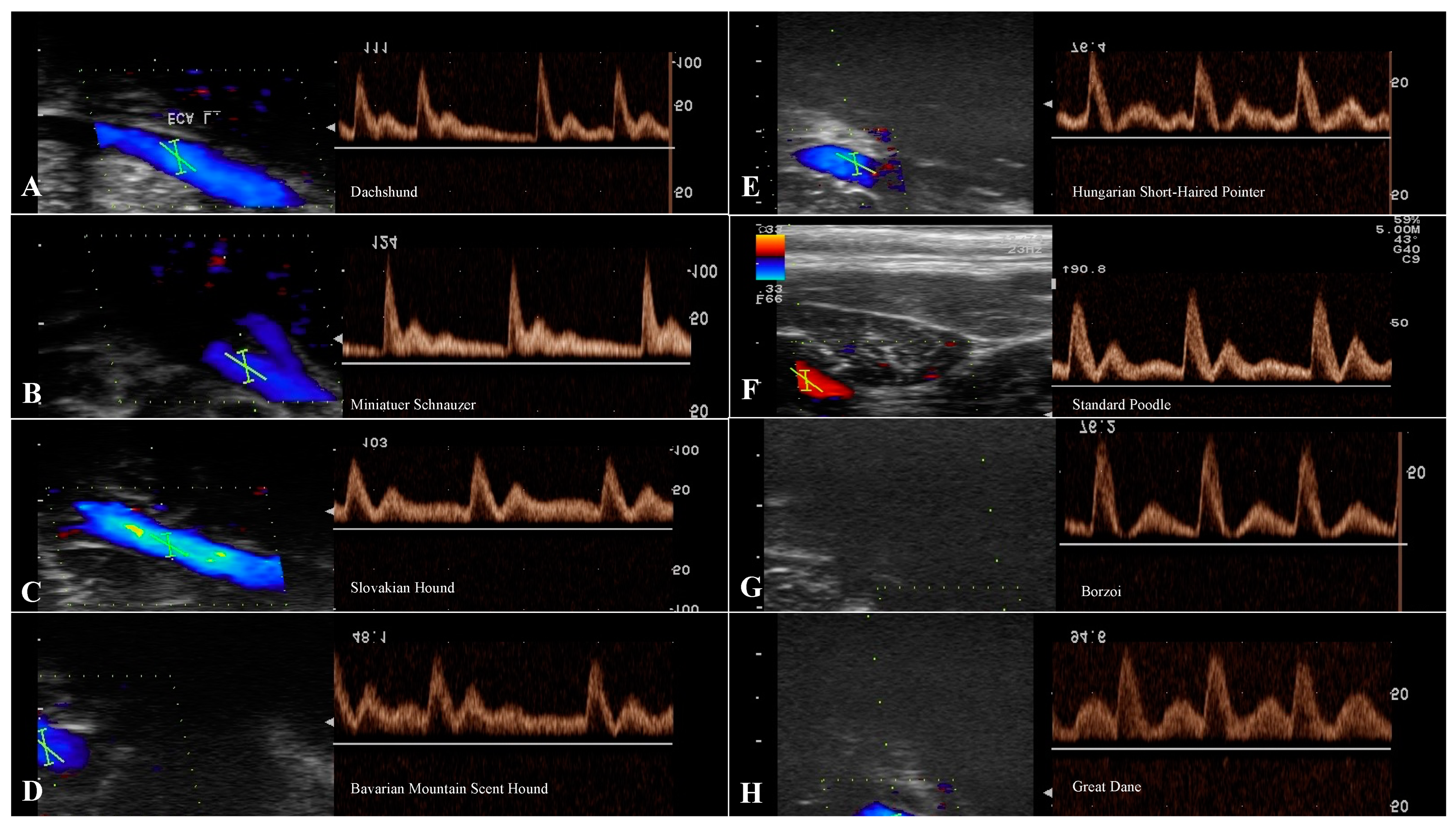
2.2. Spectral Doppler Examination
- -
- Arterial waveform nomenclature major descriptors—1. Flow direction (antegrade, retrograde, bidirectional, absent), 2. Phasicity (multiphasic, monophasic), 3. Resistance (high, intermediate, low).
