Comparison of Cassava Chips and Winged Bean Tubers with Various Starch Modifications on Chemical Composition, the Kinetics of Gas, Ruminal Degradation, and Ruminal Fermentation Characteristics Using an In Situ Nylon Bag and an In Vitro Gas Production Technique
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Station and Treatment Preparation
2.2. Experimental Design and Dietary Treatments
2.3. In Situ Nylon Bag Measurement
2.4. Ruminal Liquor Preparation and In Vitro Gas Technique
2.5. Statistical Analysis
3. Results
3.1. Chemical Compositions of Cassava Chip- and Winged Bean Tuber-Modified Starch
3.2. In Situ Degradability
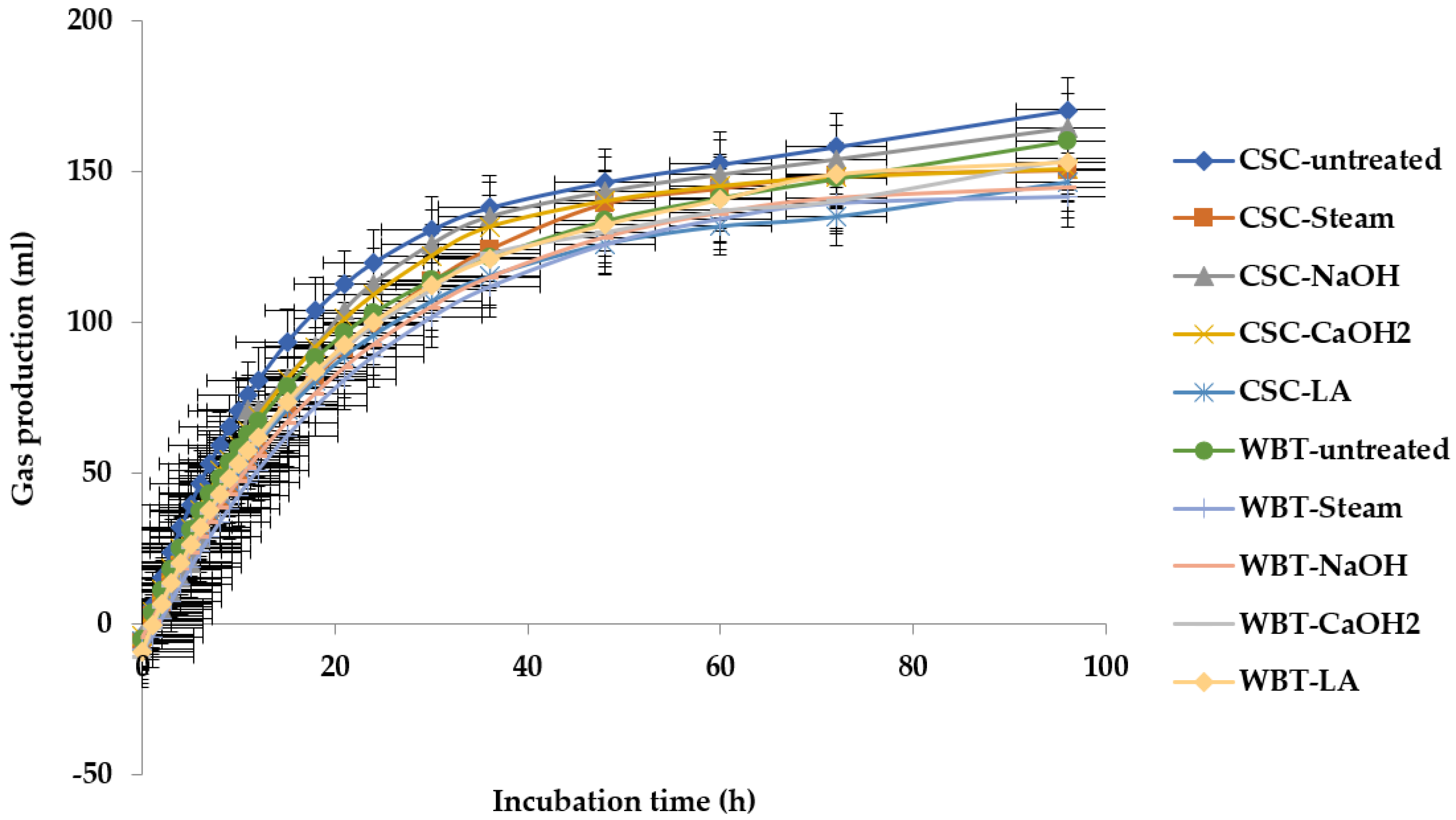
3.3. Gas of Kinetics
3.4. In Vitro Degradability
3.5. Ruminal pH, and Ammonia-Nitrogen Concentrations
3.6. In Vitro Volatile Fatty Acid (VFA) Profiles
4. Discussion
4.1. Chemical Compositions
4.2. In Situ Degradability
4.3. Kinetics of Gas
4.4. In Vitro Degradability
4.5. Ruminal pH and Ammonia-Nitrogen Concentrations
4.6. In Vitro VFA Profiles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wanapat, M.; Kang, S. Cassava chip (Manihot esculenta Crantz) as an energy source for ruminant feeding. Anim. Nutr. 2015, 1, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Khota, W.; Kaewpila, C.; Suwannasing, R.; Srikacha, N.; Maensathit, J.; Ampaporn, K.; Patarapreecha, P.; Thip-uten, S.; Sawnongbue, P.; Subepang, S.; et al. Ensiling cyanide residue and In vitro rumen fermentation of cassava root silage treated with cyanide-utilizing bacteria and cellulase. Fermentation 2023, 9, 151. [Google Scholar] [CrossRef]
- Suntara, C.; Wanapat, M.; Chankaew, S.; Khonkhaeng, B.; Supapong, C.; Chanjula, P.; Gunun, P.; Gunun, N.; Foiklang, S.; Phesatcha, K.; et al. Improvement of the nutritional quality of Psophocarpus tetragonolobus tubers by fermentation with ruminal crabtree-negative yeasts on the in vitro digestibility and fermentation in rumen fluid. Fermentation 2022, 8, 209. [Google Scholar] [CrossRef]
- Pilachai, R.; Thongdee, W.; Chumpol, Y.; Seesupa, S.; Schonewille, J.T. Effects of proportion of cassava and lactic acid-treated cassava in rations on rumen pH and plasma lipopolysaccharide-binding protein in beef cattle. Thai J. Vet. Med. 2017, 47, 173. [Google Scholar]
- Sriwichai, S.; Monkham, T.; Sanitchon, J.; Jogloy, S.; Chankaew, S. Dual-purpose of the winged bean (Psophocarpus tetragonolobus (L.) DC.), the neglected Tropical legume, based on pod and tuber yields. Plant 2021, 10, 1746. [Google Scholar] [CrossRef]
- Tanzi, A.S.; Eagleton, G.E.; Ho, W.K.; Wong, Q.N.; Mayes, S.; Massawe, F. Winged bean (Psophocarpus tetragonolobus (L.) DC.) for food and nutritional security: Synthesis of past research and future direction. Planta 2019, 250, 911–931. [Google Scholar] [CrossRef]
- Chankaew, S.; Sriwichai, S.; Rakvong, T.; Monkham, T.; Sanitchon, J.; Tangphatsornruang, S.; Kongkachana, W.; Sonthirod, C.; Pootakham, W.; Amkul, K. The first genetic linkage map of Winged bean [Psophocarpus tetragonolobus (L.) DC.] and QTL mapping for flower-, pod-, and seed-related traits. Plant 2022, 11, 500. [Google Scholar] [CrossRef]
- Srakaew, W.; Wachirapakorn, C.; Wongnen, C. Dietary modified cassava chip and corn seed: Effect on growth performance, rumen production, and blood glucose and insulin in early fattening beef bulls. Walailak J. Sci. Technol. 2021, 18, 9217. [Google Scholar] [CrossRef]
- Chanjarujit, W.; Chaiseri, S. Effect of alkaline conditions on the linalool retention ability of dried starch matrices. J. Food Sci. Technol. 2018, 13, 36–48. [Google Scholar]
- Chanjula, P.; Suntara, C.; Cherdthong, A. The Effects of oil palm fronds silage supplemented with urea-calcium hydroxide on rumen fermentation and nutrient digestibility of Thai native-Anglo Nubian goats. Fermentation 2021, 7, 218. [Google Scholar] [CrossRef]
