Impact of a Blend of Microencapsulated Organic Acids and Botanicals on the Microbiome of Commercial Broiler Breeders under Clinical Necrotic Enteritis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Husbandry
2.2. Clostridium Perfringens Preparation
2.3. Necrotic Enteritis Model
2.4. Scoring Necrotic Enteritis Lesions
2.5. DNA Extraction
2.6. Library Preparation
2.7. Bioinformatic Analyses
3. Results
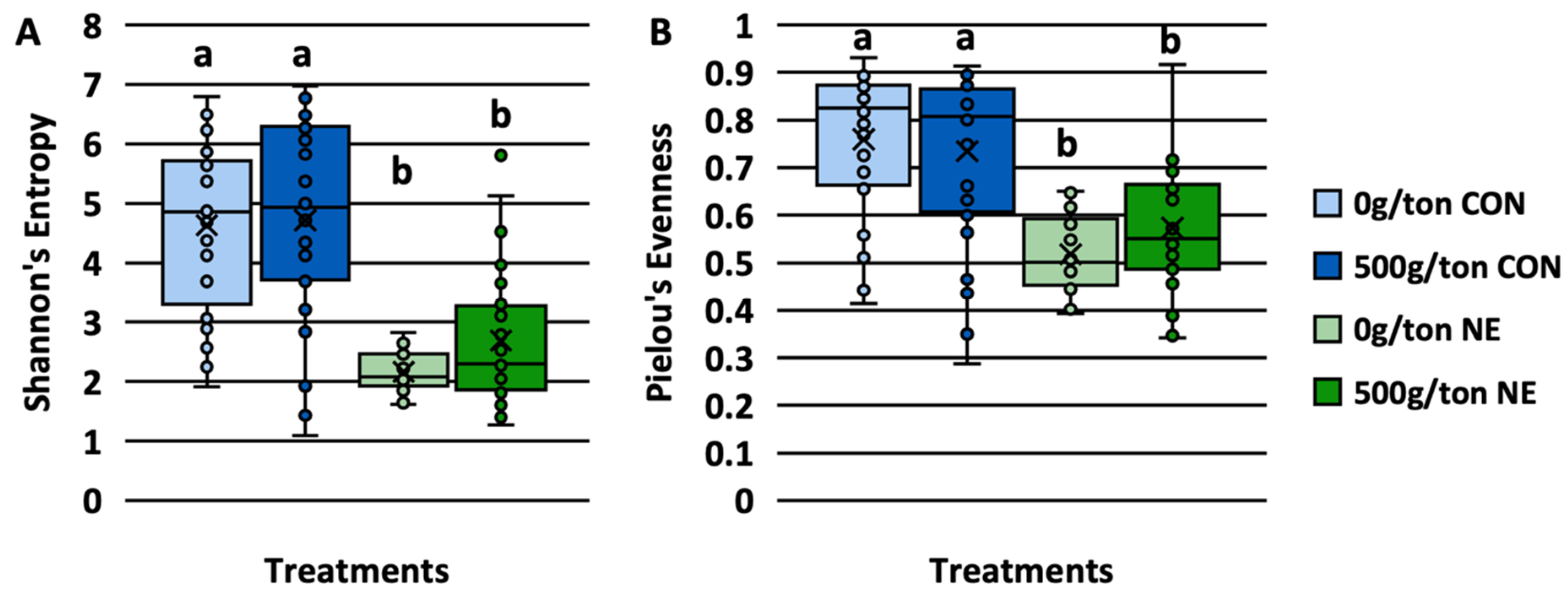
3.1. Alpha Diversity and Its Relation to Lesion Scores
3.2. Beta Diversity Strongly Related to Supplement and Necrotic Enteritis Infection
3.3. Core Communities Shifted by Feed Additive and Infection
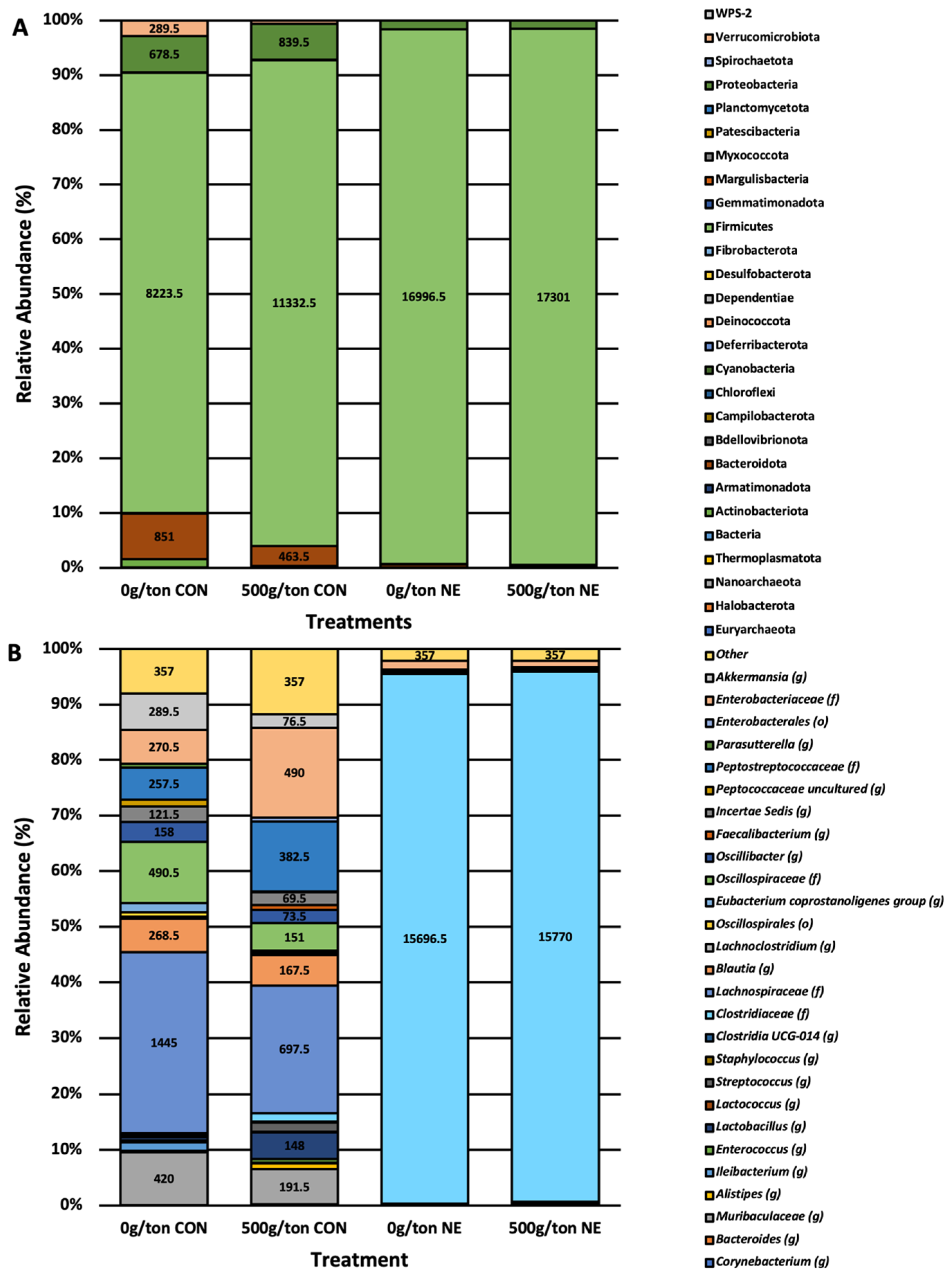
3.4. Relative Abundance of Taxa Impacted by Diet Supplementation and NE Infection
4. Discussion
4.1. Lesion Scores Impact on Richness and Evenness
4.2. Treatment and Infection on Abundance and Phylogenetic Diversity
4.3. Necrotic Enteritis Narrows Core Microbiome
4.4. Lactobacillus and Clostridiaceae Core ASVs of Microencapsulated Blend
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paiva, D.; McElroy, A. Necrotic enteritis: Applications for the poultry industry. J. Appl. Poult. Res. 2014, 23, 557–566. [Google Scholar] [CrossRef]
- Van der Sluis, W. Clostridial enteritis is an often underestimated problem. World Poult. 2000, 16, 42–43. [Google Scholar]
- Castanon, J.I.R. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. Deciphering desirable immune responses from disease models with resistant and susceptible chickens. Poult. Sci. 2019, 98, 1634–1642. [Google Scholar] [CrossRef]
- Adhikari, P.; Kiess, A.; Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]
- Wade, B.; Keyburn, A. The True Cost of Necrotic Enteritis. Available online: https://www.poultryworld.net/Meat/Articles/2015/10/The-true-cost-of-necrotic-enteritis-2699819W/ (accessed on 3 May 2023).
