Evaluation of the Horizontal Transmission of White Spot Syndrome Virus for Whiteleg Shrimp (Litopenaeus vannamei) Based on the Disease Severity Grade and Viral Shedding Rate
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Shrimp and Virus
2.2. Determination of WSSV Genome Copies in Whiteleg Shrimp
2.3. Iron Flocculation Assay to Determine WSSV Concentration in Seawater
2.4. Correlation between Disease Severity Grade and Viral Shedding Rate in WSSV-Infected Shrimp
2.4.1. Intramuscular Injection Challenge
2.4.2. Histopathological Analysis and Determination of the Disease Severity Grade
2.4.3. WSSV VP28 Gene Expression Analysis
2.5. Waterborne Transmission of WSSV under the Mimicking Natural Conditions
2.5.1. Determination of the Minimum Infective Dose via the Waterborne Route
2.5.2. Cohabitation Challenge
2.6. Statistical Analyses
3. Results
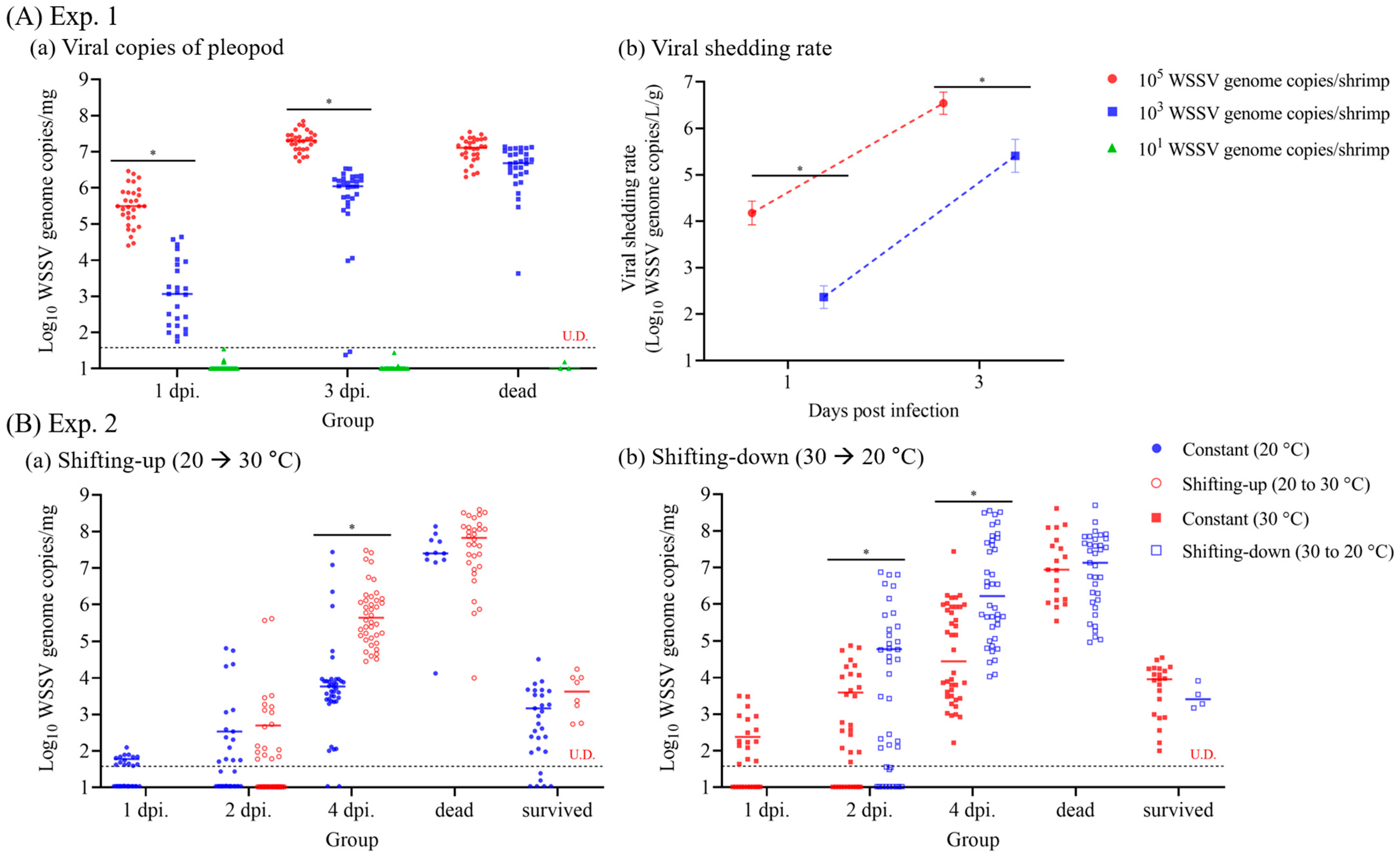
3.1. Correlation between Viral Copies of Pleopods and Viral Shedding Rate
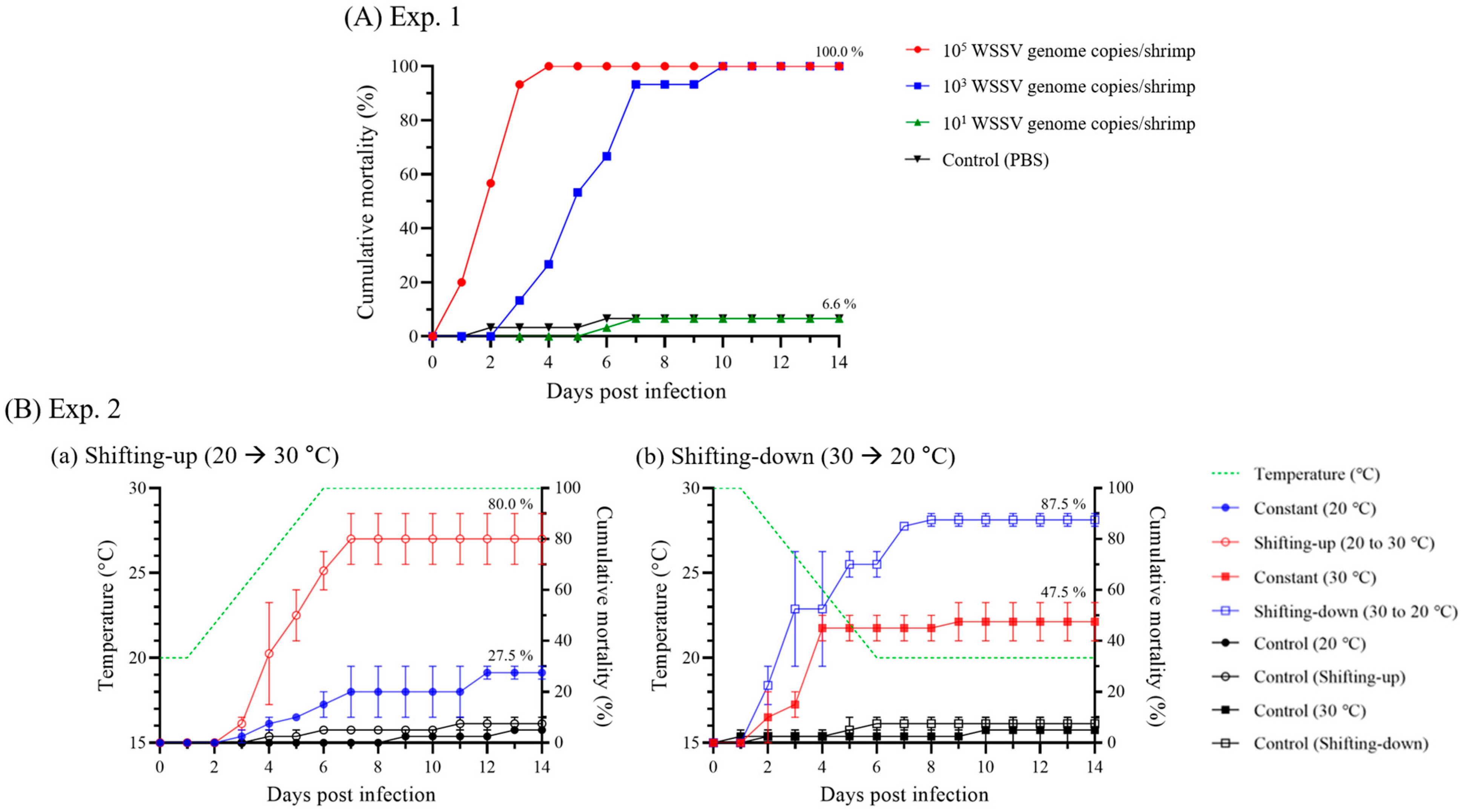
3.1.1. Cumulative Mortality in Injection-Challenged Groups
3.1.2. Viral Shedding Rate in Injection-Challenged Groups
3.1.3. Time-Course WSSV Propagation Levels in Shrimp
3.1.4. Linear Correlation between Viral Copies of Pleopods and Viral Shedding Rate
3.2. WSSV Severity Grade and Quantitative Analysis
3.2.1. Disease Severity Grade in WSSV-Infected Shrimp
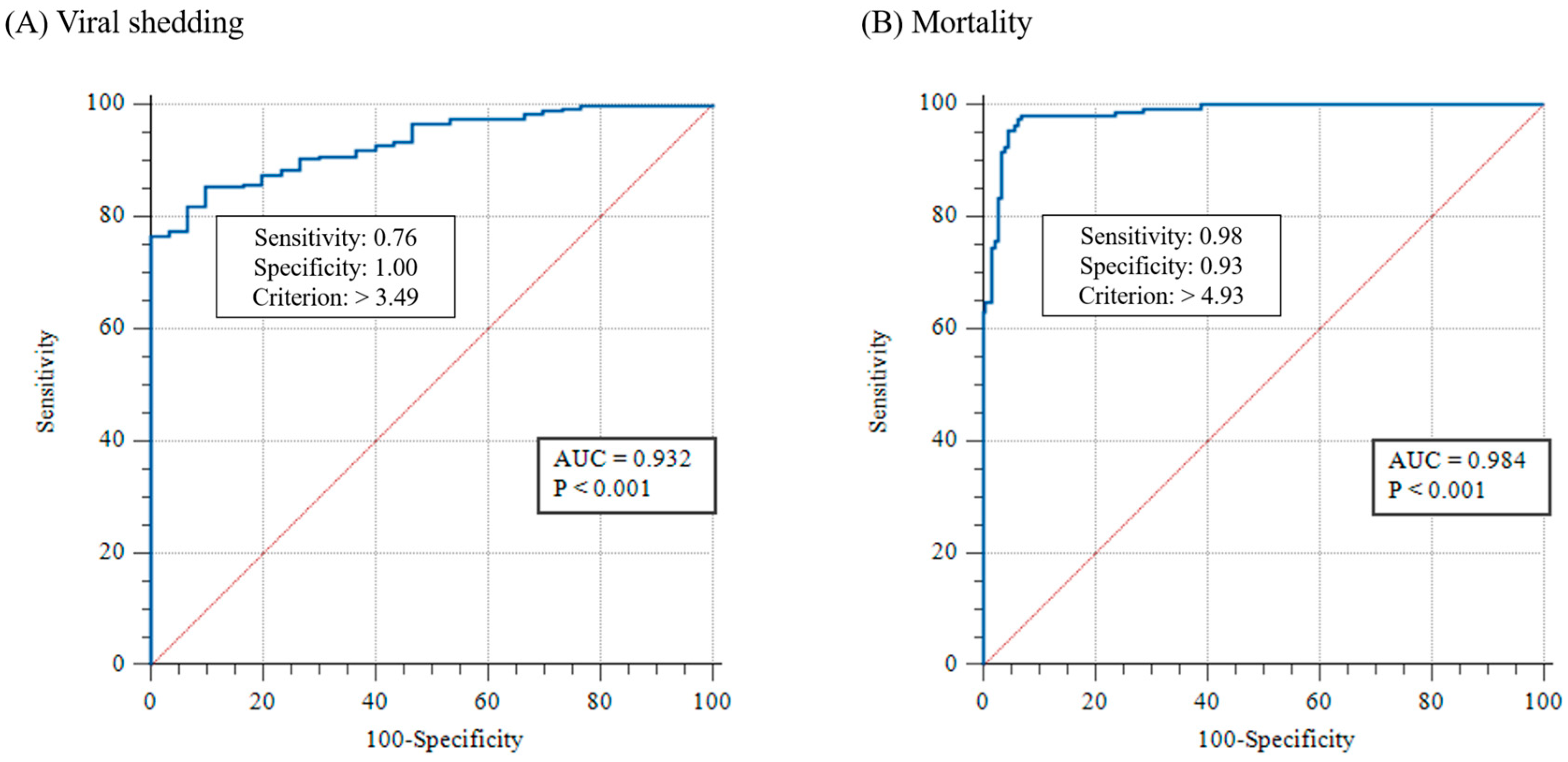
3.2.2. Threshold of Clinical Changes in WSSV-Infected Shrimp
3.2.3. Correlation between WSSV Severity Grades and Viral Copies of Pleopods
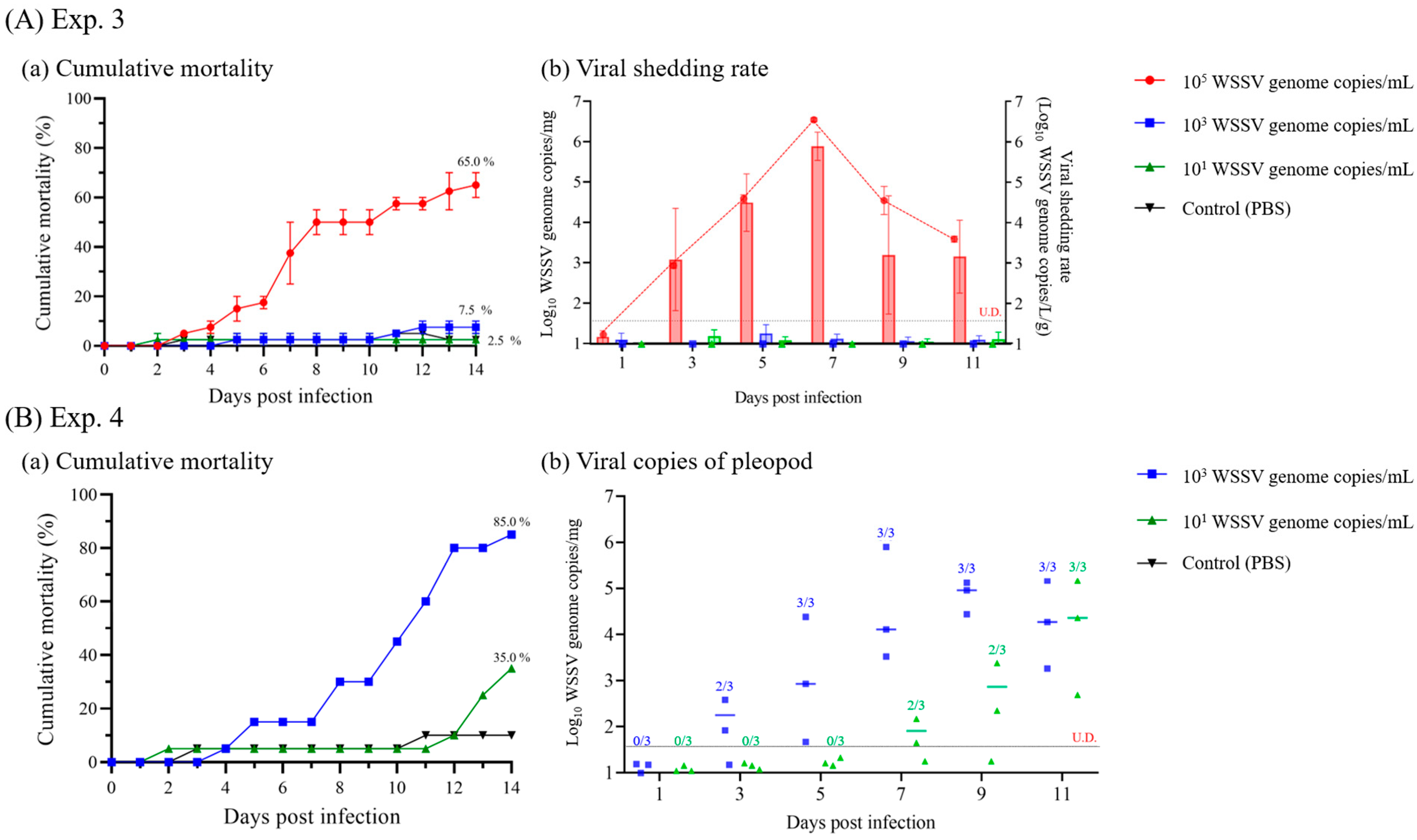
3.3. Waterborne Transmission of WSSV in Natural-Condition-Mimicking Conditions
3.3.1. Minimum Infective Dose of WSSV via the Waterborne Route
