Simplified Light’s Criteria and Acute Phase Proteins Reflect Aetiology of Feline Body Cavity Effusions Better than the Traditional Classification Scheme
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cats and Diagnostic Tests
2.2. Haematology and Biochemistry
2.3. Analysis of the Effusion
2.4. Measurement of APPs
2.5. Classification of Effusions
2.6. Statistical Analysis
3. Results
3.1. Study Cats
3.2. Classification of Effusions
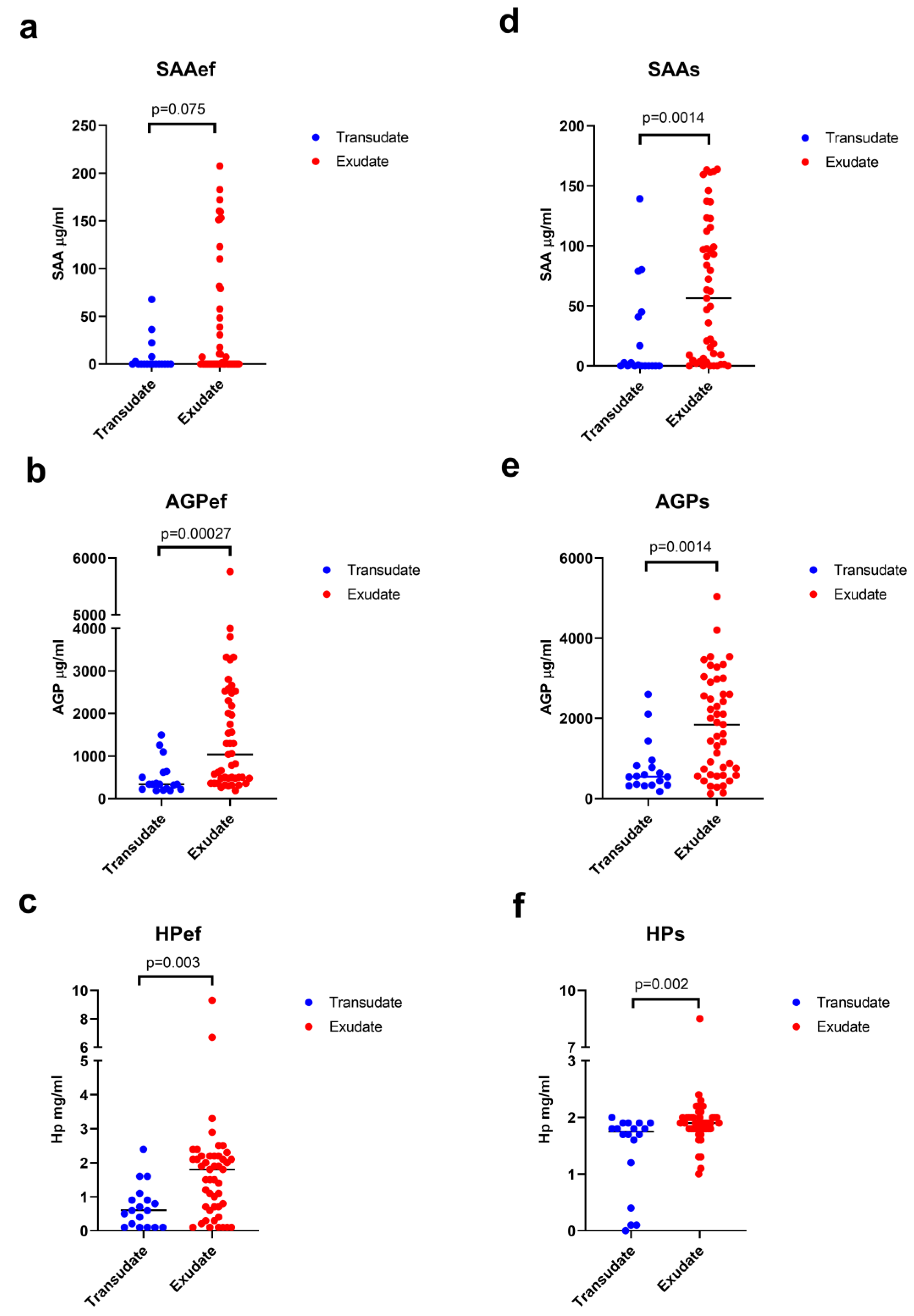
3.3. Acute Phase Proteins in Effusions and Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valenciano, A.C.; Rizzi, T.E. Abdominal, thoracic, and pericardial effusions. In Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat, 5th ed.; Valenciano, A., Cowell, R., Eds.; Mosby: St. Louis, MO, USA, 2020; pp. 229–246. [Google Scholar]
- Beatty, J.; Barrs, V. Pleural effusion in the cat. J. Feline Med. Surg. 2010, 12, 693–707. [Google Scholar] [CrossRef] [PubMed]
- König, A.; Hartmann, K.; Mueller, R.S.; Wess, G.; Schulz, B.S. Retrospective analysis of pleural effusion in cats. J. Feline Med. Surg. 2019, 21, 1102–1110. [Google Scholar] [CrossRef]
- Stokol, T. Fluid analysis. In Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat, 8th ed.; Ettinger, S.J., Feldman, E.C., Cote, E., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 292–299. [Google Scholar]
- Pedersen, N.C. An update on feline infectious peritonitis: Diagnostics and therapeutics. Vet. J. 2014, 201, 133–141. [Google Scholar] [CrossRef]
- Zoia, A.; Slater, L.A.; Heller, J.; Connolly, D.J.; Church, D.B. A new approach to pleural effusion in cats: Markers for distinguishing transudates from exudates. J. Feline Med. Surg. 2009, 11, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Zoia, A.; Drigo, M. Diagnostic value of Light’s criteria and albumin gradient in classifying the pathophysiology of pleural effusion formation in cats. J. Feline Med. Surg. 2016, 18, 666–672. [Google Scholar] [CrossRef]
- Light, R.W.; Macgregor, M.I.; Luchsinger, P.C.; Ball, W.C. Pleural effusions: The diagnostic separation of transudates and exudates. Ann. Intern. Med. 1972, 77, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Heffner, J.E.; Brown, L.K.; Barbieri, C.A. Diagnostic value of tests that discriminate between exudative and transudative pleural effusions. Chest 1997, 111, 970–980. [Google Scholar] [CrossRef]