- -
- Additional modifier terms:
- a
- Upstroke:
- -
- Rapid: Nearly vertical slope or steep rise to peak systole
- -
- Prolonged: Gradual slope to peak systole.
- b
- Sharp peak:
- -
- Sharp, single, and well-defined peak, often with maximum
- -
- Velocity, within range of the artery being interrogated.
- c
- Spectral broadening:
- -
- Widening of the velocity band in the spectral waveform, a ‘filling in’ of the clear ‘window’ under the systolic peak. Spectral broadening is commonly seen in turbulent flow but can also be seen in the absence of turbulence.
- d
- Staccato:
- -
- A very high-resistance pattern with a short ‘spike’ of velocity acceleration and deceleration followed by a short and low-amplitude diastolic signal reflecting low antegrade flow.
- e
- Dampened:
- -
- Combined finding of an abnormal upstroke (delayed) and peak (broad), often with decreased velocity.
- f
- Flow reversal:
- -
- Flow that changes direction, not as part of normal diastolic flow reversal, which may be transient (positional) or consistent with each cardiac cycle (systole/diastole).
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Gender and Breed Related Differences
3.2.1. Gender Differences
3.2.2. Breed Differences
3.3. Evaluation of the CCA
3.4. Evaluation of the ECA
3.5. Evaluation of the ICA
Inter-, Intra-Observer Reliability (ICC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Svicero, D.J.; Doiche, D.P.; Mamprim, M.J.; Heckler, M.C.T.; Amorim, R.M. Ultrasound evaluation of common carotid artery blood flow in the Labrador retriever. BMC Vet. Res. 2013, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, J.; Kiełbowicz, Z.; Zaleska-Dorobisz, U.; Atamaniuk, W.; Pietsch-Fulbiszewska, A.; Kinda, W. Resistive index (RI) obtained in renal interlobar arteries of normal dogs and cats by means of Doppler ultrasonography. Pak. Vet. J. 2016, 36, 45–48. [Google Scholar]
- Szatmári, V.; Sótonyi, P.; Vörös, K. Normal duplex Doppler waveforms of major abdominal blood vessels in dogs: A review. Vet. Radiol. Ultrasound 2001, 42, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Choi, M.; Yoon, J.; Jung, J. Spectral waveform analysis of major arteries in conscious dogs by Doppler ultrasonography. Vet. Radiol. Ultrasound 2004, 45, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Mahadappa, P.; Jeyaraja, K. Doppler Ultrasonography of Abdominal Vasculature in Canines. Int. J. Adv. Res. 2015, 3, 1167–1171. [Google Scholar]
- Neelis, D.A.; Mattoon, J.S.; Nyland, T.G. Chapter 6—Neck, Small Animal Diagnostic Ultrasound, 3rd ed.; John, S., Mattoon, T., Nyland, G., Saunders, W.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 155–187. ISBN 9781416048671. [Google Scholar] [CrossRef]
- Pati, P.M.; Patra, B.; Jena, B. Hemodynamic Changes in Uterine Artery during Pyometra in Bitches. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 381–385. [Google Scholar] [CrossRef]
- Carrillo, J.D.; Soler, M.; Lucas, X.; Agut, A. Colour and pulsed Doppler ultrasonographic study of the canine testis. Reprod. Domest. Anim. 2012, 47, 655–659. [Google Scholar] [CrossRef]