3.3.2. Pathogenicity and Viral Load Dynamics in Cohabitation Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flegel, T.W. Historic emergence, impact and current status of shrimp pathogens in Asia. J. Invertebr. Pathol. 2012, 110, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.C.; James, R.; Rajan, L.A.; Surendran, P.K.; Lalitha, K.V. White spot syndrome virus infection: Threat to crustacean biodiversity in Vembanad Lake, India. Appl. Biotechnol. Rep. 2015, 7, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.; Huang, C.; Wang, C.; Chiang, H.; Lo, C. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis. Aquat. Org. 1995, 23, 165–173. [Google Scholar] [CrossRef]
- Lightner, D.V.; Hasson, K.W.; White, B.L.; Redman, R.M. Experimental infection of western hemisphere penaeid shrimp with Asian white spot syndrome virus and Asian yellow head virus. J. Aquat. Anim. Health 1998, 10, 271–281. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lo, C.F.; Chang, P.S.; Kou, G.H. Experimental infection of white spot baculovirus in some cultured and wild decapods in Taiwan. Aquaculture 1998, 164, 221–231. [Google Scholar] [CrossRef]
- Chou, H.Y.; Huang, C.Y.; Lo, C.F.; Kou, G.H. Studied on transmission of white spot syndrome associated baculovirus (WSBV) in Penaeus monodon and P. japonicus via waterborne contact and oral ingestion. Aquaculture 1998, 164, 263–276. [Google Scholar] [CrossRef]
- Hershberger, P.; Gregg, J.; Grady, C.; Collins, R.; Winton, J. Kinetics of viral shedding provide insights into the epidemiology of viral hemorrhagic septicemia in Pacific herring. Mar. Ecol. Prog. Ser. 2010, 400, 187–193. [Google Scholar] [CrossRef]