- Van Immerseel, F.; De Buck, J.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef]
- Cooper, K.K.; Songer, J.G. Necrotic enteritis in chickens: A paradigm of enteric infection by Clostridium perfringens type A. Anaerobe 2009, 15, 55–60. [Google Scholar] [CrossRef]
- Geier, M.; Mikkelsen, L.; Torok, V.; Allison, G.; Olnood, C.; Boulianne, M.; Hughes, R.; Choct, M. Comparison of alternatives to in-feed antimicrobials for the prevention of clinical necrotic enteritis. J. Appl. Microbiol. 2010, 109, 1329–1338. [Google Scholar] [CrossRef]
- Piva, A.; Pizzamiglio, V.; Morlacchini, M.; Tedeschi, M.; Piva, G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine 1, 2. J. Anim. Sci. 2007, 85, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Svihus, B. Function of the digestive system. J. Appl. Poult. Res. 2014, 23, 306–314. [Google Scholar] [CrossRef]
- Svihus, B. Starch digestion capacity of poultry. Poult. Sci. 2014, 93, 2394–2399. [Google Scholar] [CrossRef] [PubMed]
- Feye, K.M.; Swaggerty, C.L.; Kogut, M.H.; Ricke, S.C.; Piva, A.; Grilli, E. The biological effects of microencapsulated organic acids and botanicals induces tissue-specific and dose-dependent changes to the Gallus gallus microbiota. BMC Microbiol. 2020, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Grilli, E.; Tugnoli, B.; Passey, J.L.; Stahl, C.H.; Piva, A.; Moeser, A.J. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. BMC Vet. Res. 2015, 11, 96. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Byrd, J.A.; Arsenault, R.J.; Perry, F.; Johnson, C.N.; Genovese, K.J.; He, H.; Kogut, M.H.; Piva, A.; Grilli, E. A blend of microencapsulated organic acids and botanicals reduces necrotic enteritis via specific signaling pathways in broilers. Poult. Sci. 2022, 101, 101753. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Arsenault, R.J.; Johnson, C.; Piva, A.; Grilli, E. Dietary supplementation with a microencapsulated blend of organic acids and botanicals alters the kinome in the ileum and jejunum of Gallus gallus. PLoS ONE 2020, 15, e0236950. [Google Scholar] [CrossRef] [PubMed]
- Swaggerty, C.L.; He, H.; Genovese, K.J.; Callaway, T.R.; Kogut, M.H.; Piva, A.; Grilli, E. A microencapsulated feed additive containing organic acids, thymol, and vanillin increases in vitro functional activity of peripheral blood leukocytes from broiler chicks. Poult. Sci. 2020, 99, 3428–3436. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- AgGuide. Guide for the Care and Use of Agricultural Animals in Research and Teaching. Available online: https://www.aaalac.org/pub/?id=E900BDB6-CCCF-AB13-89B6-DA98A4B52218 (accessed on 3 May 2023).