- Bielsa, S.; Porcel, J.M.; Castellote, J.; Mas, E.; Esquerda, A.; Light, R.W. Solving the Light’s criteria misclassification rate of cardiac and hepatic transudates. Respirology 2012, 17, 721–726. [Google Scholar] [CrossRef]
- Elis, A.; Meisel, S.; Tishler, T.; Kitai, Y.; Lishner, M. Ascitic fluid to serum bilirubin concentration ratio for the classification of transudates or exudates. Am. J. Gastroenterol. 1998, 93, 401–403. [Google Scholar] [CrossRef]
- Anchinmane, V.T.; Puranik, G.V. The diagnostic separation of transudates and exudates in ascitic fluid and pleural fluid. Bombay Hosp. J. 2011, 53, 166–172. [Google Scholar]
- Kushner, I. The Phenomenon of the Acute Phase Response. Ann. N. Y. Acad. Sci. 1982, 389, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S. The feline acute phase reaction. Vet. J. 2008, 177, 26–35. [Google Scholar] [CrossRef]
- Duthie, S.; Eckersall, P.D.; Addie, D.D.; Lawrence, C.E.; Jarrett, O. Value of alpha1-acid glycoprotein in the diagnosis of feline infectious peritonitis. Vet. Rec. 1997, 141, 299–303. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Giordano, A.; Tranquillo, V.; Guazzetti, S. Critical Assessment of the Diagnostic Value of Feline α1-Acid Glycoprotein for Feline Infectious Peritonitis Using the Likelihood Ratios Approach. J. Vet. Diagn. Investig. 2007, 19, 266–272. [Google Scholar] [CrossRef]
- Selting, K.A.; Ogilvie, G.K.; Lana, S.E.; Fettman, M.J.; Mitchener, K.L.; Hansen, L.A.; Richardson, K.L.; Walton, J.A.; Scherk, M.A. Serum alhpa 1-acid glycoprotein concentrations in healthy and tumor-bearing cats. J. Vet. Intern. Med. 2000, 14, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ma, Z.; Khatlani, T.S.; Okuda, M.; Inokuma, H.; Onishi, T. Evaluation of feline serum amyloid A (SAA) as an inflammatory marker. J. Vet. Med. Sci. 2003, 65, 545–548. [Google Scholar] [CrossRef]
- Petini, M.; Drigo, M.; Zoia, A. Prognostic value of systemic inflammatory response syndrome and serum concentrations of acute phase proteins, cholesterol, and total thyroxine in cats with panleukopenia. J. Vet. Intern. Med. 2020, 34, 719–724. [Google Scholar] [CrossRef]