- Park, J.; Oh, S.-K.; Chung, H.-J.; Shin, D.S.; Choi, I.; Park, H.J. Effect of steaming and roasting on the quality and resistant starch of brown rice flour with high amylose content. LWT Food Sci. Technol. 2022, 167, 113801. [Google Scholar] [CrossRef]
- Shen, J.; Song, L.; Sun, H.; Wang, B.; Chai, Z.; Chacher, B.; Liu, J. Effects of corn and soybean meal types on rumen fermentation, nitrogen metabolism and productivity in dairy cows. J. Anim. Sci. 2015, 28, 351. [Google Scholar] [CrossRef]
- Gómez, L.M.; Posada, S.L.; Olivera, M. Starch in ruminant diets: A review. Rev. Colomb. Cienc. Pecu. 2016, 29, 77–90. [Google Scholar] [CrossRef]
- Khol-Parisini, A.; Humer, E.; Sizmaz, Ö.; Abdel-Raheem, S.M.; Gruber, L.; Gasteiner, J.; Zebeli, Q. Ruminal disappearance of phosphorus and starch, reticulo-ruminal pH and total tract nutrient digestibility in dairy cows fed diets differing in grain processing. Anim. Feed Sci. Technol. 2015, 210, 74–85. [Google Scholar] [CrossRef]
- Wanapat, M.; Polyorach, S.; Boonnop, K.; Mapato, C.; Cherdthong, A. Effects of treating rice straw with urea or urea and calcium hydroxide upon intake, digestibility, rumen fermentation and milk yield of dairy cows. Livest. Sci. 2009, 125, 238–243. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analysis Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Holtshausen, L. Effect of Nonfiber Carbohydrates on Product Yield and Fiber Digestion in Fermentations with Mixed Ruminal Microbes. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2004. [Google Scholar]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Sommart, K.; Parker, D.; Rowlinson, P.; Wanapat, M. Fermentation characteristics and microbial protein synthesis in an in vitro system using cassava, rice straw and dried ruzi grass as substrates. Asian-Australas. J. Anim. Sci. 2000, 13, 1084–1093. [Google Scholar] [CrossRef]
- Fawcett, J.; Scott, J. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960, 13, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.; Murray, R. The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods. Grass Forage Sci. 2001, 56, 405–411. [Google Scholar] [CrossRef]
- Gunun, P.; Wanapat, M.; Gunun, N.; Cherdthong, A.; Sirilaophaisan, S.; Kaewwongsa, W. Effects of condensed tannins in Mao (Antidesma thwaitesianum Muell. Arg.) seed meal on rumen fermentation characteristics and nitrogen utilization in goats. Asian-Australas. J. Anim. Sci. 2016, 29, 1111–1119. [Google Scholar] [CrossRef]
- Tilley, J.; Terry, D.R. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Coxe, S.; West, S.G.; Aiken, L.S. Generalized linear models. In The Oxford Handbook of Quantitative Methods: Statistical Analysis; Little, T.D., Ed.; Oxford University Press: Oxford, UK, 2013; pp. 26–51. [Google Scholar]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1980; Volume 2, p. 633. [Google Scholar]
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; Van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Srakaew, W.; Wachirapakorn, C.; Cherdthong, A.; Wongnen, C. Ruminal degradability and bypass nutrients of alkaline or steam-treated cassava chip and corn grain. Trop. J. Anim. Sci. 2021, 44, 451–461. [Google Scholar] [CrossRef]
- Ruiiz, E.; Srikaeo, K.; de la Revilla, L.S. Effects of heat moisture treatment on physicochemical properties and starch digestibility of rice flours differing in amylose content. Food Appl. Biosci. J. 2018, 6, 140–153. [Google Scholar]
- Offner, A.; Bach, A.; Sauvant, D. Quantitative review of in situ starch degradation in the rumen. Anim. Feed Sci. Technol. 2003, 106, 81–93. [Google Scholar] [CrossRef]
