Expression and Functional Analysis of AMT1 Gene Responding to High Ammonia Stress in Razor Clam (Sinonovacula constricta)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Cultivation
2.2. Ammonia Challenge
2.3. Real-Time Quantitative PCR Analysis
2.4. Temporal Expression of Sc-AMT1 by Western Blotting Analysis
2.5. Sequence Analysis of Sc-AMT1
2.6. Paraffin Section and Immunofluorescence Staining
2.7. RNA Interference Assay
2.8. Determination of Hemolymph Ammonia Concentration
2.9. SNP Validation
2.10. Statistical Analysis
3. Results
3.1. SNP Validation
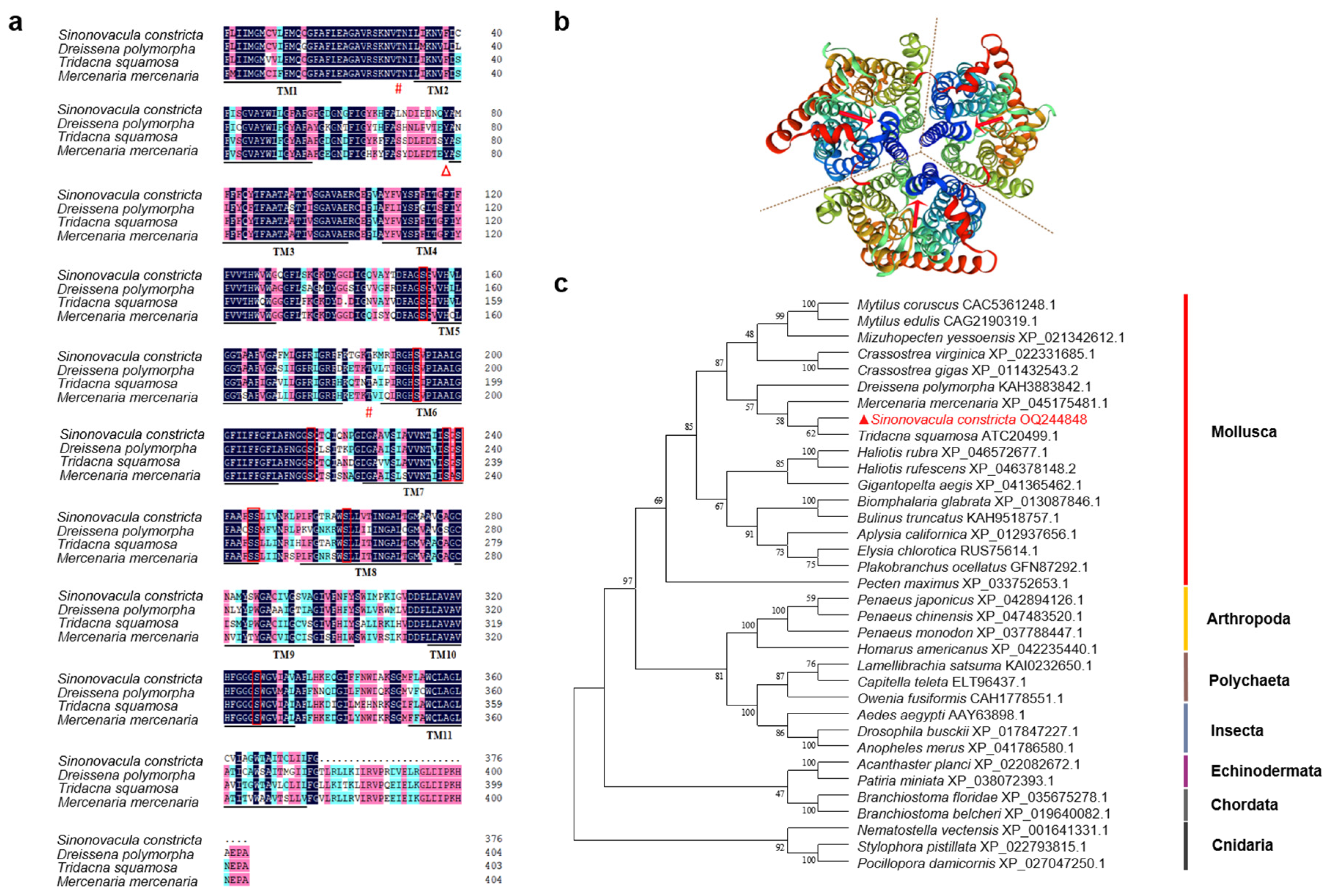
3.2. Deduced Amino Acid Sequence and Phylogenetic Analysis of Sc-AMT1
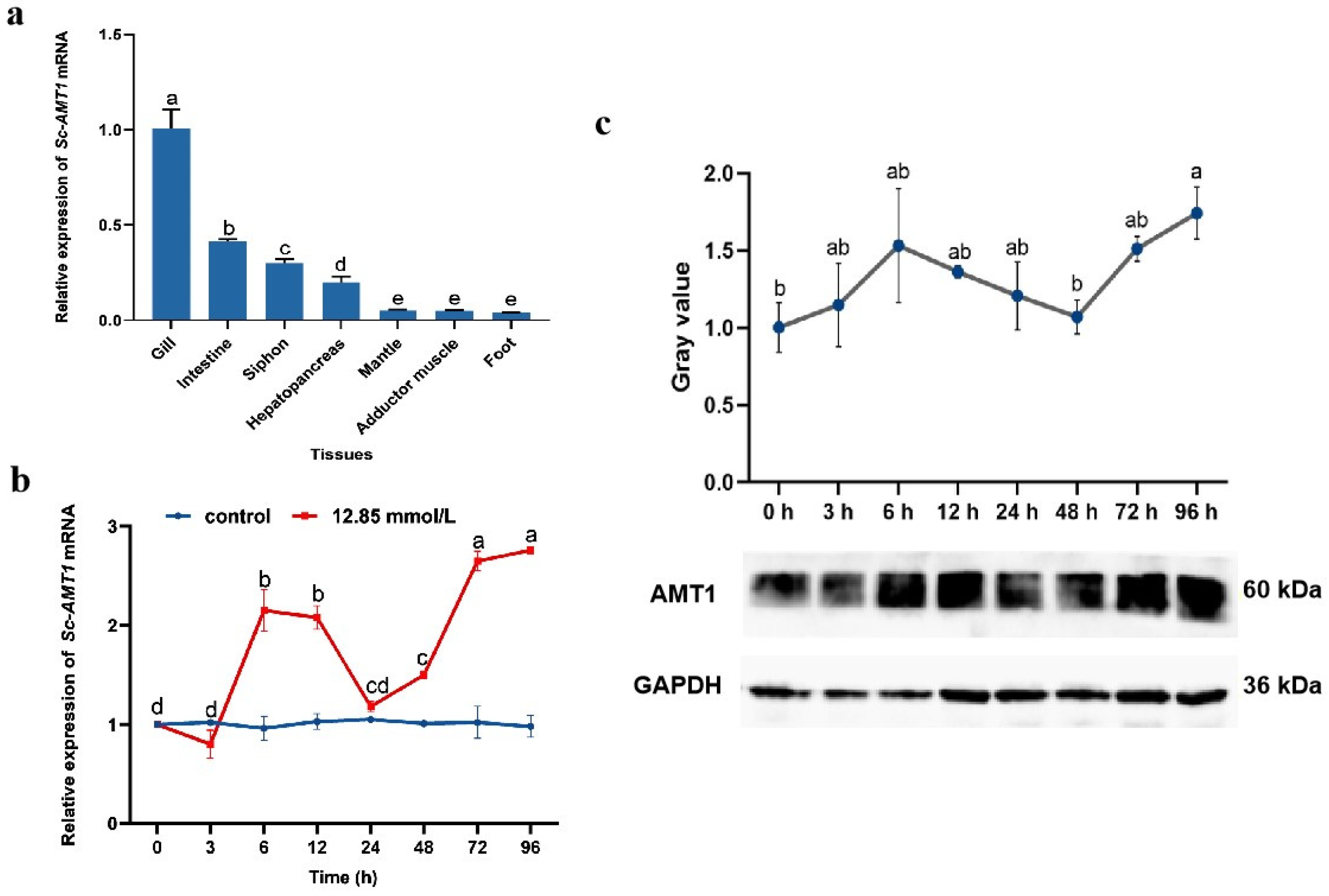
3.3. Expression Analysis and Tissue Localization of Sc-AMT1
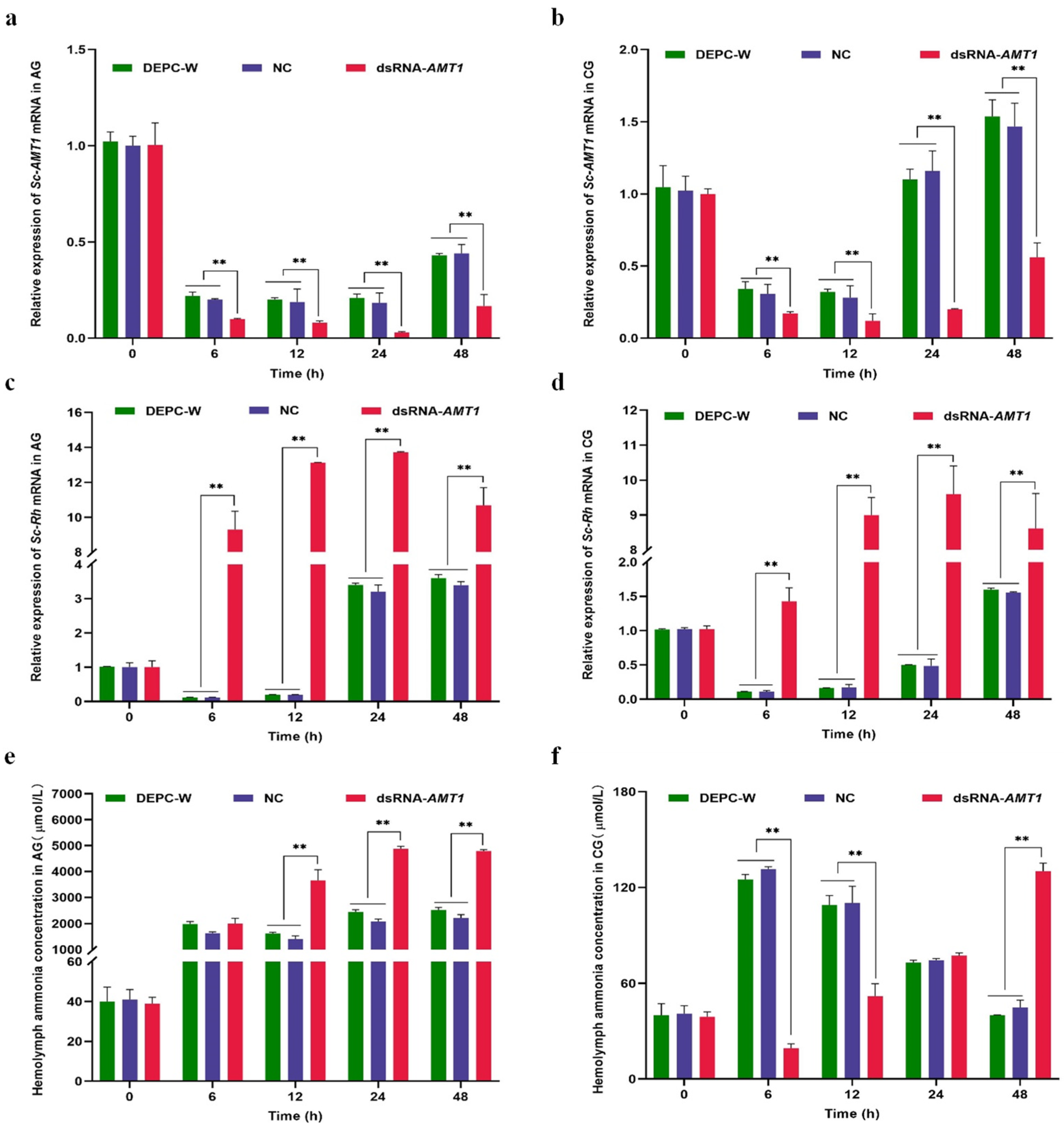
3.4. Effects of Sc-AMT1 RNAi on the Rh Expression and Hemolymph Ammonia Concentration
4. Discussion
4.1. Association between the SNP and Ammonia Tolerance
4.2. Sequence Characteristics and Phylogenetic Relationship of Sc-AMT1
4.3. Spatio–Temporal Expression Pattern of Sc-AMT1
4.4. Tissue Localization of Sc-AMT1 in Gill
4.5. Effects of Sc-AMT1 RNAi on Sc-Rh Expression and Hemolymph Ammonia Concentration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Pan, L.Q.; Xu, L.J.; Si, L.J. Effects of ammonia-N exposure on the concentrations of neurotransmitters, hemocyte intracellular signaling pathways and immune responses in white shrimp Litopenaeus vannamei. Fish Shellfish. Immunol. 2018, 75, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wu, Y.; Wu, L.; Bai, Y.Z.; Zhou, Y.; Wang, Z.F. The effects of ammonia stress exposure on protein degradation, immune response, degradation of nitrogen-containing compounds and energy metabolism of Chinese mitten crab. Mol. Biol. Rep. 2022, 49, 6053–6061. [Google Scholar] [CrossRef] [PubMed]
- Walker, V. Ammonia metabolism and hyperammonemic disorders. Adv. Clin. Chem. 2014, 67, 73–150. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.D.; Verlander, J.W. Ammonia transporters and their role in acid-base balance. Physiol. Rev. 2017, 97, 465–494. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.D.; Verlander, J.W. Role of NH3 and NH4+ transporters in renal acid-base transport. Am. J. Physiol. Ren. 2011, 300, 11–23. [Google Scholar] [CrossRef]