- Held, S. Genauigkeit Diagnostischer Tests Für Feline Infektiöse Peritonitis (FIP) Bei Katzen Mit Einem Körperhöhlenerguss. Ph.D. Thesis, Justus-Liebig-Universität, Gießen, Germany, 2013. [Google Scholar]
- Pfannschmidt, K.; Hüllermeier, E.; Held, S.; Neiger, R. Evaluating Tests in Medical Diagnosis: Combining Machine Learning with Game-Theoretical Concepts. In Information Processing and Management of Uncertainty in Knowledge-Based Systems, Proceedings of the IPMU 2016, Communications in Computer and Information Science; Carvalho, J., Lesot, M., Kaymak, U., Vieira, S., Bouchon-Meunier, B., Yager, R., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Hazuchova, K.; Held, S.; Neiger, R. Usefulness of acute phase proteins in differentiating between feline infectious peritonitis and other diseases in cats with body cavity effusions. J. Feline Med. Surg. 2017, 19, 809–816. [Google Scholar] [CrossRef]
- Luis Fuentes, V.; Abbott, J.; Chetboul, V.; Cote, E.; Fox, P.R.; Häggström, J.; Kittleson, M.D.; Schober, K.; Stern, J.A. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J. Vet. Intern. Med. 2020, 34, 1062–1077. [Google Scholar] [CrossRef]
- Giordano, A.; Spagnolo, V.; Colombo, A.; Paltrinieri, S. Changes in some acute phase protein and immunoglobulin concentrations in cats affected by feline infectious peritonitis or exposed to feline coronavirus infection. Vet. J. 2004, 167, 38–44. [Google Scholar] [CrossRef]
- Giori, L.; Giordano, A.; Giudice, C.; Grieco, V.; Paltrinieri, S. Performances of different diagnostic tests for feline infectious peritonitis in challenging clinical cases. J. Small Anim. Pract. 2011, 52, 152–157. [Google Scholar] [CrossRef]
- Jacobsen, S.; Kjelgaard-Hansen, M.; Hagbard Petersen, H.; Jensen, A.L. Evaluation of a commercially available human serum amyloid A (SAA) turbidometric immunoassay for determination of equine SAA concentrations. Vet. J. 2006, 172, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Tamamoto, T.; Ohno, K.; Ohmi, A.; Goto-Koshino, Y.; Tsujimoto, H. Verification of measurement of the feline serum amyloid