- Pu, H.; Liu, G.; Huang, M.; Zhang, C.; Niu, W.; Chen, X.; Huang, J. Effects of Annealing on ultra-high pressure induced gelatinization of corn starch. Innov. Food Sci. Emerg. Technol. 2021, 74, 102849. [Google Scholar] [CrossRef]
- Narwojsz, A.; Borowska, E.J.; Polak-Śliwińska, M.; Danowska-Oziewicz, M. Effect of different methods of thermal treatment on starch and bioactive compounds of potato. Plant Foods Hum. Nutr. 2020, 75, 298–304. [Google Scholar] [CrossRef]
- Tan, N.D.; Wanapat, M.; Uriyapongson, S.; Cherdthong, A.; Pilajun, R. Enhancing mulberry leaf meal with urea by pelleting to improve rumen fermentation in cattle. Asian-Australas. J. Anim. Sci. 2012, 25, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, H.; Ma, Y.; Mai, S.; Li, C. Effects of extrusion on starch molecular degradation, order–disorder structural transition and digestibility—A review. Foods 2022, 11, 2538. [Google Scholar] [CrossRef]
- Wachirapakorn, C.; Pilachai, K.; Wanapat, M.; Pakdee, P.; Cherdthong, A. Effect of ground corn cobs as a fiber source in total mixed ration on feed intake, milk yield and milk composition in tropical lactating crossbred Holstein cows. Anim. Nutr. 2016, 2, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Hendriks, W.H.; Xiong, B.; Pellikaan, W.F. Starch and cellulose degradation in the rumen and applications of metagenomics on ruminal microorganisms. Animals 2022, 12, 3020. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Haisan, J.; Inabu, Y.; Sugino, T.; Oba, M. Effects of starch concentration of close-up diets on rumen pH and plasma metabolite responses of dairy cows to grain challenges after calving. J. Dairy Sci. 2020, 103, 11461–11471. [Google Scholar] [CrossRef]
- Karami, M.; Palizdar, M.; Almasi, M.J. The effect of different processing of corn grain on gas production kinetics and in vitro digestibility in Taleshi cows. J. Livest. Sci. 2018, 9, 101–106. [Google Scholar]
- Iommelli, P.; Zicarelli, F.; Musco, N.; Sarubbi, F.; Grossi, M.; Lotito, D.; Lombardi, P.; Infascelli, F.; Tudisco, R. Effect of cereals and legumes processing on in situ rumen protein degradability: A review. Fermentation 2022, 8, 363. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the most important methods of improving the processing properties of starch toward non-food applications. Polymers 2021, 13, 832. [Google Scholar] [CrossRef]
- Deckardt, K.; Khol-Parisini, A.; Zebeli, Q. Peculiarities of enhancing resistant starch in ruminants using chemical methods: Opportunities and challenges. Nutrients 2013, 5, 1970–1988. [Google Scholar] [CrossRef]
- Östman, E.M.; Nilsson, M.; Elmståhl, H.L.; Molin, G.; Björck, I. On the effect of lactic acid on blood glucose and insulin responses to cereal products: Mechanistic studies in healthy subjects and in vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Kaewpila, C.; Gunun, P.; Kesorn, P.; Pholsen, S.; Higgs, D.; Cai, Y.; Cherdthong, A.; Khota, W. Improving ensiling characteristics by adding lactic acid bacteria modifies In vitro digestibility and methane production of forage-sorghum mixture silage. Sci. Rep. 2021, 11, 1968. [Google Scholar] [CrossRef]
- Humer, E.; Zebeli, Q. Grains in ruminant feeding and potentials to enhance their nutritive and health value by chemical processing. Anim. Feed Sci. Technol. 2017, 226, 133–151. [Google Scholar] [CrossRef]
- Rojas, O.J.; Stein, H.H. Processing of ingredients and diets and effects on nutritional value for pigs. J. Anim. Sci. 2017, 8, 48. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.; Fernando, N.M.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.; Kulatunga, A.K. Development of starch-based materials using current modification techniques and their applications: A review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef]