- Tresguerres, M.; Clifford, A.M.; Harter, T.S.; Roa, J.N.; Thies, A.B.; Yee, D.P.; Brauner, C.J. Evolutionary links between intra- and extracellular acid-base regulation in fish and other aquatic animals. J. Exp. Zool A 2020, 333, 449–465. [Google Scholar] [CrossRef]
- Loqué, D.; Wirén, N.V. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 2004, 55, 1293–1305. [Google Scholar] [CrossRef]
- Huang, C.H.; Peng, J.B. Evolutionary conservation and diversification of Rh family genes and proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 15512–15517. [Google Scholar] [CrossRef]
- Hall, J.A.; Yan, D. The molecular basis of K+ exclusion by the Escherichia coli ammonium channel AmtB. J. Biol. Chem. 2013, 288, 14080–14086. [Google Scholar] [CrossRef]
- Huang, L.L.; Li, J.Z.; Zhang, B.; Hao, Y.Y.; Ma, F.W. Genome-wide identification and expression analysis of AMT gene family in apple (Malus domestica Borkh). Horticulturae 2022, 8, 457. [Google Scholar] [CrossRef]
- Pitts, R.J.; Derryberry, S.L.; Pulous, F.E.; Zwiebel, L.J. Antennal-expressed ammonium transporters in the malaria vector mosquito Anopheles gambiae. PLoS ONE 2014, 9, e111858. [Google Scholar] [CrossRef]
- Chasiotis, H.; Ionescu, A.; Misyura, L.; Bui, P.; Fazio, K.; Wang, J.; Patrick, M.; Weihrauch, D.; Donini, A. An animal homolog of plant Mep/Amt transporters promotes ammonia excretion by the anal papillae of the disease vector mosquito Aedes aegypti. J. Exp. Biol. 2016, 219, 1346–1355. [Google Scholar] [CrossRef]
- Boo, M.V.; Hiong, K.C.; Goh-Enan, J.K.; Choo-Celine, Y.L.; Wong, W.P.; Chew, S.F.; Ip, Y.K. The ctenidium of the giant clam, Tridacna squamosa, expresses an ammonium transporter 1 that displays light-suppressed gene and protein expression and may be involved in ammonia excretion. J. Comp. Physiol. B 2018, 188, 765–777. [Google Scholar] [CrossRef]