- Urquhart, K.; Murray, A.G.; Gregory, A.; O’Dea, M.; Munro, L.A.; Smail, D.A.; Shanks, A.M.; Raynard, R.S. Estimation of infectious dose and viral shedding rates for infectious pancreatic necrosis virus in Atlantic salmon, Salmo salar L., post-smolts. J. Fish Dis. 2008, 31, 879–887. [Google Scholar] [CrossRef]
- Dixon, P.; Paley, R.; Alegria-Moran, R.; Oidtmann, B. Epidemiological characteristics of infectious hematopoietic necrosis virus (IHNV): A review. Vet. Res. 2016, 47, 63. [Google Scholar] [CrossRef]
- Garver, K.A.; Mahony, A.A.; Stucchi, D.; Richard, J.; Van Woensel, C.; Foreman, M. Estimation of parameters influencing waterborne transmission of infectious hematopoietic necrosis virus (IHNV) in Atlantic salmon (Salmo salar). PLoS ONE 2013, 8, e82296. [Google Scholar] [CrossRef]
- Kawato, Y.; Mekata, T.; Inada, M.; Ito, T. Application of environmental DNA for monitoring Red Sea bream Iridovirus at a fish farm. Microbiol. Spectr. 2021, 9, e00796-21. [Google Scholar] [CrossRef]
- Krishnan, R.; Qadiri, S.S.N.; Kim, J.O.; Oh, M.J. Infection dynamics and shedding kinetics of nervous necrosis virus in juvenile seven band grouper using an intraperitoneal infection-cohabitation model. Aquaculture 2021, 530, 735957. [Google Scholar] [CrossRef]
- Durand, S.V.; Lightner, D.V. Quantitative real time PCR for the measurement of white spot syndrome virus in shrimp. J. Fish Dis. 2002, 25, 381–389. [Google Scholar] [CrossRef]
- Kumar, S.S.; Bharathi, R.A.; Rajan, J.J.S.; Alavandi, S.V.; Poornima, M.; Balasubramanian, C.P.; Ponniah, A.G. Viability of white spot syndrome virus (WSSV) in sediment during sun-drying (drainable pond) and under non-drainable pond conditions indicated by infectivity to shrimp. Aquaculture 2013, 402, 119–126. [Google Scholar] [CrossRef]