- Rasyada, A.R.; Azhim, A. Flow Velocity in Common Carotid Artery, Carotid Artery; IntechOpen: Rijeka, Croatia, 2018; Volume 4. [Google Scholar]
- Beach, K.W.; Bergelin, R.O.; Leotta, D.F.; Primozich, J.F.; Sevareid, P.M.; Stutzman, E.T.; Zierler, R.E. Standardized ultrasound evaluation of carotid stenosis for clinical trials: University of Washington Ultrasound Reading Center. Cardiovasc Ultrasound 2010, 8, 39. [Google Scholar] [CrossRef]
- Kim, E.S.; Sharma, A.M.; Scissons, R.; Dawson, D.; Eberhardt, R.T.; Gerhard-Herman, M.; Hughes, J.P.; Knight, S.; Marie Kupinski, A.; Mahe, G.; et al. Interpretation of peripheral arterial and venous Doppler waveforms: A consensus statement from the Society for Vascular Medicine and Society for Vascular Ultrasound. Vasc. Med. 2020, 25, 484–506. [Google Scholar] [CrossRef]
- Karen-Quirk, D.; Bandyk, F. Interpretation of carotid duplex testing. Semin. Vasc. Surg. 2013, 26, 72–85. [Google Scholar] [CrossRef]
- Oglat, A.A.; Matjafri, M.Z.; Suardi, N.; Oqlat, M.A.; Abdelrahman, M.A.; Oqlat, A.A. A review of medical doppler ultrasonography of blood flow in general and especially in common carotid artery. J. Med. Ultrasound 2018, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Panagiotis, G.; Xenoulis, J.; Steiner, M. Lipid metabolism and hyperlipidemia in dogs. Vet. J. 2010, 183, 12–21. [Google Scholar]
- Kelly, D.F. Classification of naturally occurring arterial disease in the dog. Toxicol. Pathol. 1989, 17 Pt 2, 77–93. [Google Scholar] [CrossRef]
- Hess, R.S.; Kass, P.H.; Van Winkle, T.J. Association between diabetes mellitus, hypothyroidism or hyperadrenocorticism, and atherosclerosis in dogs. J. Vet. Intern. Med. 2003, 17, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.S.G.; Nahar, T.; Kalashyan, H.; Becher, H.; Nanda, N.C. Ultrasound assessment of carotid arteries: Current concepts, methodologies, diagnostic criteria, and technological advancements. Echocardiography 2018, 35, 2079–2091. [Google Scholar] [CrossRef] [PubMed]
- Batton, D.G.; Nardis, E.E. The effect of intraventricular blood on cerebral blood flow in newborn dogs. Pediatr. Res. 1987, 21, 511–515. [Google Scholar] [CrossRef]
- Werner, C.; Kochs, E.; Hoffman, W.E.; Blanc, I.F.; Esch, J.S.A. Cerebral blood flow and cerebral blood flow velocity during angiotensin-induced arterial hypertension in dogs. Can. J. Anaesth. 1993, 40, 755–760. [Google Scholar] [CrossRef]
- Lee, W. General principles of carotid Doppler ultrasonography. Ultrasonography 2014, 33, 11–17. [Google Scholar] [CrossRef]
- Wood, M.M.; Romine, L.E.; Lee, Y.K.; Richman, K.M.; O’Boyle, M.K.; Paz, D.A.; Chu, P.K.; Pretorius, D.H. Spectral Doppler signature waveforms in ultrasonography: A review of normal and abnormal waveforms. Ultrasound Q. 2010, 26, 83–99. [Google Scholar] [CrossRef]
- Meyer, J.I.; Khalil, R.M.; Obuchowski, N.A.; Baus, L.K. Common carotid artery: Variability of Doppler US velocity measurements. Radiology 1997, 204, 339–341. [Google Scholar] [CrossRef]