- Harder, H.; Khol-Parisini, A.; Zebeli, Q. Treatments with organic acids and pullulanase differently affect resistant starch and fiber composition in flour of various barley genotypes (Hordeum vulgare L.). Starch-Stärke 2015, 67, 512–520. [Google Scholar] [CrossRef]
- Shaikh, F.; Ali, T.M.; Mustafa, G.; Hasnain, A. Comparative study on effects of citric and lactic acid treatment on morphological, functional, resistant starch fraction and glycemic index of corn and sorghum starches. Int. J. Biol. Macromol. 2019, 135, 314–327. [Google Scholar] [CrossRef]
- Pilachai, R.; Kaewwongsa, W.; Petlum, A.; Chumpol, Y.; Thongdee, W. Rumen digestibility of lactic acid steeped cassava using nylon bag technique. Khon Kaen Agric. J. 2014, 42, 567–576. [Google Scholar]
- Huhtanen, P.; Ahvenjärvi, S.; Broderick, G.; Reynal, S.; Shingfield, K. Quantifying ruminal digestion of organic matter and neutral detergent fiber using the omasal sampling technique in cattle—A meta-analysis. J. Dairy Sci. 2010, 93, 3203–3215. [Google Scholar] [CrossRef] [PubMed]
- Shreck, A.L. Use of Alkaline Treated Crop Residues as Partial Grain Replacements for Finishing Cattle. Ph.D. Thesis, The University of Nebraska, Lincoln, NE, USA, 2013. [Google Scholar]
- Chen, P.; Li, Y.; Shen, Y.; Cao, Y.; Li, Q.; Wang, M.; Liu, M.; Wang, Z.; Huo, Z.; Ren, S. Effect of dietary rumen-degradable starch to rumen-degradable protein ratio on In vitro rumen fermentation characteristics and microbial protein synthesis. Animals 2022, 12, 2633. [Google Scholar] [CrossRef] [PubMed]
- Supapong, C.; Cherdthong, A.; Seankamsorn, A.; Khonkhaeng, B.; Wanapat, M.; Uriyapongson, S.; Gunun, N.; Gunun, P.; Chanjula, P.; Polyorach, S. In vitro fermentation, digestibility and methane production as influenced by Delonix regia seed meal containing tannins and saponins. J. Anim. Feed Sci. 2017, 26, 123–130. [Google Scholar] [CrossRef]
- Hung, L.V.; Wanapat, M.; Cherdthong, A. Effects of Leucaena leaf pellet on bacterial diversity and microbial protein synthesis in swamp buffalo fed on rice straw. Livest. Sci. 2013, 151, 188–197. [Google Scholar] [CrossRef]
- Beckett, L.; Gleason, C.; Bedford, A.; Liebe, D.; Yohe, T.; Hall, M.; Daniels, K.; White, R. Rumen volatile fatty acid molar proportions, rumen epithelial gene expression, and blood metabolite concentration responses to ruminally degradable starch and fiber supplies. J. Dairy Sci. 2021, 104, 8857–8869. [Google Scholar] [CrossRef]
- Cherdthong, A.; Wanapat, M. Manipulation of in vitro ruminal fermentation and digestibility by dried rumen digesta. Livest. Sci. 2013, 153, 94–100. [Google Scholar] [CrossRef]
| Items | DM (g/kg) | CP | EE | NDF | ADF | Ash | NFC | GE (kcal/g DM) | |
|---|---|---|---|---|---|---|---|---|---|
| (g/kg of DM) | |||||||||
| CSC | Raw | 909.72 ± 9.44 | 20.42 ± 0.40 | 7.66 ± 0.82 | 275.00 ± 7.28 | 40.11 ± 0.09 | 29.45 ± 0.49 | 667.46 ± 7.34 | 3.73 ± 0.05 |
| Steam | 913.20 ± 10.24 | 19.47 ± 0.08 | 7.71 ± 0.35 | 271.09 ± 7.82 | 37.05 ± 0.14 | 28.86 ± 0.53 | 672.88 ± 6.85 | 3.76 ± 0.06 | |
| NaOH | 912.27 ± 0.62 | 18.14 ± 0.15 | 7.53 ± 0.40 | 267.59 ± 3.65 | 37.77 ± 0.63 | 57.86 ± 5.57 | 648.88 ± 1.66 | 3.75 ± 0.05 | |
| CaOH2 | 905.99 ± 3.12 | 19.44 ± 0.47 | 7.55 ± 0.01 | 269.05 ± 5.59 | 31.41 ± 2.19 | 65.23 ± 0.12 | 638.74 ± 4.99 | 3.72 ± 0.07 | |
| LA | 900.98 ± 5.31 | 20.29 ± 0.69 | 7.67 ± 0.58 | 271.40 ± 5.06 | 32.23 ± 0.38 | 27.74 ± 1.99 | 672.90 ± 4.33 | 3.74 ± 0.02 | |
| WBT | Raw | 906.70 ± 1.49 | 190.25 ± 0.75 | 12.66 ± 2.03 | 233.70 ± 4.05 | 59.43 ± 2.00 | 32.33 ± 1.44 | 531.06 ± 4.21 | 3.78 ± 0.03 |
| Steam | 917.94 ± 0.76 | 183.66 ± 0.34 | 11.77 ± 1.51 | 239.69 ± 3.74 | 63.02 ± 2.80 | 31.75 ± 0.31 | 533.12 ± 5.91 | 3.79 ± 0.03 | |