- Andrade, S.L.; Dickmanns, A.; Ficner, R.; Einsle, O. Crystal structure of the archaeal ammonium transporter Amt-1 from Archaeoglobus fulgidus. Proc. Natl. Acad. Sci. USA 2005, 102, 14994–14999. [Google Scholar] [CrossRef]
- Durant, A.C.; Donini, A. Ammonia excretion in an osmoregulatory syncytium is facilitated by AeAmt2, a novel ammonia transporter in Aedes aegypti larvae. Front. Physiol. 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.R.; Parker, M.D.; Toye, A.M.; Boron, W.F.; Musa-Aziz, R. Relative CO2/NH3 permeabilities of human RhAG, RhBG and RhCG. J. Membr. Biol. 2013, 246, 915–926. [Google Scholar] [CrossRef]
- Si, L.J.; Pan, L.Q.; Wang, H.D.; Zhang, X. Identification of the role of Rh protein in ammonia excretion of the swimming crab Portunus trituberculatus. J. Exp. Biol. 2018, 221, jeb184655. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Chai, X.R.; Zhang, D.X.; Xu, W.J.; He, J. Effect of Sinonovacula constricta on sediment microbial numbers and easily degradable organics in shrimp-crab polyculture systems. Front. Mar. Sci. 2022, 9, 1807. [Google Scholar] [CrossRef]
- Weihrauch, D.; Morris, S.; Towle, D.W. Ammonia excretion in aquatic and terrestrial crabs. J. Exp. Biol. 2004, 207, 4491–4504. [Google Scholar] [CrossRef]
- Liu, X.J.; He, X.G.; Huang, G.Q.; Zhou, Y.; Lai, J.X. Bioremediation by the mullet Mugil cephalus feeding on organic deposits produced by intensive shrimp mariculture. Aquaculture 2021, 541, 736674. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Chen, J.Q.; Shen, W.L.; Chen, C.F.; Lin, Z.H.; Li, C.H. Molecular characterization of a novel sulfide: Quinone oxidoreductase from the razor clam Sinonovacula constricta and its expression response to sulfide stress. Comp. Biochem. Physiol. B 2020, 239, 110367. [Google Scholar] [CrossRef]
- Lv, L.Y.; Ren, J.F.; Zhang, H.; Sun, C.S.; Dong, Y.H.; Lin, Z.H. Transcriptomic analysis of gill and hepatopancreas in razor clam (Sinonovacula constricta) exposed to acute ammonia. Front. Mar. Sci. 2022, 9, 832494. [Google Scholar] [CrossRef]