- Rohren, E.M.; Kliewer, M.A.; Carroll, B.A.; Hertzberg, B.S. A spectrum of Doppler waveforms in the carotid and vertebral arteries. AJR Am. J. Roentgenol. 2003, 181, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Donboklang, L.; Chhunthang, D.; Aman, Y.K.; Evarisalin, M.; Ibamelaker, T. Effects of dynamic range variations on the Doppler flow velocities of common carotid arteries. Artery Res. 2018, 22, 18–23. [Google Scholar]
- Scissons, R. Characterizing Triphasic, Biphasic, and Monophasic Doppler Waveforms: Should a Simple Task Be So Difficult? J. Diagn. Med. Sonogr. 2008, 24, 269–276. [Google Scholar] [CrossRef]
- Hoskins, P.R. A review of the measurement of blood velocity and related quantities using Doppler ultrasound. Proc. Inst. Mech. Eng. Part H 1999, 213, 391–400. [Google Scholar] [CrossRef]
- Unal, B.; Bagcier, S.; Simsir, I.; Bilgili, Y.; Kara, S. Evaluation of differences between observers and automatic-manual measurements in calculation of Doppler parameters. J. Ultrasound Med. 2004, 23, 1041–1048. [Google Scholar] [CrossRef]
- Mikkonen, R.H.; Kreula, J.M.; Virkkunen, P.J. Reliability of Doppler ultrasound in follow-up studies. Acta Radiol. 1998, 39, 193–199. [Google Scholar] [CrossRef]
- Park, M.Y.; Jung, S.E.; Choi, J.I. Optimization of beam-flow angles for Doppler ultrasound flow velocity measurements using slanted gel pads. SpringerPlus 2016, 5, 328. [Google Scholar] [CrossRef]
- Zoli, M.; Merkel, C.; Sabbà, C.; Sacerdoti, D.; Gaiani, S.; Ferraioli, G.; Bolondi, L. Interobserver and inter-equipment variability of echo-Doppler sonographic evaluation of the superior mesenteric artery. J. Ultrasound Med. 1996, 15, 99–106. [Google Scholar] [CrossRef]
- Revzin, M.V.; Imanzadeh, A.; Menias, C.; Pourjabbar, S.; Mustafa, A.; Nezami, N.; Spektor, M.; Pellerito, J.S. Optimizing Image Quality When Evaluating Blood Flow at Doppler US: A Tutorial. RadioGraphics 2019, 39, 1501–1523. [Google Scholar] [CrossRef]
- Mahé, G.; Boulon, C.; Desormais, I.; Lacroix, P.; Bressollette, L.; Guilmot, J.L.; Le Hello, C.; Sevestre, M.A.; Pernod, G.; Constans, J.; et al. Statement for Doppler waveforms analysis. Vasa 2017, 46, 337–345. [Google Scholar] [CrossRef]
- Miyajima, T.; Yokoyama, H.; Taira, H.; Tsuji, Y. Quantitative estimation of renal blood flow by power Doppler ultrasonography in renovascular hypertensive dogs. Kidney Int. 2005, 68, 2781–2786. [Google Scholar] [CrossRef] [PubMed]
- Meurs, K.M.; Miller, M.W.; Slater, M.R.; Glaze, K. Arterial blood pressure measurement in a population of healthy geriatric dogs. J. Am. Anim. Hosp. Assoc. 2000, 36, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Pascala, R.; Yvesa, F.; Bronwyn, K.; Ahimastos, A. Gender differences in artery wall biomechanical properties throughout life. J. Hypertens. 2011, 29, 1023–1033. [Google Scholar]