A (SAA) concentration by human SAA turbidimetric immunoassay and its clinical application. J. Vet. Med. Sci. 2008, 70, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Kjelgaard-Hansen, M.; Jacobsen, S. Assay validation and diagnostic applications of major acute-phase protein testing in companion animals. Clin. Lab. Med. 2011, 31, 51–70. [Google Scholar] [CrossRef]
- Burgess, L.J. Biochemical analysis of pleural, peritoneal and pericardial effusions. Clin. Chim. Acta 2004, 343, 61–84. [Google Scholar] [CrossRef]
- Hatch, A.; Jandrey, K.E.; Tenwolde, M.C.; Kent, M.S. Incidence of chyloabdomen diagnosis in dogs and cats and corresponding clinical signs, clinicopathologic test results, and outcomes: 53 cases (1984–2014). J. Am. Vet. Med. Assoc. 2018, 253, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Doelken, P.; Sahn, S.A. Pleural fluid analysis in chylous pleural effusion. Chest 2008, 133, 1436–1441. [Google Scholar] [CrossRef]
- Vives, M.; Porcel, J.M.; Vicente de Vera, M.C.; Ribelles, E.; Rubio, M. A study of Light’s criteria and possible modifications for distinguishing exudative from transudative pleural effusions. Chest 1996, 109, 1503–1507. [Google Scholar] [CrossRef]
- Stockham, S.L.; Scott, M.A. Cavitary effusions. In Fundamentals of Veterinary Clinical Pathology, 2nd ed.; Stockham, S.L., Scott, M.A., Eds.; Blackwell Publishing: Ames, IA, USA, 2008; pp. 831–868. [Google Scholar]
- Hanley, J.A.; Mcneil, B.J. Meaning and and use of of the the area under a receiver operating curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1998; p. 567. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Dempsey, S.M.; Ewing, P.J. A review of the pathophysiology, classification, and analysis of canine and feline cavitary effusions. J. Am. Anim. Hosp. Assoc. 2011, 47, 1–11. [Google Scholar] [CrossRef]
- Gavazza, A.; Turinelli, V.; Lubas, G. Effusion in the cat: Classification of 396 fluids according to a problem-oriented scheme. Comp. Clin. Path. 2013, 22, 517–521. [Google Scholar] [CrossRef]
- Light, R.W. The Light Criteria: The Beginning and Why they are Useful 40 Years Later. Clin. Chest Med. 2013, 34, 21–26. [Google Scholar] [CrossRef]