- Chen, K.F.; Dong, Y.H.; Yao, H.H.; Lin, Z.H.; Sun, C.S. Identification and characteristic expression analysis of miR-8245a-5p target gene related to ammonia nitrogen stress in Sinonovacula constricta. Oceanol. Limnol. Sin. 2020, 51, 388–394. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, Y.H.; Yao, H.H.; Yu, K.J.; Hu, Y.Y.; Lin, Z.H. Cloning of GST and HSP90 genes of Sinonovacula constricta and analysis of their expression characteristics under ammonia nitrogen stress. Haiyang Xuebao 2020, 42, 66–78. [Google Scholar] [CrossRef]
- Sun, G.G.; Dong, Y.H.; Sun, C.S.; Yao, H.H.; Lin, Z.H. Vital role of glutamate dehydrogenase gene in ammonia detoxification and the association between its SNPs and ammonia tolerance in Sinonovacula constricta. Front. Physiol. 2021, 12, 664804. [Google Scholar] [CrossRef]
- Zhao, X.L.; Fu, J.P.; Jiang, L.T.; Zhang, W.W.; Shao, Y.N.; Jin, C.H.; Xiong, J.B.; Li, C.H. Transcriptome-based identification of the optimal reference genes as internal controls for quantitative RT-PCR in razor clam (Sinonovacula constricta). Genes Genom. 2018, 40, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.G.; Zhang, H.; Yao, H.H.; Dai, W.F.; Lin, Z.H.; Dong, Y.H. Characteristics of glutathione peroxidase gene and its responses to ammonia-N stress in razor clam Sinonovacula constricta. Comp. Biochem. Physiol. B 2022, 261, 110752. [Google Scholar] [CrossRef]
- Larsen, E.H.; Deaton, L.E.; Onken, H.; O’Donnell, M.; Grosell, M.; Dantzler, W.H.; Weihrauch, D. Osmoregulation and excretion. Compr. Physiol. 2014, 4, 405–573. [Google Scholar] [CrossRef]
- Sun, G.G.; Sun, C.S.; He, J.; Yao, H.H.; Dai, W.F.; Lin, Z.H.; Dong, Y.H. Characterizing the role of glutamine synthetase gene on ammonia nitrogen detoxification metabolism of the razor clam Sinonovacula constricta. Front. Mar. Sci. 2021, 8, 1718. [Google Scholar] [CrossRef]
- Wright, P.A. Nitrogen excretion: Three end products, many physiological roles. J. Exp. Biol. 1995, 198, 273–281. [Google Scholar] [CrossRef]
- Peng, J.; Huang, C.H. Rh proteins vs Amt proteins: An organismal and phylogenetic perspective on CO2 and NH3 gas channels. Transfus. Clin. Biol. 2006, 13, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Boeckstaens, M.; André, B.; Marini, A.M. Distinct transport mechanisms in yeast ammonium transport/sensor proteins of the Mep/Amt/Rh family and impact on filamentation. J. Biol. Chem. 2008, 283, 21362–21370. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Lu, C.R. A review on native and denaturing purification methods for non-coding RNA (ncRNA). J. Chromatogr. B 2019, 1120, 71–79. [Google Scholar] [CrossRef]