- Qayoom, U.; Gireesh-Babu, P.; Kumar, G.; Chaudhari, A. WSSV susceptibility in the early life stages of penaeus vannamei shows relationship with bodyweight. J. Invertebr. Pathol. 2023, 198, 107912. [Google Scholar] [CrossRef]
- Oidtmann, B.; Stentiford, G.D. White spot syndrome virus (WSSV) concentrations in crustacean tissues–a review of data relevant to assess the risk associated with commodity trade. Transbound. Emerg. Dis. 2011, 58, 469–482. [Google Scholar] [CrossRef]
- Lightner, D.V. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp; Lightner, D.V.: Baton Rouge, LA, USA, 1996. [Google Scholar]
- Tang, K.F.; Lightner, D.V. Quantification of white spot syndrome virus DNA through a competitive polymerase chain reaction. Aquaculture 2000, 189, 11–21. [Google Scholar] [CrossRef]
- De la Peña, L.D.; Arboleda, J.I.; Castellano, J.L.A. Establishment of threshold infection levels of WSSV in different weight ranges of Penaeus vannamei using quantitative PCR (qPCR). In Proceedings of the International Workshop on the Promotion of Sustainable Aquaculture, Aquatic Animal Health, and Resource Enhancement in Southeast Asia, Iloilo City, Philippines, 25–27 June 2019; Aquaculture Department, Southeast Asian Fisheries Development Center: Palembang, Indonesia, 2021; pp. 218–228. [Google Scholar]
- Talukder, A.S.; Punom, N.J.; Eshik, M.M.E.; Begum, M.K.; Islam, H.R.; Hossain, Z.; Rahman, M.S. Molecular identification of white spot syndrome virus (WSSV) and associated risk factors for white spot disease (WSD) prevalence in shrimp (Penaeus monodon) aquaculture in Bangladesh. J. Invertebr. Pathol. 2021, 179, 107535. [Google Scholar] [CrossRef]