- McReynolds, J.L.; Byrd, J.A.; Genovese, K.J.; Poole, T.L.; Duke, S.E.; Farnell, M.B.; Nisbet, D.J. Dietary Lactose and its Effect on the Disease Condition of Necrotic Enteritis. Poult. Sci. 2007, 86, 1656–1661. [Google Scholar] [CrossRef]
- Prescott, J.F. The Prevention of Experimentally Induced Necrotic Enteritis in Chickens by Avoparcin. Avian Dis. 1979, 23, 1072. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Dillon, M.R.; Zhang, Y.; Rideout, J.R.; Bolyen, E.; Li, H.; Albert, P.S.; Caporaso, J.G. q2-longitudinal: Longitudinal and Paired-Sample Analyses of Microbiome Data. Msystems 2018, 3, e00219-18. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Shannon, C.A. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Jaccard, P. The Distribution of the Flora in the Alpine Zone.1. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.Å.; Knight, R.T.; Peddada, S.D. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2013, 42, D643–D648. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, A.S.; Leo, L. Microbiomeutilities: Utilities for Microbiome Analytics; R Package Version 1.00.16; GitHub: San Francisco, CA, USA, 2020. [Google Scholar]
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed. Sci. Technol. 2018, 250, 32–40. [Google Scholar] [CrossRef]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J Anim Sci Biotechnol. 2020, 11, 18. [Google Scholar] [CrossRef]
- Caly, D.L.; D’Inca, R.; Auclair, E.; Drider, D. Alternatives to Antibiotics to Prevent Necrotic Enteritis in Broiler Chickens: A Microbiologist’s Perspective. Front. Microbiol. 2015, 6, 1336. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Calik, A.; White, M.B.; Kimminau, E.A.; Dalloul, R.A. Effect of Probiotics and Multi-Component Feed Additives on Microbiota, Gut Barrier and Immune Responses in Broiler Chickens During Subclinical Necrotic Enteritis. Front. Veter. Sci. 2020, 7, 572142. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Keyburn, A.L.; Denman, S.E.; Moore, R.J. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Veter. Microbiol. 2012, 159, 155–162. [Google Scholar] [CrossRef]
- Stanley, D.; Wu, S.-B.; Rodgers, N.; Swick, R.; Moore, R.J. Differential Responses of Cecal Microbiota to Fishmeal, Eimeria and Clostridium perfringens in a Necrotic Enteritis Challenge Model in Chickens. PLoS ONE 2014, 9, e104739. [Google Scholar] [CrossRef]
- Lacey, J.A.; Stanley, D.; Keyburn, A.L.; Ford, M.; Chen, H.; Johanesen, P.; Lyras, D.; Moore, R.J. Clostridium perfringens-mediated necrotic enteritis is not influenced by the pre-existing microbiota but is promoted by large changes in the post-challenge microbiota. Vet. Microbiol. 2018, 227, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kiu, R.; Brown, J.; Bedwell, H.; Leclaire, C.; Caim, S.; Pickard, D.; Dougan, G.; Dixon, R.A.; Hall, L.J. Genomic analysis on broiler-associated Clostridium perfringens strains and exploratory caecal microbiome investigation reveals key factors linked to poultry necrotic enteritis. Anim. Microbiome 2019, 1, 12. [Google Scholar] [CrossRef]
- Hargis, B. Overview of Necrotic Enteritis in Poultry. Available online: https://www.merckvetmanual.com/poultry/necrotic-enteritis/overview-of-necrotic-enteritis-in-poultry (accessed on 8 November 2022).