- Juonala, M.; Kähönen, M.; Laitinen, T.; Hutri-Kähönen, N.; Jokinen, E.; Taittonen, L.; Pietikäinen, M.; Helenius, H.; Viikari, J.S.; Raitakari, O.T. Effect of age and sex on carotid intima-media thickness, elasticity and brachial endothelial function in healthy adults: The cardiovascular risk in Young Finns Study. Eur. Heart J. 2008, 29, 1198–1206. [Google Scholar] [CrossRef]
- Krejza, J.; Arkuszewski, M.; Kasner, S.E.; Weigele, J.; Ustymowicz, A.; Hurst, R.W.; Cucchiara, B.L.; Messe, S.R. Carotid artery diameter in men and women and the relation to body and neck size. Stroke 2006, 37, 1103–1105. [Google Scholar] [CrossRef]
- Hamdon, Z.; Azhim, A.; Saleh, M.; Baghepour, P.; Kinouchi, Y.; Ibrahim, F. Assessment of First Derivative of Doppler Blood Flow Velocity in Vascular Aging. IFMBE Proc. 2014, 43, 68–71. [Google Scholar]
- Trihan, J.E.; Perez-Martin, A.; Guillaumat, J.; Lanéelle, D. Normative and pathological values of hemodynamic and Doppler ultrasound arterial findings in children. Vasa 2020, 49, 264–274. [Google Scholar] [CrossRef]
- Zhang, X.; Haneishi, H.; Liu, H. Multiscale modeling of the cardiovascular system for infants, children, and adolescents: Age-related alterations in cardiovascular parameters and hemodynamics. Comput. Biol. Med. 2019, 108, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Kozàkovà, M.; Palombo, C.; Morizzo, C.; Nolan, J.J.; Konrad, T.; Dekker, J.M.; Balkau, B.; Nilsson, P.M. Gender-specific differences in carotid intima-media thickness and its progression over three years: A multicenter European study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 151–158. [Google Scholar] [CrossRef]





| CCA | |||||||
|---|---|---|---|---|---|---|---|
| n | Ø (mm) | RI | PSV (cm/s) | EDV (cm/s) | S/D | ||
| Small breeds | Dachshund | 13 | 2.5 | 0.84 | 130.6 ± 15.56 | 17.98 ± 4.48 | 7.84 ± 2.1 |
| Miniature Schnauzer | 13 | 2.3 | 0.84 | 127.3 ± 13.49 | 19.57 ± 3.68 | 6.85 ± 1.7 | |
| Medium breeds | Slovakian Hound | 13 | 3.5 | 0.81 | 108.1 ± 17.39 | 19.96 ± 6.83 | 6.47 ± 2.9 |
| Bavarian Mountain Scent Hound | 13 | 3.6 | 0.76 | 115.4 ± 21.7 | 30.71 ± 7.1 | 3.88 ± 0.4 | |
| Large breeds | Hungarian Short-Haired Pointer | 13 | 3.5 | 0.76 | 119.9 ± 16.07 | 27.52 ± 5.63 | 4.63 ± 0.98 |
| Standard Poodle | 13 | 3.5 | 0.79 | 106.7 ± 9.2 | 22.31 ± 4.49 | 5 ± 0.77 | |
| Giant breeds | Borzoi | 13 | 3.2 | 0.78 | 96.54 ± 6.21 | 22.3 ± 1.56 | 4.49 ± 0.54 |
| Great Dane | 13 | 5.7 | 0.66 | 94.37 ± 18.7 | 31.43 ± 12 | 3.40 ± 0.95 | |
| ECA | ||||||
|---|---|---|---|---|---|---|
| n | RI | PSV (cm/s) | EDV (cm/s) | S/D | ||
| Small breeds | Dachshund | 13 | 0.79 | 76.21 ± 13.77 | 17.71 ± 4.91 | 4.77 ± 1.34 |
| Miniature Schnauzer | 13 | 0.79 | 78.81 ± 19.23 | 18.05 ± 6.02 | 4.76 ± 1.17 | |
| Medium breeds | Slovakian Hound | 13 | 0.74 | 73.08 ± 13.76 | 19.78 ± 5.29 | 3.89 ± 0.92 |
| Bavarian Mountain Scent Hound | 13 | 0.71 | 80.45 ± 17.78 | 24.21 ± 7.91 | 3.57 ± 0.87 | |
| Large breeds | Hungarian Short-Haired Pointer | 13 | 0.73 | 71.74 ± 15.42 | 19.14 ± 2.58 | 3.86 ± 0.79 |
| Standard Poodle | 13 | 0.73 | 65.98 ± 15.01 | 20.42 ± 5.62 | 3.34 ± 0.54 | |