- Walker, P.J.; Gudkovs, N.; Mohan, C.V.; Raj, V.S.; Pradeep, B.; Sergeant, E.; Mohan, A.B.; Ravibabu, G.; Karunasagar, I.; Santiago, T.C. Longitudinal disease studies in small-holder black tiger shrimp (Penaeus monodon) ponds in Andhra Pradesh, India. II. Multiple WSSV genotypes associated with disease outbreaks in ponds seeded with uninfected postlarvae. Aquaculture 2011, 319, 18–24. [Google Scholar] [CrossRef]
- Lo, C.F.; Ho, C.H.; Peng, S.E.; Chen, C.H.; Hsu, H.C.; Chiu, Y.L.; Chang, C.F.; Liu, K.F.; Su, M.S.; Wang, C.H.; et al. White spot syndrome baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis. Aquat. Org. 1996, 27, 215–225. [Google Scholar] [CrossRef]
- Kim, M.J.; Baek, E.J.; Kim, K.I. Application of iron flocculation to concentrate white spot syndrome virus in seawater. J. Virol. Methods 2022, 306, 114554. [Google Scholar] [CrossRef]
- Bell, T.A.; Lightner, D.V. A Handbook of Normal Penaeid Shrimp Histology; Bell, T.A.; Lightner, D.V.: Baton Rouge, LA, USA, 1988. [Google Scholar]
- Jia, X.H.; Zhang, C.L.; Shi, D.J.; Zhuang, M.M.; Wang, X.; Jia, R.; Zhang, Z.Y.; Huang, J.; Sun, Y.H.; Qian, W.Y.; et al. Oral administration of Anabaenaexpressed VP28 for both drug and food against white spot syndrome virus in shrimp. J. Appl. Psychol. 2016, 28, 1001–1009. [Google Scholar]
- Anirudhan, A.; Okomoda, V.T.; Iryani, M.T.M.; Andriani, Y.; Abd Wahid, M.E.; Tan, M.P.; Danish-Daniel, M.; Wong, L.L.; Tengku-Muhammad, T.S.; Mok, W.J.; et al. Pandanus tectorius fruit extract promotes Hsp70 accumulation, immune-related genes expression and Vibrio parahaemolyticus tolerance in the whiteleg shrimp Penaeus vannamei. Fish Shellfish Immunol. 2021, 109, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71. [Google Scholar]
- World Organisation for Animal Health (WOAH). Manual of Diagnostic Tests for Aquatic Animals; World Organisation for Animal Health (WOAH): Paris, France, 2018. [Google Scholar]