- Boyer, T.D.; Kahn, A.M.; Reynolds, T.B. Diagnostic Value of Ascitic Fluid Lactic Dehydrogenase, Protein, and WBC Levels. Arch. Intern. Med. 1978, 138, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Burgess, L.J.; Maritz, F.J.; Taljaard, J.J.F. Comparative analysis of the biochemical parameters used to distinguish between pleural transudates and exudates. Chest 1995, 107, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.; Engelmann, A.M.; Jaguezeski, A.M.; da Silva, C.B.; Barbosa, N.V.; de Andrade, C.M. Retrospective study of the aetiopathological diagnosis of pleural or peritoneal effusion exams of dogs and cats. Comp. Clin. Path. 2021, 30, 811–820. [Google Scholar] [CrossRef]
- Kogan, Y.; Sabo, E.; Odeh, M. Role of c-reactive protein in discrimination between transudative and exudative pleural effusions. Diagnostics 2021, 11, 3–11. [Google Scholar] [CrossRef]
- Okino, A.M.; Bürger, C.; Cardoso, J.R.; Lavado, E.L.; Lotufo, P.A.; Campa, A. The acute-phase proteins serum amyloid A and C reactive protein in transudates and exudates. Mediat. Inflamm. 2006, 2006, 047297. [Google Scholar] [CrossRef]
- Alexandrakis, M.G.; Coulocheri, S.A.; Bouros, D.; Vlachonikolis, I.G.; Eliopoulos, G.D. Significance of alpha-2-macroglobulin, alpha-1-acid glycoprotein, and C-reactive protein in pleural effusion differentiation. Respiration 2000, 67, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Calikoglu, M.; Sezer, C.; Unlü, A.; Kanik, A.; Tamer, L.; Calikoglu, I. Use of acute phase proteins in pleural effusion discrimination. Tuberk. Toraks 2004, 52, 122–129. [Google Scholar]
- Shanthaveeranna, G.K.; Thykadavil, V.; D’Souza, G. Use of pleural fluid ceruloplasmin in the differentiation of exudative and transudative pleural effusion. Lung India 2015, 32, 11–15. [Google Scholar] [CrossRef]
- Tamamoto, T.; Ohno, K.; Takahashi, M.; Nakashima, K.; Fujino, Y.; Tsujimoto, H. Serum amyloid A as a prognostic marker in cats with various diseases. J. Vet. Diagn. Investig. 2013, 25, 428–432. [Google Scholar] [CrossRef]