| Giant breeds | Borzoi | 13 | 0.79 | 76.13 ± 4.53 | 17.14 ± 1.23 | 4.55 ± 0.39 |
| Great Dane | 13 | 0.65 | 68.19 ± 10.15 | 22.6 ± 5.09 | 3.12 ± 0.59 | |
| ICA | ||||||
|---|---|---|---|---|---|---|
| n | RI | PSV (cm/s) | EDV (cm/s) | S/D | ||
| Small breeds | Dachshund | 13 | 0.56 | 29.52 ± 8.86 | 12.82 ± 4.52 | 2.40 ± 0.47 |
| Miniature Schnauzer | 13 | 0.60 | 32.75 ± 8.47 | 12.29 ± 1.99 | 2.66 ± 0.44 | |
| Medium breeds | Slovakian Hound | 13 | 0.60 | 28.55 ± 7.87 | 11.51 ± 3.43 | 2.56 ± 0.33 |
| Bavarian Mountain Scent Hound | 13 | 0.58 | 31.99 ±16.22 | 11.54 ± 2.61 | 2.7 ± 0.74 | |
| Large breeds | Hungarian Short-Haired Pointer | 13 | 0.66 | 24.55 ± 7.19 | 8.84 ± 3.58 | 2.98 ± 0.49 |
| Standard Poodle | 13 | 0.64 | 24.42 ± 7.71 | 9.31 ± 3.43 | 2.73 ± 0.25 | |
| Giant breeds | Borzoi | 13 | 0.64 | 43.26 ± 12.3 | 17.2 ± 3.18 | 2.55 ± 0.36 |
| Great Dane | 13 | 0.66 | 29.27 ± 8.8 | 10.17 ± 4.08 | 3.01 ± 0.48 | |
| Variable | Male (n = 51) | Female (n = 53) | p < 0.05 |
|---|---|---|---|
| Age (years) | 2.45 ± 0.98 | 2.6 ± 1.1 | ns |
| Weight (kg) | 26.46 ± 17.14 | 21.8 ± 13.18 | ns |
| Diameter CCA (mm) | 3.56 ± 1.09 | 3.25 ± 0.92 | 0.001 |
| Intima media CCA (mm) | 0.32 ± 0.04 | 0.29 ± 0.05 | 0.05 |
| RI CCA | 0.78 ± 0.07 | 0.8 ± 0.06 | ns |
| PSV CCA (cm/s) | 113.3 ± 21.6 | 112.5 ± 16.5 | ns |
| EDV CCA (cm/s) | 24.9 ± 8.4 | 23.2 ± 6.72 | ns |
| RI ECA (cm/s) | 0.74 ± 0.07 | 0.73 ± 0.06 | ns |
| PSV ECA (cm/s) | 76.42 ± 14.32 | 72.04 ± 12.57 | ns |
| EDV ECA (cm/s) | 20.34 ± 5.31 | 19.57 ± 4.86 | ns |
| RI ICA (cm/s) | 0.63 ± 0.07 | 0.62 ± 0.06 | ns |
| PSV ICA (cm/s) | 31.66 ± 12.35 | 29.18 ± 8.85 | ns |
| EDV ICA (cm/s) | 12.05 ± 4.23 | 11.26 ± 3.86 | ns |
| Breed-Related Differences in PSV, EDV and RI CCA Data | |||
|---|---|---|---|
| Breed | PSV CCA p < 0.05 | EDV CCA p < 0.05 | RI CCA p < 0.05 |
| Dachshund vs. Standard Poodle | 0.05 | ns | ns |
| Dachshund vs. Bavarian Mountain Scent Hound | ns | 0.001 | 0.001 |
| Dachshund vs. Hungarian Short-Haired Pointer | ns | 0.01 | 0.001 |
| Dachshund vs. Borzoi | 0.01 | ns | 0.01 |
| Dachshund vs. Great Dane | 0.05 | 0.0001 | 0.001 |
| Miniature Schnauzer vs. Hungarian Short-Haired Pointer | ns | 0.01 | ns |
| Miniature Schnauzer vs. Standard Poodle | 0.01 | ns | ns |
| Miniature Schnauzer vs. Borzoi | 0.01 | ns | ns |
| Miniature Schnauzer vs. Great Dane | 0.05 | 0.01 | 0.05 |
| Slovakian Hound vs. Bavarian Mountain Scent Hound | ns | 0.01 | ns |
| Slovakian Hound vs. Great Dane | ns | 0.01 | 0.05 |
| Hungarian Short-Haired Pointer vs. Borzoi | 0.05 | ns | ns |
| Hungarian Short-Haired Pointer vs. Great Dane | ns | ns | 0.05 |
| Standard Poodle vs. Great Dane | ns | 0.05 | 0.05 |
| Borzoi vs. Great Dane | ns | 0.05 | 0.05 |
| Breed-Related Differences in RI ECA Data | ||
|---|---|---|
| Breed | RI ECA | p < 0.05 |
| Dachshund vs. Great Dane | 0.77 ± 0.06 vs. 0.66 ± 0.05 | 0.001 |
| Miniature Schnauzer vs. Great Dane | 0.77 ± 0.06 vs. 0.66 ± 0.05 | 0.001 |
| Bavarian Mountain Scent Hound vs. Borzoi | 0.70 ± 0.08 vs. 0.80 ± 0.13 | 0.01 |