| NaOH | 902.89 ± 2.10 | 177.97 ± 4.28 | 11.70 ± 1.07 | 227.64 ± 5.95 | 62.71 ± 0.86 | 55.58 ± 8.78 | 527.11 ± 2.53 | 3.78 ± 0.05 | |
| CaOH2 | 906.61 ± 0.95 | 189.06 ± 2.00 | 11.84 ± 0.35 | 228.62 ± 5.79 | 61.02 ± 4.08 | 61.71 ± 1.66 | 508.76 ± 5.78 | 3.77 ± 0.07 | |
| LA | 908.39 ± 8.17 | 184.94 ± 2.31 | 11.86 ± 1.47 | 224.36 ± 5.83 | 63.29 ± 4.11 | 30.94 ± 2.30 | 547.90 ± 7.28 | 3.78 ± 0.06 | |
| SEM | 6.54 | 1.70 | 0.11 | 8.66 | 2.56 | 3.50 | 3.46 | 0.008 | |
| p-value | 0.60 | 0.05 | 0.97 | 0.84 | 0.16 | 0.78 | 0.06 | 0.41 | |
| Source of starch | CSC | 908.43 ± 4.49 | 19.55 b ± 0.81 | 7.62 b ± 0.07 | 270.83 a ± 2.51 | 35.71 b ± 3.35 | 41.83 ± 16.27 | 660.17 a ± 13.88 | 3.74 ± 0.06 |
| WBT | 908.51 ± 5.05 | 185.18 a ± 436 | 11.97 a ± 0.35 | 230.80 b ± 5.36 | 61.89 a ± 1.46 | 42.46 ± 13.36 | 529.59 b ± 12.57 | 3.78 ± 0.05 | |
| Starch modification methods | Raw | 908.21 ± 1.51 | 105.34 a ± 84.91 | 10.16 ± 2.50 | 254.35 ± 2.65 | 49.77 ± 9.66 | 30.89 b ± 1.44 | 599.26 ab ± 68.20 | 3.74 b ± 0.03 |
| Steam | 915.57 ± 2.37 | 101.56 ab ± 82.10 | 9.74 ± 2.03 | 255.39 ± 1.57 | 50.03 ± 12.98 | 30.31 b ± 1.45 | 603.00 ab ± 69.88 | 3.77 a ± 0.08 | |
| NaOH | 907.58 ± 4.69 | 98.06 b ± 79.91 | 9.61 ± 2.08 | 247.62 ± 1.99 | 50.24 ± 12.47 | 56.72 a ± 1.14 | 588.00 bc ± 60.89 | 3.75 b ± 0.07 | |
| CaOH2 | 906.30 ± 0.31 | 104.25 a ± 84.81 | 9.70 ± 2.15 | 248.83 ± 2.35 | 46.22 ± 14.80 | 63.47 a ± 1.76 | 573.75 c ± 64.99 | 3.76 ab ± 0.05 | |
| LA | 904.68 ± 3.70 | 102.62 a ± 82.32 | 9.76 ± 2.10 | 247.88 ± 2.35 | 47.76 ± 15.53 | 29.34 b ± 1.60 | 610.40 a ± 62.50 | 3.76 ab ± 0.03 | |
| Items | In Situ Degradation Characteristics | Effective Dry Matter Degradability (%) | Effective Organic Matter Degradability (%) | |||
|---|---|---|---|---|---|---|
| a (%) | b (%) | c (h−1) | ||||
| CSC | Raw | 49.79 a ± 0.13 | 57.17 d ± 1.08 | 0.20 ± 0.07 | 94.44 a ± 3.34 | 95.69 a ± 2.46 |
| Steam | 11.12 f ± 1.09 | 67.90 a ± 1.83 | 0.09 ± 0.01 | 54.37 d ± 2.99 | 74.49 c ± 1.78 | |
| NaOH | 13.95 de ± 0.56 | 64.92 bc ± 0.56 | 0.17 ± 0.01 | 64.03 c ± 0.056 | 75.40 c ± 0.91 | |
| CaOH2 | 14.99 cd ± 0.32 | 65.71 bc ± 0.57 | 0.18 ± 0.04 | 65.65 c ± 2.93 | 75.81 c ± 1.03 | |
| LA | 16.17 c ± 0.82 | 64.07 c ± 1.22 | 0.18 ± 0.02 | 65.94 c ± 1.32 | 75.74 c ± 0.62 | |
| WBT | Raw | 21.93 b ± 0.93 | 65.51 bc ± 1.84 | 0.19 ± 0.01 | 73.95 b ± 0.37 | 84.22 b ± 0.94 |
| Steam | 5.73 g ± 1.02 | 63.94 c ± 3.91 | 0.06 ± 0.04 | 37.92 e ± 6.24 | 73.71 c ± 2.31 | |
| NaOH | 12.27 f ± 0.16 | 65.54 bc ± 1.17 | 0.11 ± 0.00 | 57.56 d ± 0.55 | 73.99 c ± 2.50 | |
| CaOH2 | 11.89 f ± 0.78 | 66.05 bc ± 1.24 | 0.10 ± 0.02 | 55.98 d ± 1.71 | 75.03 c ± 1.65 | |
| LA | 12.53 ef ± 0.36 | 65.37 bc ± 1.39 | 0.13 ± 0.02 | 57.63 d ± 1.78 | 76.15 c ± 2.41 | |
| SEM | 0.89 | 1.67 | 0.06 | 2.57 | 1.77 | |
| p-value | <0.01 | <0.01 | 0.57 | <0.01 | <0.01 | |
| Source of starch | CSC | 21.20 ± 14.39 | 63.95 ± 3.62 | 0.16 a ± 0.04 | 68.89 ± 13.47 | 79.01 ± 8.37 |
| WBT | 12.73 ± 5.19 | 64.97 ± 0.71 | 0.14 b ± 0.04 | 55.59 ± 11.42 | 76.00 ± 3.66 | |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Starch modification methods | Raw | 35.86 ± 13.39 | 61.34 ± 4.17 | 0.22 a ± 0.00 | 84.20 ± 10.24 | 89.96 ± 5.74 |
| Steam | 8.43 ± 2.69 | 65.92 ± 1.98 | 0.09 c ± 0.01 | 46.14 ± 8.23 | 75.15 ± 0.66 | |
| NaOH | 13.11 ± 0.84 | 65.23 ± 0.31 | 0.13 b ± 0.03 | 60.79 ± 3.24 | 74.69 ± 0.71 | |
| CaOH2 | 13.44 ± 1.55 | 65.88 ± 0.17 | 0.14 b ± 0.04 | 60.82 ± 4.84 | 74.37 ± 0.66 | |
| LA | 14.35 ± 1.82 | 64.72 ± 0.65 | 0.14 b ± 0.02 | 61.78 ± 4.16 | 75.95 ± 0.20 | |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Items | Kinetics of Gas | ||||
|---|---|---|---|---|---|
| a | b | c | |a| + b | ||
| CSC | Raw | −3.50 a ± 0.71 | 157.40 ± 6.64 | 0.064 a ± 0.00 | 160.90 ± 5.78 |