- Pang, H.; Wang, G.; Zhou, S.; Wang, J.; Zhao, J.; Hoare, R.; Monaghan, S.J.; Wang, Z.; Sun, C. Survival and immune response of white shrimp Litopenaeus vannamei following single and concurrent infections with WSSV and Vibrio parahaemolyticus. Fish Shellfish Immunol. 2019, 92, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.R.; Álvarez, D.A.G.; Cano, F.M.; Garcia, T.E.; Molina, D.E.C.; Clark, G.P.; Marques, M.R.F.; Barajas, F.J.M.; López, J.H. Water temperature influences viral load and detection of White Spot Syndrome Virus (WSSV) in Litopenaeus vannamei and wild crustaceans. Aquaculture 2012, 326, 9–14. [Google Scholar] [CrossRef]
- Rahman, M.M.; Escobedo-Bonilla, C.M.; Corteel, M.; Dantas-Lima, J.J.; Wille, M.; Alday Sanz, V.; Pensaert, M.B.; Sorgeloos, P.; Nauwynck, H.J. Effect of high water temperature (33 °C) on the clinical and virological outcome of experimental infections with white spot syndrome virus (WSSV) in specific pathogen-free (SPF) Litopenaeus vannamei. Aquaculture 2006, 261, 842–849. [Google Scholar] [CrossRef]
- Sun, Y.; Li, F.; Xiang, J. Analysis on the dynamic changes of the amount of WSSV in Chinese shrimp Fenneropenaeus chinensis during infection. Aquaculture 2013, 376, 124–132. [Google Scholar] [CrossRef]
- Jang, I.K.; Gopalakannan, A.; Suriakala, K.; Kim, J.S.; Kim, B.R.; Cho, Y.R.; Seo, H.C. Real-time PCR quantification of White Spot Syndrome Virus (WSSV) and Hepatopancreatic Parvovirus (HPV) loads in shrimp and seawaters of shrimp ponds on the West Coast of South Korea. Fish. Aquatic Sci. 2008, 11, 195–204. [Google Scholar] [CrossRef]
- Kou, G.H. Effect of temperature shifts on shrimp lightly infected with white spot syndrome virus (WSSV). Acta Zool. 2000, 11, 63–81. [Google Scholar]