| Standard Poodle vs. Borzoi | 0.70 ± 0.04 vs. 0.80 ± 0.13 | 0.05 |
| Borzoi vs. Great Dane | 0.80 ± 0.13 vs. 0.66 ± 0.05 | <0.0001 |
| Breed-Related Differences in PSV, EDV, and RI ICA Data | |||
|---|---|---|---|
| PSV ICA | EDV ICA | RI ICA | |
| Breed | p < 0.05 | p < 0.05 | p < 0.05 |
| Dachshund vs. Hungarian Short-Haired Pointer | ns | ns | 0.01 |
| Dachshund vs. Borzoi | 0.05 | ns | 0.01 |
| Dachshund vs. Great Dane | ns | ns | 0.01 |
| Miniature Schnauzer vs. Borzoi | ns | 0.045 | ns |
| Slovakian Hound vs. Borzoi | 0.05 | 0.0105 | ns |
| Bavarian Mountain Hound vs. Borzoi | ns | 0.01 | ns |
| Hungarian Short-Haired Pointer vs. Borzoi | 0.001 | <0.0001 | ns |
| Standard Poodle vs. Borzoi | 0.01 | <0.0001 | ns |
| Borzoi vs. Great Dane | 0.05 | 0.001 | ns |
| ICC Inter-Observer | |||||||
|---|---|---|---|---|---|---|---|
| PSV | EDV | ||||||
| CCA | ECA | ICA | CCA | ECA | ICA | ||
| Small breeds | Dachshund | 0.75 | 0.68 | 0.76 | 0.79 | 0.69 | 0.79 |
| Miniature Schnauzer | 0.85 | 0.79 | 0.92 | 0.88 | 0.8 | 0.91 | |
| Medium breeds | Slovakian Hound | 0.62 | 0.57 | 0.63 | 0.65 | 0.61 | 0.62 |
| Bavarian Mountain Scent Hound | 0.86 | 0.81 | 0.79 | 0.83 | 0.83 | 0.81 | |
| Large breeds | Hungarian Short-Haired Pointer | 0.88 | 0.87 | 0.85 | 0.76 | 0.83 | 0.8 |
| Standard Poodle | 0.76 | 0.77 | 0.8 | 0.79 | 0.69 | 0.8 | |
| Giant breeds | Borzoi | 0.91 | 0.86 | 0.9 | 0.9 | 0.89 | 0.92 |
| Great Dane | 0.93 | 0.89 | 0.91 | 0.89 | 0.92 | 0.9 | |
| ICC Intra-Observer | |||||||
| PSV | EDV | ||||||
| CCA | ECA | ICA | CCA | ECA | ICA | ||
| Observer 1 | 0.92 | 0.93 | 0.87 | 0.91 | 0.89 | 0.93 | |
| Observer 2 | 0.9 | 0.89 | 0.91 | 0.9 | 0.92 | 0.91 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ševčíková, M.K.; Figurová, M.; Ševčík, K.; Hluchý, M.; Domaniža, M.; Lapšanská, M.; Drahovská, Z.; Žert, Z. Ultrasound Evaluation of Extracranial Cerebral Circulation (The Common, External and Internal Carotid Artery) in Different Breeds of Dogs. Animals 2023, 13, 1584. https://doi.org/10.3390/ani13101584
Ševčíková MK, Figurová M, Ševčík K, Hluchý M, Domaniža M, Lapšanská M, Drahovská Z, Žert Z. Ultrasound Evaluation of Extracranial Cerebral Circulation (The Common, External and Internal Carotid Artery) in Different Breeds of Dogs. Animals. 2023; 13(10):1584. https://doi.org/10.3390/ani13101584
Chicago/Turabian StyleŠevčíková, Marieta K., Mária Figurová, Karol Ševčík, Marián Hluchý, Michal Domaniža, Mária Lapšanská, Zuzana Drahovská, and Zdeněk Žert. 2023. "Ultrasound Evaluation of Extracranial Cerebral Circulation (The Common, External and Internal Carotid Artery) in Different Breeds of Dogs" Animals 13, no. 10: 1584. https://doi.org/10.3390/ani13101584
APA StyleŠevčíková, M. K., Figurová, M., Ševčík, K., Hluchý, M., Domaniža, M., Lapšanská, M., Drahovská, Z., & Žert, Z. (2023). Ultrasound Evaluation of Extracranial Cerebral Circulation (The Common, External and Internal Carotid Artery) in Different Breeds of Dogs. Animals, 13(10), 1584. https://doi.org/10.3390/ani13101584