| Steam | −6.00 ab ± 1.63 | 169.58 ± 7.05 | 0.041 d ± 0.00 | 175.58 ± 7.46 | |
| NaOH | −3.66 a ± 0.18 | 141.36 ± 1.91 | 0.051 c ± 0.00 | 163.03 ± 1.74 | |
| CaOH2 | −3.78 a ± 0.56 | 163.77 ± 3.17 | 0.049 c ± 0.00 | 167.55 ± 2.06 | |
| LA | −5.53 a ± 1.02 | 144.50 ± 4.35 | 0.050 c ± 0.00 | 150.03 ± 5.35 | |
| WBT | Raw | −4.57 a ± 0.95 | 144.69 ± 9.55 | 0.057 b ± 0.00 | 149.26 ± 1.33 |
| Steam | −10.84 d ± 1.15 | 158.41 ± 1.48 | 0.041 d ± 0.00 | 169.25 ± 1.89 | |
| NaOH | −5.32 a ± 1.50 | 153.83 ± 3.88 | 0.042 d ± 0.00 | 159.15 ± 4.79 | |
| CaOH2 | −9.11 cd ± 1.03 | 153.49 ± 6.60 | 0.051 d ± 0.00 | 162.59 ± 5.73 | |
| LA | −8.11 bc ± 0.90 | 154.30 ± 2.47 | 0.050 d ± 0.00 | 162.42 ± 3.46 | |
| SEM | 0.93 | 5.84 | 0.002 | 6.90 | |
| p-value | <0.01 | 0.10 | <0.01 | 0.14 | |
| Source of starch | CSC | −4.49 ± 1.05 | 155.32 ± 1.68 | 0.051± | 163.42 ± 1.54 |
| WBT | −8.34 ± 2.34 | 155.01 ± 1.91 | 0.048± | 163.35 ± 1.99 | |
| p-value | 0.05 | 0.16 | <0.01 | 0.06 | |
| Starch modification methods | Raw | −4.49 ± 0.53 | 147.59 b ± 1.55 | 0.060 ± 0.01 | 155.08 b ± 7.03 |
| Steam | −8.42 ± 2.42 | 164.00 a ± 6.21 | 0.041 ± 0.01 | 172.42 a ± 2.55 | |
| NaOH | −4.49 ± 0.83 | 147.59 b ± 9.00 | 0.046 ± 0.00 | 161.09 ab ± 9.96 | |
| CaOH2 | −6.45 ± 2.66 | 158.63 ab ± 9.34 | 0.050 ± 0.00 | 165.07 ab ± 3.35 | |
| LA | −6.82 ± 1.29 | 149.40 b ± 1.16 | 0.050 ± 0.00 | 156.22 b ± 0.62 | |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | |
| Items | IVDMD (g/kg DM) | IVOMD (g/kg DM) | |||||
|---|---|---|---|---|---|---|---|
| 12 h | 24 h | Means | 12 h | 24 h | Means | ||
| CSC | Raw | 548.60 ± 3.40 | 677.60 ± 0.80 | 613.10 ± 4.50 | 748.70 ± 0.90 | 838.40 ± 2.80 | 793.55 ± 9.95 |
| Steam | 532.20 ± 3.20 | 666.50 ± 1.10 | 599.35 ± 7.15 | 735.80 ± 0.80 | 825.80 ± 3.45 | 780.80 ± 5.00 | |
| NaOH | 519.60 ± 6.40 | 661.90 ± 0.90 | 590.75 ± 1.15 | 725.90 ± 4.90 | 831.80 ± 5.00 | 778.85 ± 2.95 | |
| CaOH2 | 511.70 ± 4.10 | 661.10 ± 0.30 | 586.40 ± 4.70 | 719.50 ± 8.50 | 834.50 ± 2.10 | 777.00 ± 7.50 | |
| LA | 504.40 ± 2.20 | 651.97 ± 6.93 | 578.19 ± 3.79 | 699.80 ± 8.80 | 808.60 ± 10.60 | 754.20 ± 9.30 | |
| WBT | Raw | 546.30 ± 3.50 | 681.64 ± 7.04 | 613.97 ± 7.67 | 738.40 ± 7.36 | 854.90 ± 9.60 | 796.65 ± 0.90 |
| Steam | 536.20 ± 3.20 | 670.80 ± 1.80 | 603.50 ± 7.30 | 712.90 ± 2.90 | 838.90 ± 9.30 | 775.90 ± 3.00 | |
| NaOH | 519.80 ± 3.00 | 666.70 ± 2.90 | 593.25 ± 3.45 | 720.60 ± 6.40 | 847.50 ± 10.30 | 784.05 ± 3.45 | |
| CaOH2 | 521.50 ± 4.10 | 664.62 ± 5.56 | 593.06 ± 1.56 | 691.50 ± 2.50 | 845.90 ± 9.30 | 768.70 ± 7.20 | |
| LA | 517.40 ± 8.80 | 655.64 ± 10.16 | 586.52 ± 9.12 | 688.80 ± 6.40 | 820.20 ± 1.10 | 754.50 ± 3.05 | |
| SEM | 12.15 | 6.93 | 12.63 | 11.21 | 11.72 | 10.09 | |
| p-value | 0.81 | 0.74 | 0.71 | 0.97 | 0.96 | 0.59 | |
| Source of starch | CSC | 523.30 ± 5.65 | 663.81 ± 8.35 | 593.56 ± 11.92 | 725.94 ± 6.37 | 827.82 ± 10.45 | 776.88 ± 4.07 |
| WBT | 528.24 ± 1.17 | 667.88 ± 8.48 | 598.06 ± 9.63 | 710.44 ± 8.54 | 841.48 ± 11.79 | 775.96 ± 5.41 | |
| p-value | 0.86 | 0.53 | 0.78 | 0.18 | 0.38 | 0.95 | |
| Starch modification methods | Raw | 547.45 a ± 1.15 | 679.62 a ± 0.02 | 613.54 ± 0.44 | 743.55 a ± 5.15 | 846.65 ± 5.80 | 795.10 ± 0.32 |
| Steam | 534.20 ab ± 2.00 | 668.65 ab ± 2.15 | 601.43 ± 2.07 | 724.35 b ± 11.45 | 832.35 ± 6.55 | 778.35 ± 2.45 | |
| NaOH | 519.70 bc ± 0.10 | 664.30 b ± 2.40 | 592.00 ± 1.25 | 723.25 b ± 2.65 | 839.65 ± 7.85 | 781.45 ± 2.60 | |
| CaOH2 | 516.60 bc ± 4.90 | 662.86 b ± 1.76 | 589.73 ± 3.33 | 705.50 c ± 14.00 | 840.20 ± 5.70 | 772.85 ± 4.15 | |
| LA | 510.90 c ± 6.50 | 653.81 c ± 1.84 | 582.35 ± 4.17 | 694.30 c ± 5.50 | 814.40 ± 8.25 | 754.35 ± 1.37 | |
| p-value | <0.01 | <0.01. | 0.98 | <0.01 | 0.12 | 1.00 | |
| Items | pH | NH3-N (mg/dL) | |||
|---|---|---|---|---|---|