- Thalmeier, S.; Güssow, A.; Häuser, M.K.; Bauer, N.; Hazuchova, K. Cat alpha-1-acid glycoprotein enzyme-linked immunosorbent assay: Performance characteristics and reference intervals. J. Feline Med. Surg. 2023, 25, 1098612X231162836. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.; Vinther, A.M.; Kjelgaard-Hansen, M.; Nielsen, L.N. Validation of an equine serum amyloid A assay with an unusually broad working range. BMC Vet. Res. 2019, 15, 462. [Google Scholar] [CrossRef] [PubMed]

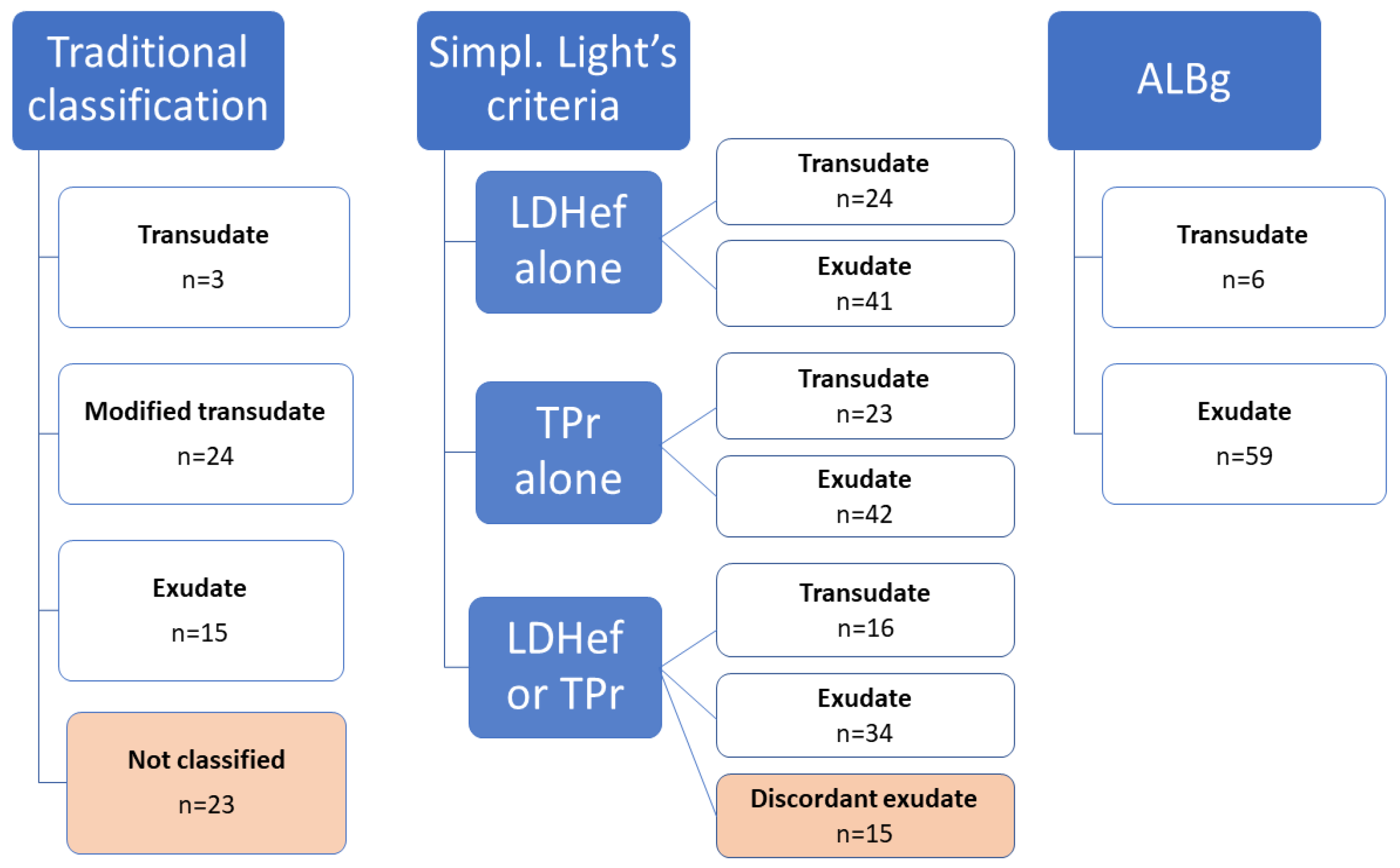

| Transudate (n = 18) | Exudate (n = 47) | |||
|---|---|---|---|---|
| Pleural effusion (n = 13) | Pericardial effusion (n = 1) | Ascites (n = 4) | Pleural effusion (n = 18) | Ascites (n = 29) |
|
|
|
|
|
| Classification Scheme | Sensitivity | Specificity | Accuracy | Misclassified Transudates n (%) | Misclassified Exudates n (%) |
|---|---|---|---|---|---|
| Traditional veterinary scheme *# | 39% | 73% | 48% | 3/11 (27%) | 19/31 (61%) |
| LDHef (IU/L) | 81% | 83% | 82% | 3/18 (17%) | 9/47 (19%) |
| TPr | 79% | 72% | 77% | 5/18 (28%) | 10/47 (21%) |
| Simplified Light’s criteria | 87% | 56% | 79% | 8/18 (44%) | 6/47 (13%) |
| ALBg | 98% | 28% | 75% | 13/18 (72%) | 1/47 (2%) |
| Classification Scheme | APPs | Transudate | Exudate | p-Value | Cohen’s d | Power% |
|---|---|---|---|---|---|---|
| Aetiological classification | n = 18 | n = 47 | ||||
| SAAef [μg/mL] | 0.1 (0.1–67.7) | 0.1 (0.1–207.4) | 0.075 | 0.40 | 28 | |
| AGPef [μg/mL] | 340 (190–1500) | 1040 (190–5760) | 0.00027 | 0.79 | 78 | |
| HPef [mg/mL] | 0.6 (0.1–2.4) | 1.8 (0.1–9.3) | 0.003 | 0.79 | 78 | |
| SAAs [μg/mL] | 0.45 (0.1–139.2) | 56.4 (0.1–163.8) | 0.0014 | 0.86 | 85 | |
| AGPs [μg/mL] | 550 (180–2600) | 1840 (120–5040) | 0.0014 | 0.86 | 85 | |
| HPs [mg/mL] | 1.75 (0–2) | 1.9 (1–8.5) | 0.002 | 0.82 | 82 | |
| Traditional veterinary scheme * | n = 27 | n = 15 | ||||
| SAAef [μg/mL] | 0.1 (0.1–159.4) | 0.1 (0.1–182.7) | 0.71 | 0.10 | 6 | |
| AGPef [μg/mL] | 580 (190–3260) | 480 (190–3800) | 0.36 | 0.28 | 13 | |
| HPef [mg/mL] | 0.8 (0.1–2.5) | 1 (0.1–2.2) | 0.25 | 0.37 | 19 | |
| SAAs [μg/mL] | 15.3 (0.1–162.1) | 35.8 (0.1–163.8) | 0.65 | 0.13 | 7 | |
| AGPs [μg/mL] | 820 (140–3540) | 780 (120–3320) | 0.56 | 0.18 | 8 | |
| HPs [mg/mL] | 1.8 (0–2.2) | 1.9 (1.3–2.3) | 0.15 | 0.45 | 27 |
| Classification | APPs | AUC (95% CI) | p-Value | Best Cut-Off (Sensitivity%, Specificity%) |