- Bakhtiarizadeh, M.R.; Salehi, A.; Rivera, R.M. Genome-wide identification and analysis of A-to-I RNA editing events in bovine by transcriptome sequencing. PLoS ONE 2018, 13, e0193316. [Google Scholar] [CrossRef]
- Levran, O.; Randesi, M.; Rotrosen, J.; Ott, J.; Adelson, M.; Kreek, M.J. A 3′ UTR SNP rs885863, a cis-eQTL for the circadian gene VIPR2 and lincRNA 689, is associated with opioid addiction. PLoS ONE 2019, 14, e0224399. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, D.; Joseph, G.; Allen, P. Ammonia excretion in aquatic invertebrates: New insights and questions. J. Exp. Biol. 2018, 221, jeb169219. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.Q.; Zhao, J.L.; Huang, S.Y.; Hao, Y.Y.; Cheng, Y.M.; Cao, X.Y. Ammonia transporter expression of Rh protein gene in gills of Nile tilapia Oreochromis niloticus under stress of alkali. Fish. Sci. 2019, 38, 194–200. [Google Scholar] [CrossRef]
- Wright, P.A.; Wood, C.M. A new paradigm for ammonia excretion in aquatic animals: Role of Rhesus (Rh) glycoproteins. J. Exp. Biol. 2009, 212, 2303–2312. [Google Scholar] [CrossRef]
- Zhang, L.; Michele, N.C.; De, B.G.; Wood, C.M. Rh protein expression in branchial neuroepithelial cells, and the role of ammonia in ventilatory control in fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 186, 39–51. [Google Scholar] [CrossRef]
- Liu, S.T.; Horng, J.L.; Lin, L.Y. Role of the basolateral Na+/H+ exchanger-2 (NHE2) in ionocytes of seawater-acclimated medaka (Oryzias latipes). Front. Physiol. 2022, 278, 375–379. [Google Scholar] [CrossRef]
- Wacker, T.; Garcia-Celma, J.J.; Lewe, P.; Andrade, S.L.; Kabaca, H.R. Direct observation of electrogenic NH4+ transport in ammonium transport (Amt) proteins. Proc. Natl. Acad. Sci. USA 2018, 111, 9995–10000. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.P.; Goldstein, L. 5 Formation of Excretory Products. Fish Physiol. 1969, 1, 313–350. [Google Scholar] [CrossRef]