| 4 h | 2 h | 4 h | Means | ||
| CSC | Raw | 6.86 ± 0.02 | 16.58 ± 1.17 | 19.42 ± 0.01 | 18.00 ± 1.42 |
| Steam | 6.90 ± 0.03 | 15.31 ± 0.10 | 18.61 ± 0.40 | 16.96 ± 1.65 | |
| NaOH | 6.94 ± 0.01 | 15.61 ± 0.20 | 18.91 ± 0.70 | 17.26 ± 1.65 | |
| CaOH2 | 7.06 ± 0.00 | 16.01 ± 0.60 | 18.41 ± 0.40 | 17.21 ± 1.20 | |
| LA | 6.93 ± 0.00 | 15.81 ± 0.20 | 18.92 ± 0.30 | 17.36 ± 1.56 | |
| WBT | Raw | 6.96 ± 0.00 | 16.61 ± 0.30 | 19.91 ± 0.20 | 18.01 ± 1.40 |
| Steam | 6.96 ± 0.00 | 16.01 ± 0.40 | 19.22 ± 0.40 | 17.61 ± 1.60 | |
| NaOH | 6.99 ± 0.00 | 16.31 ± 0.10 | 18.01 ± 0.30 | 17.41 ± 1.10 | |
| CaOH2 | 7.02 ± 0.01 | 16.55 ± 0.80 | 18.79 ± 0.54 | 17.67 ± 1.12 | |
| LA | 6.97 ± 0.01 | 16.05 ± 0.60 | 18.61 ± 0.16 | 17.33 ± 1.28 | |
| SEM | 0.03 | 0.55 | 0.39 | 1.41 | |
| p = value | 0.32 | 0.96 | 0.65 | 1.00 | |
| Source of starch | CSC | 6.93 ± 0.07 | 15.86 ± 0.43 | 18.85 ± 0.34 | 17.36 ± 0.34 |
| WBT | 6.98 ± 0.06 | 16.31 ± 0.25 | 18.91 ± 0.35 | 17.61 ± 0.24 | |
| p-value | 0.21 | 0.23 | 0.83 | 0.79 | |
| Starch modification methods | Raw | 6.85 c ± 0.01 | 16.59 ± 0.20 | 19.41 ± 0.00 | 18.00 ± 0.01 |
| Steam | 6.94 b ± 0.03 | 15.66 ± 0.35 | 18.92 ± 0.30 | 17.29 ± 0.33 | |
| NaOH | 6.96 b ± 0.02 | 15.96 ± 0.35 | 18.71 ± 0.20 | 17.34 ± 0.08 | |
| CaOH2 | 7.04 a ± 0.02 | 16.28 ± 0.27 | 18.60 ± 0.19 | 17.44 ± 0.23 | |
| LA | 6.95 b ± 0.02 | 15.93 ± 0.12 | 18.76 ± 0.16 | 17.35 ± 0.02 | |
| p-value | <0.01 | 0.52 | 0.32 | 0.98 | |
| Items | Total VFA (mmol/L) | C2, % | C3, % | C4, % | C2 to C3 Ratio | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h | 4 h | Mean | 2 h | 4 h | Mean | 2 h | 4 h | Mean | 2 h | 4 h | Mean | 2 h | 4 h | Mean | ||
| CSC | Raw | 91.97 ± 1.02 | 96.81 ± 2.15 | 94.39 ± 0.12 | 66.90 ± 0.15 | 66.56 ± 0.06 | 66.73 ± 0.10 | 21.99 ± 0.11 | 22.84 ± 0.04 | 22.42 ± 0.02 | 11.110 ± 0.15 | 10.60 ± 0.13 | 10.85 ± 0.24 | 3.04 ± 0.02 | 2.91 ± 0.02 | 2.98 ± 0.18 |
| Steam | 90.84 ± 0.40 | 95.09 ± 1.30 | 92.96 ± 0.38 | 66.81 ± 0.23 | 66.64 ± 0.05 | 66.73 ± 0.09 | 21.82 ± 0.14 | 22.31 ± 0.05 | 22.06 ± 0.04 | 11.37 ± 0.18 | 11.05 ± 0.14 | 11.21 ± 1.25 | 3.06 ± 0.03 | 2.99 ± 0.04 | 3.02 ± 0.15 | |
| NaOH | 90.37 ± 0.80 | 96.08 ± 2.05 | 93.22 ± 0.52 | 66.70 ± 0.18 | 66.74 ± 0.18 | 66.72 ± 0.07 | 21.34 ± 0.21 | 22.11 ± 0.02 | 21.73 ± 0.02 | 11.96 ± 0.10 | 11.15 ± 0.05 | 11.55 ± 1.62 | 3.13 ± 0.04 | 3.02 ± 0.03 | 3.07 ± 0.12 | |
| CaOH2 | 90.40 ± 0.65 | 95.88 ± 0.35 | 93.14 ± 0.63 | 67.09 ± 0.20 | 66.79 ± 0.14 | 66.94 ± 0.26 | 22.09 ± 0.18 | 22.16 ± 0.05 | 22.12 ± 0.05 | 10.82 ± 0.08 | 11.05 ± 0.08 | 10.94 ± 0.17 | 3.04 ± 0.05 | 3.01 ± 0.04 | 3.03 ± 0.07 | |
| LA | 90.55 ± 0.45 | 95.19 ± 0.40 | 92.87 ± 0.45 | 67.58 ± 0.25 | 65.93 ± 0.26 | 66.76 ± 0.13 | 21.53 ± 0.10 | 22.67 ± 0.03 | 22.10 ± 0.06 | 10.89 ± 0.07 | 11.40 ± 0.10 | 11.14 ± 0.14 | 3.14 ± 0.05 | 2.91 ± 0.04 | 3.02 ± 0.08 | |
| WBT | Raw | 91.88 ± 0.30 | 96.73 ± 0.60 | 94.30 ± 0.85 | 66.87 ± 015 | 66.91 ± 0.52 | 66.89 ± 0.70 | 21.90 ± 0.21 | 22.01 ± 0.04 | 21.96 ± 0.02 | 11.23 ± 0.10 | 11.08 ± 0.16 | 11.15 ± 0.21 | 3.05 ± 0.07 | 3.04 ± 0.03 | 3.05 ± 0.11 |
| Steam | 90.61 ± 0.10 | 94.84 ± 0.25 | 92.72 ± 0.15 | 66.53 ± 0.16 | 66.24 ± 0.32 | 66.39 ± 0.40 | 22.26 ± 0.09 | 22.74 ± 0.05 | 22.50 ± 0.05 | 11.21 ± 0.12 | 11.02 ± 0.02 | 11.11 ± 0.90 | 2.99 ± 0.06 | 2.91 ± 0.02 | 2.95 ± 0.10 | |
| NaOH | 90.36 ± 0.25 | 96.00 ± 0.50 | 93.18 ± 0.35 | 66.56 ± 0.20 | 66.13 ± 0.11 | 66.35 ± 0.53 | 21.78 ± 0.10 | 22.78 ± 0.05 | 22.28 ± 0.04 | 11.66 ± 0.15 | 11.09 ± 0.03 | 11.37 ± 1.02 | 3.06 ± 0.04 | 2.90 ± 0.05 | 2.98 ± 0.09 | |
| CaOH2 | 91.08 ± 0.20 | 94.82 ± 0.40 | 92.95 ± 0.48 | 66.35 ± 0.14 | 66.22 ± 0.18 | 66.29 ± 0.47 | 22.01 ± 0.12 | 22.17 ± 0.02 | 22.09 ± 0.03 | 11.64 ± 0.20 | 11.61 ± 0.17 | 11.62 ± 1.52 | 3.01 ± 0.05 | 2.99 ± 0.05 | 3.00 ± 0.14 | |