|---|---|---|---|---|
| Aetiological classification | SAAef #§ [μg/mL] | 0.63 (0.5–0.75) | 0.07 | >36.2 (Sens. 32%, Spec. 94%) |
| AGPef # [μg/mL] | 0.79 (0.68–0.88) | <0.0001 | >340 (Sens. 87%, Spec. 61%) | |
| HPef [mg/mL] | 0.74 (0.61–0.84) | 0.0002 | >0.9 (Sens. 68%, Spec. 78%) | |
| SAAs [μg/mL] | 0.76 (0.63–0.85) | 0.0001 | >2.7 (Sens. 81%, Spec. 67%) | |
| AGPs § [μg/mL] | 0.76 (0.64–0.85) | <0.0001 | >960 (Sens. 64%, Spec. 83%) | |
| HPs [mg/mL] | 0.75 (0.63–0.85) | 0.0001 | >1.7 (Sens. 83%, Spec. 50%) | |
| Traditional veterinary scheme * | SAAef [μg/mL] | 0.53 (0.37–0.69) | 0.75 | ≤7.5 (Sens. 80%, Spec. 37%) |
| AGPef [μg/mL] | 0.59 (0.42–0.74) | 0.36 | ≤620 (Sens. 80%, Spec. 48%) | |
| HPef [mg/mL] | 0.61 (0.45–0.76) | 0.23 | ≤1.6 (Sens. 93%, Spec. 37%) | |
| SAAs [μg/mL] | 0.54 (0.38–0.7) | 0.68 | >122.9 (Sens. 27%, Spec. 96%) | |
| AGPs [μg/mL] | 0.55 (0.39–0.7) | 0.57 | ≤440 (Sens. 40%, Spec. 82%) | |
| HPs [mg/mL] | 0.64 (0.47–0.78) | 0.12 | >1.7 (Sens. 87%, Spec. 41%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazuchova, K.; Held, S.; Klemm, I.; Bauer, N. Simplified Light’s Criteria and Acute Phase Proteins Reflect Aetiology of Feline Body Cavity Effusions Better than the Traditional Classification Scheme. Animals 2023, 13, 1918. https://doi.org/10.3390/ani13121918
Hazuchova K, Held S, Klemm I, Bauer N. Simplified Light’s Criteria and Acute Phase Proteins Reflect Aetiology of Feline Body Cavity Effusions Better than the Traditional Classification Scheme. Animals. 2023; 13(12):1918. https://doi.org/10.3390/ani13121918
Chicago/Turabian StyleHazuchova, Katarina, Susanne Held, Isabell Klemm, and Natali Bauer. 2023. "Simplified Light’s Criteria and Acute Phase Proteins Reflect Aetiology of Feline Body Cavity Effusions Better than the Traditional Classification Scheme" Animals 13, no. 12: 1918. https://doi.org/10.3390/ani13121918
APA StyleHazuchova, K., Held, S., Klemm, I., & Bauer, N. (2023). Simplified Light’s Criteria and Acute Phase Proteins Reflect Aetiology of Feline Body Cavity Effusions Better than the Traditional Classification Scheme. Animals, 13(12), 1918. https://doi.org/10.3390/ani13121918