- Dumouhtsidou, G.P.; Dimitriadis, V.K. Lysosomal, tissue and cellular alterations in the gills, palps and intestine of the mussel Mytilus galloprovincialis, in relation to pollution. Mar. Biol. 2004, 145, 109–120. [Google Scholar] [CrossRef]
- José, A.O.D.; Renato, B.S.; Carmem, S.F. Fine structure of Mytella falcata (Bivalvia) gill filaments. Micron 2008, 39, 329–336. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.E. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Transfus. Med. Rev. 2001, 15, 245. [Google Scholar] [CrossRef]
- Durant, A.C.; Donini, A. Ammonium transporter expression in sperm of the disease vector Aedes aegypti mosquito influences male fertility. Proc. Natl. Acad. Sci. USA 2020, 117, 29712–29719. [Google Scholar] [CrossRef]
- Weihrauch, D.; Donini, A.; O’Donnell, M.J. Ammonia transport by terrestrial and aquatic insects. J. Insect Physiol. 2012, 58, 473–487. [Google Scholar] [CrossRef]
- Lecompte, M.; Cattaert, D.; Vincent, A.; Birman, S.; Chérif-Zahar, B. Drosophila ammonium transporter Rh50 is required for integrity of larval muscles and neuromuscular system. J. Comp. Neurol. 2020, 528, 81–94. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, F.; Sun, H.H.; Barker, M.; Pitts, R.J.; Zwiebel, L.J. Heterogeneous expression of the ammonium transporter AgAmt in chemosensory appendages of the malaria vector, Anopheles gambiae. Insect Biochem. Mol. Biol. 2020, 120, 103360. [Google Scholar] [CrossRef]
- Talbot, K.; Kwong, R.W.M.; Gilmour, K.M.; Perry, S.F. The water channel aquaporin-1a1 facilitates movement of CO2 and ammonia in zebrafish (Danio rerio) larvae. J. Exp. Biol. 2015, 218, 3931–3940. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Piermarini, P.M. Molecular expression of aquaporin mRNAs in the northern house mosquito, Culex pipiens. J. Insect Physiol. 2017, 96, 35–44. [Google Scholar] [CrossRef] [PubMed]

| Primers | Primer Sequence (5′-3′) | Tm (°C) | Product Size (bp) | Genbank Accession |
|---|---|---|---|---|
| qRT-PCR | ||||
| AMT1-F | GTGGTTCGTGGGGTGTGA | 59.5 | 246 | OQ244848 |