| LA | 91.04 ± 0.30 | 95.05 ± 0.75 | 93.04 ± 0.95 | 66.96 ± 0.28 | 66.14 ± 0.10 | 66.55 ± 0.18 | 21.85 ± 0.15 | 22.79 ± 0.03 | 22.32 ± 0.03 | 11.19 ± 0.06 | 11.07 ± 0.04 | 11.13 ± 1.74 | 3.06 ± 0.04 | 2.90 ± 0.03 | 2.98 ± 0.08 | |
| SEM | 1.35 | 0.71 | 2.39 | 0.41 | 0.40 | 0.41 | 0.36 | 0.25 | 0.38 | 0.41 | 0.34 | 0.39 | 0.44 | 0.29 | 0.07 | |
| p-value | 0.99 | 0.94 | 0.89 | 0.69 | 0.69 | 0.69 | 0.54 | 0.14 | 0.87 | 0.39 | 0.64 | 0.88 | 0.81 | 0.81 | 0.36 | |
| Source of starch | CSC | 90.83 ± 0.50 | 95.81 ± 0.15 | 93.32 ± 1.85 | 67.02 ± 0.30 | 66.53 ± 0.10 | 66.78 ± 0.30 | 21.75 ± 0.10 | 22.42 ± 0.03 | 22.09 ± 0.04 | 11.23 ± 0.07 | 11.05 ± 0.16 | 11.14 ± 1.55 | 3.08 ± 0.06 | 2.97 ± 0.04 | 3.02 ± 0.05 |
| WBT | 90.99 ± 0.54 | 95.49 ± 0.25 | 93.24 ± 2.62 | 66.65 ± 0.12 | 66.33 ± 0.16 | 66.49 ± 0.24 | 21.96 ± 0.12 | 22.50 ± 0.05 | 22.23 ± 0.02 | 11.39 ± 0.08 | 11.17 ± 0.10 | 11.28 ± 1.25 | 3.04 ± 0.02 | 2.95 ± 0.05 | 2.99 ± 0.04 | |
| p value | 0.85 | 0.49 | 0.96 | 0.50 | 0.38 | 0.16 | 0.41 | 0.80 | 0.53 | 0.83 | 0.67 | 0.74 | 0.30 | 0.56 | 0.41 | |
| Starch modification methods | Raw | 91.93 ± 0.06 | 96.77 ± 1.30 | 94.35 ± 0.20 | 66.89 ± 0.18 | 66.74 ± 0.27 | 66.81± | 21.95 ± 0.14 | 22.43 ± 0.06 | 22.19 ± 0.02 | 11.17 ± 0.20 | 10.84 ± 0.04 | 11.00 ± 1.95 | 3.05 ± 0.02 | 2.98± | 3.01 ± 0.03 |
| Steam | 90.73 ± 0.15 | 94.97 ± 1.74 | 92.84 ± 0.40 | 66.67 ± 0.20 | 66.44 ± 0.25 | 66.56± | 22.04 ± 0.20 | 22.53 ± 0.05 | 22.28 ± 0.02 | 11.29 ± 0.16 | 11.04 ± 0.08 | 11.16 ± 1.65 | 3.03 ± 0.03 | 2.95 ± 0.02 | 2.99 ± 0.03 | |
| NaOH | 90.37 ± 0.20 | 96.04 ± 3.58 | 93.20 ± 0.30 | 66.63 ± 0.23 | 66.44 ± 0.41 | 66.54± | 21.56 ± 0.11 | 22.45 ± 0.06 | 22.01 ± 0.03 | 11.81 ± 0.14 | 11.12 ± 0.10 | 11.46 ± 1.47 | 3.09 ± 0.05 | 2.96 ± 0.04 | 3.03 ± 0.02 | |
| CaOH2 | 90.74 ± 0.15 | 95.35 ± 1.05 | 93.05 ± 0.20 | 66.72 ± 0.14 | 66.51 ± 0.30 | 66.62± | 22.05 ± 0.14 | 22.17 ± 0.02 | 22.11 ± 0.04 | 11.23 ± 0.06 | 11.33 ± 0.16 | 11.28 ± 0.18 | 3.03 ± 0.04 | 3.00 ± 0.05 | 3.01 ± 0.05 | |
| LA | 90.80 ± 0.30 | 95.12 ± 1.64 | 92.96 ± 0.60 | 67.27 ± 0.17 | 66.04 ± 0.25 | 66.66± | 21.69 ± 0.17 | 22.73 ± 0.02 | 22.21 ± 0.02 | 11.04 ± 0.03 | 11.24 ± 0.18 | 11.14 ± 0.05 | 3.10 ± 0.05 | 2.91 ± 0.02 | 3.00 ± 0.04 | |
| p-value | 0.82 | 0.13 | 0.97 | 0.97 | 0.49 | 0.43 | 0.62 | 0.74 | 0.94 | 0.16 | 0.83 | 0.19 | 0.65 | 0.57 | 0.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unnawong, N.; Suriyapha, C.; Khonkhaeng, B.; Chankaew, S.; Rakvong, T.; Polyorach, S.; Cherdthong, A. Comparison of Cassava Chips and Winged Bean Tubers with Various Starch Modifications on Chemical Composition, the Kinetics of Gas, Ruminal Degradation, and Ruminal Fermentation Characteristics Using an In Situ Nylon Bag and an In Vitro Gas Production Technique. Animals 2023, 13, 1640. https://doi.org/10.3390/ani13101640
Unnawong N, Suriyapha C, Khonkhaeng B, Chankaew S, Rakvong T, Polyorach S, Cherdthong A. Comparison of Cassava Chips and Winged Bean Tubers with Various Starch Modifications on Chemical Composition, the Kinetics of Gas, Ruminal Degradation, and Ruminal Fermentation Characteristics Using an In Situ Nylon Bag and an In Vitro Gas Production Technique. Animals. 2023; 13(10):1640. https://doi.org/10.3390/ani13101640
Chicago/Turabian StyleUnnawong, Narirat, Chaichana Suriyapha, Benjamad Khonkhaeng, Sompong Chankaew, Teppratan Rakvong, Sineenart Polyorach, and Anusorn Cherdthong. 2023. "Comparison of Cassava Chips and Winged Bean Tubers with Various Starch Modifications on Chemical Composition, the Kinetics of Gas, Ruminal Degradation, and Ruminal Fermentation Characteristics Using an In Situ Nylon Bag and an In Vitro Gas Production Technique" Animals 13, no. 10: 1640. https://doi.org/10.3390/ani13101640