- Dey, B.K.; Dugassa, G.H.; Hinzano, S.M.; Bossier, P. Causative agent, diagnosis and management of white spot disease in shrimp: A review. Rev. Aquac. 2020, 12, 822–865. [Google Scholar] [CrossRef]
- Song, J.H.; Choo, Y.J.; Cho, J.C. Quantification of white spot syndrome virus (WSSV) in seawaters using real-time PCR and correlation analyses between WSSV and environmental parameters. Korean J. Microbiol. 2008, 44, 49–55. [Google Scholar]





| Severity Grades of WSSV Infection | Criteria of Categorization |
|---|---|
| G0 | All tissues are normal without any sign |
| G1 | Intracellular inclusion bodies could be seen in less than 10% |
| G2 | Intracellular inclusion bodies could be seen in less than 30–40% |
| G3 | Intracellular inclusion bodies could be seen in less than 40–50% |
| G4 | Intracellular inclusion bodies could be seen greater than 80% |
| Group | Severity Grade of WSSV 1 (n = 3) | |
|---|---|---|
| Administered Dose (WSSV Genome Copies/Shrimp) | Sampling Days | |
| 105 | 1 dpi 2 | G2 |
| 3 dpi | G3–G4 | |
| 103 | 1 dpi | G1–G2 |
| 3 dpi | G2–G3 | |
| 101 | 1 dpi | G0 |
| 3 dpi | G0 | |
| Groups | Sampling Days | Severity Grade of WSSV 1 (n = 5) | Groups | Sampling Days | Severity Grade of WSSV (n = 5) |
|---|---|---|---|---|---|
| Constant (20 °C) | 1 dpi 2 | G0 | Shifting-up (20 to 30) | 1 dpi | - |
| 2 dpi | G0–G1 | 2 dpi | G0–G1 | ||
| 4 dpi | G1–G2 | 4 dpi | G2–G3 | ||
| survived | G0–G1 | survived | G0–G2 | ||
| Constant (30 °C) | 1 dpi | G0–G1 | Shifting-down (30 to 20) | 1 dpi | - |
| 2 dpi | G0–G2 | 2 dpi | G1–G2 | ||
| 4 dpi | G1–G2 | 4 dpi | G3–G4 | ||
| survived | G0–G2 | survived | G0–G1 |