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B.; et al. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.D.; Adhikari, B.; Park, S.H.; Teague, K.D.; Graham, L.E.; Mahaffey, B.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Kwon, Y.M.; Ricke, S.C.; et al. Evaluation of the Epithelial Barrier Function and Ileal Microbiome in an Established Necrotic Enteritis Challenge Model in Broiler Chickens. Front. Veter. Sci. 2018, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic Acids and Potential for Modifying the Avian Gastrointestinal Tract and Reducing Pathogens and Disease. Front. Veter. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- Stanley, D.; Hughes, R.J.; Geier, M.S.; Moore, R.J. Bacteria within the Gastrointestinal Tract Microbiota Correlated with Improved Growth and Feed Conversion: Challenges Presented for the Identification of Performance Enhancing Probiotic Bacteria. Front. Microbiol. 2016, 7, 187. [Google Scholar] [CrossRef]



| Infection | Diet | Organisms | ASV |
|---|---|---|---|
| Control | 0 g/MT | Lachnospiraceae (2 unique ASVs) | 10650515e35dcecde52015f8c34a4a06 |
| Lachnospiraceae_Blautia | 0b6c85919018ef580a0e1111b794c86b | ||
| Lachnospiraceae_Blautia_Lachnospiraceae_bacterium | 74e7c89ab2da511c0003f650773f9c2f | ||
| Lachnospiraceae | 492f2e502cce64557efbfad55333f9ca | ||
| Erysipelotrichaceae_Ileibacterium_Ileibacterium_valens | a922583a2cd922f6e2361c1df16702bb | ||
| Enterobacteriaceae | 20cfe7f61d18f6525cc71caae0ab28dc | ||
| Sutterellaceae_Parasutterella | cf39b94723be8d7c4e5b41f4b20dab44 | ||
| Akkermansiaceae_Akkermansia | b6b05223adf86d071fd279f79dc2533c | ||
| Peptostreptococcaceae | ee59a70b1b832493583e7bc2d6488ec7 | ||
| Peptostreptococcaceae | 931dbe33cb8438cfd863ac9acfb5d17f | ||
| 500 g/MT | Lachnospiraceae_Blautia | 0b6c85919018ef580a0e1111b794c86b | |
| Lachnospiraceae_ | 492f2e502cce64557efbfad55333f9ca | ||
| Clostridiaceae_ | dc85940d84ddbd7315db16b14390cb8d | ||
| Lactobacillaceae_Lactobacillus | 44d9fc4de8898b6c82c69654435a9f0b | ||
| Enterobacteriaceae_ | 20cfe7f61d18f6525cc71caae0ab28dc | ||
| Peptostreptococcaceae_ | 931dbe33cb8438cfd863ac9acfb5d17f | ||
| NE-Induced | 0 g/MT | Clostridiaceae | 3be4ebb35ff7f6217fae945cb0ad5413 |
| Clostridiaceae | 2acc5f779e35ac658d8389859048ff61 | ||
| Clostridiaceae | dc85940d84ddbd7315db16b14390cb8d | ||
| Clostridiaceae | 3b55963ebca3b90a7dcf9a27ef40a76a | ||
| Enterobacteriaceae | 20cfe7f61d18f6525cc71caae0ab28dc | ||
| 500 g/MT | Clostridiaceae | 3be4ebb35ff7f6217fae945cb0ad5413 | |
| Clostridiaceae | 2acc5f779e35ac658d8389859048ff61 | ||
| Clostridiaceae | 9dece060ffcff93473a7023dcee0defd | ||
| Clostridiaceae | dc85940d84ddbd7315db16b14390cb8d | ||
| Clostridiaceae | e52b990e8b8ae704003a5ff38b791dbf | ||
| Clostridiaceae | 3b55963ebca3b90a7dcf9a27ef40a76a | ||
| Enterobacteriaceae | 20cfe7f61d18f6525cc71caae0ab28dc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittoe, D.K.; Johnson, C.N.; Byrd, J.A., II; Ricke, S.C.; Piva, A.; Grilli, E.; Swaggerty, C.L. Impact of a Blend of Microencapsulated Organic Acids and Botanicals on the Microbiome of Commercial Broiler Breeders under Clinical Necrotic Enteritis. Animals 2023, 13, 1627. https://doi.org/10.3390/ani13101627
Dittoe DK, Johnson CN, Byrd JA II, Ricke SC, Piva A, Grilli E, Swaggerty CL. Impact of a Blend of Microencapsulated Organic Acids and Botanicals on the Microbiome of Commercial Broiler Breeders under Clinical Necrotic Enteritis. Animals. 2023; 13(10):1627. https://doi.org/10.3390/ani13101627
Chicago/Turabian StyleDittoe, Dana K., Casey N. Johnson, James A. Byrd, II, Steven C. Ricke, Andrea Piva, Ester Grilli, and Christina L. Swaggerty. 2023. "Impact of a Blend of Microencapsulated Organic Acids and Botanicals on the Microbiome of Commercial Broiler Breeders under Clinical Necrotic Enteritis" Animals 13, no. 10: 1627. https://doi.org/10.3390/ani13101627
APA StyleDittoe, D. K., Johnson, C. N., Byrd, J. A., II, Ricke, S. C., Piva, A., Grilli, E., & Swaggerty, C. L. (2023). Impact of a Blend of Microencapsulated Organic Acids and Botanicals on the Microbiome of Commercial Broiler Breeders under Clinical Necrotic Enteritis. Animals, 13(10), 1627. https://doi.org/10.3390/ani13101627