| AMT1-R | GGTAGGCGGGCTCGTTAT | 60.0 | ||
| Rh-F | TGCGGACCACATCCAGAA | 59.4 | 106 | OQ244849 |
| Rh-R | GGCGACGGACCCCATAAC | 61.2 | ||
| RS9-F | TGAAGTCTGGCGTGTCAAGT | 60.2 | 117 | OQ244850 |
| RS9-R | CGTCTCAAAAGGGCATTACC | 60.4 | ||
| RNA Interference | ||||
| dsRNA-AMT1-F | GCAGCAAGAAUGUCACAAATT | \ | \ | OQ244848 |
| dsRNA-AMT1-R | UUUGUGACAUUCUUGCUGCTT | \ | \ | |
| NC-F | UUCUCCGAACGUGUCACGUTT | \ | \ | \ |
| NC-R | ACGUGACACGUUCGGAGAATT | \ | \ | \ |
| SNP Validation | ||||
| AMT1-SNP-F1 | GAAGGTGACCAAGTTCATGCTTCATTAGAAATAAAATGCCGCAGAA | 58.7 | 83 | \ |
| AMT1-SNP-F2 | GAAGGTCGGAGTCAACGGATTTCATTAGAAATAAAATGCCGCAGAT | 58.2 | ||
| AMT1-SNP-R | AATAGGATGTAACGCCATTCCTTACCTTG | 60.3 |
| Locus | Genotype | Number/Genotype Frequency (%) | Allele | Allele Frequency (%) | χ2/p-Value | Variation Type | ||
|---|---|---|---|---|---|---|---|---|
| TG | SG | TG | SG | |||||
| g.15211125A > T | AA | 144/96 | 107/71.33 | A | 96 | 77 | 34.954/0.000 ** | Transversion |
| AT | 0/0 | 17/11.34 | T | 4 | 23 | |||
| TT | 6/4 | 26/17.33 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Dai, W.; Zhu, X.; Yao, H.; Lin, Z.; Dong, Y.; Lv, L. Expression and Functional Analysis of AMT1 Gene Responding to High Ammonia Stress in Razor Clam (Sinonovacula constricta). Animals 2023, 13, 1638. https://doi.org/10.3390/ani13101638
Hu C, Dai W, Zhu X, Yao H, Lin Z, Dong Y, Lv L. Expression and Functional Analysis of AMT1 Gene Responding to High Ammonia Stress in Razor Clam (Sinonovacula constricta). Animals. 2023; 13(10):1638. https://doi.org/10.3390/ani13101638
Chicago/Turabian StyleHu, Chenxin, Wenfang Dai, Xiaojie Zhu, Hanhan Yao, Zhihua Lin, Yinghui Dong, and Liyuan Lv. 2023. "Expression and Functional Analysis of AMT1 Gene Responding to High Ammonia Stress in Razor Clam (Sinonovacula constricta)" Animals 13, no. 10: 1638. https://doi.org/10.3390/ani13101638
APA StyleHu, C., Dai, W., Zhu, X., Yao, H., Lin, Z., Dong, Y., & Lv, L. (2023). Expression and Functional Analysis of AMT1 Gene Responding to High Ammonia Stress in Razor Clam (Sinonovacula constricta). Animals, 13(10), 1638. https://doi.org/10.3390/ani13101638