| WSSV Severity Grades Based on the Histopathology 1 | n 2 | Viral Genome Copies/mg (Median [IQR 3]) | Threshold of Clinical Changes in WSSV-Infected Shrimp 4 |
|---|---|---|---|
| G0 | 23 | 7.9 × 101 (4.3 × 101 to 2.6 × 103) | - |
| G1 | 35 | 3.1 × 103 (7.4 × 102 to 9.9 × 103) | Viral shedding (3.1 × 103 WSSV genome copies/mg) |
| G2 | 16 | 7.5 × 104 (1.3 × 104 to 5.5 × 105) | Mortality (8.5 × 104 WSSV genome copies/mg) |
| G3 | 10 | 1.9 × 106 (1.4 × 105 to 1.5 × 107) | - |
| G4 | 4 | 1.9 × 108 (6.7 × 107 to 3.2 × 108) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-J.; Kim, J.-O.; Jang, G.-I.; Kwon, M.-G.; Kim, K.-I. Evaluation of the Horizontal Transmission of White Spot Syndrome Virus for Whiteleg Shrimp (Litopenaeus vannamei) Based on the Disease Severity Grade and Viral Shedding Rate. Animals 2023, 13, 1676. https://doi.org/10.3390/ani13101676
Kim M-J, Kim J-O, Jang G-I, Kwon M-G, Kim K-I. Evaluation of the Horizontal Transmission of White Spot Syndrome Virus for Whiteleg Shrimp (Litopenaeus vannamei) Based on the Disease Severity Grade and Viral Shedding Rate. Animals. 2023; 13(10):1676. https://doi.org/10.3390/ani13101676
Chicago/Turabian StyleKim, Min-Jae, Jae-Ok Kim, Gwang-Il Jang, Mun-Gyeong Kwon, and Kwang-Il Kim. 2023. "Evaluation of the Horizontal Transmission of White Spot Syndrome Virus for Whiteleg Shrimp (Litopenaeus vannamei) Based on the Disease Severity Grade and Viral Shedding Rate" Animals 13, no. 10: 1676. https://doi.org/10.3390/ani13101676
APA StyleKim, M.-J., Kim, J.-O., Jang, G.-I., Kwon, M.-G., & Kim, K.-I. (2023). Evaluation of the Horizontal Transmission of White Spot Syndrome Virus for Whiteleg Shrimp (Litopenaeus vannamei) Based on the Disease Severity Grade and Viral Shedding Rate. Animals, 13(10), 1676. https://doi.org/10.3390/ani13101676






