A Review of Sperm Ultrastructural Characters in the Opecoelidae (Digenea) and Their Phylogenetic Implications, with New Data on Peracreadium characis, a Parasite of Diplodus puntazzo in Tunisia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
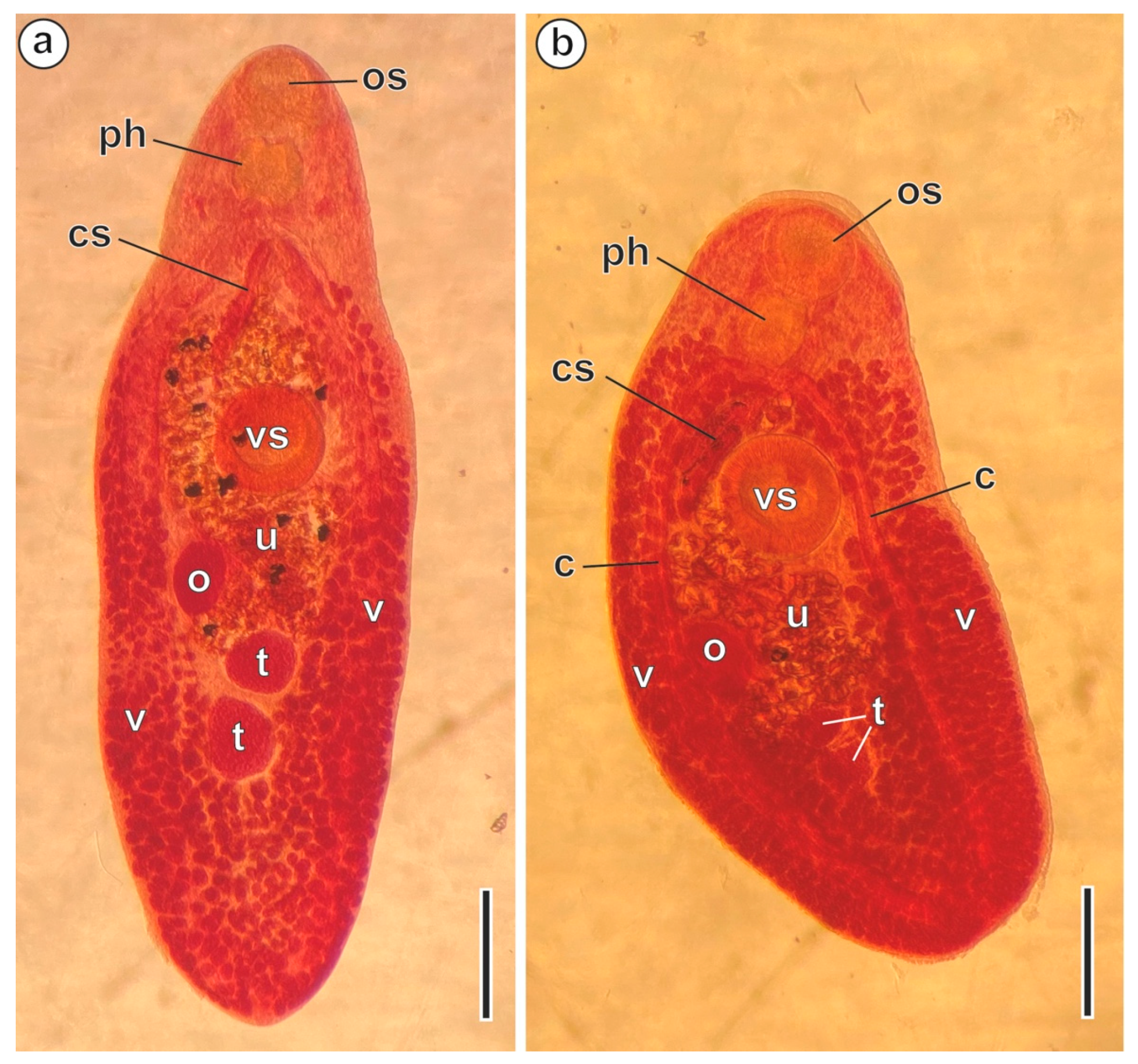
2.1. Specimens
2.2. Transmission Electron Microscopy
2.3. Cytochemistry
3. Results
3.1. Region I: Anterior Spermatozoon Extremity (Figure 2a–h and Figure 4I)
3.2. Region II: Middle Spermatozoon Region (Figure 2i–k and Figure 4II)



3.3. Region III: Posterior Spermatozoon Extremity (Figure 2l, Figure 3a–f and Figure 4III)
4. Discussion
4.1. Anterior Extremity: Anterior Dense Material
4.2. Axonemes
4.3. External Ornamentation
4.4. Cortical Microtubules
4.5. Spine-Like Bodies
4.6. Mitochondrion
4.7. Posterior Region of the Spermatozoon
5. Concluding Remarks: Contribution of Spermatological Characteristics to Opecoelidean Phylogenetic Inferences
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, R.A.; Cribb, T.H.; Littlewood, D.T.J.; Waeschenbach, A. The molecular phylogeny of the digenean family Opecoelidae Ozaki, 1925 and the value of morphological characters, with the erection of a new subfamily. Folia Parasitol. 2016, 63, 013. [Google Scholar] [CrossRef] [Green Version]
- Cribb, T.H. Family Opecoelidae Ozaki, 1925. In Keys to the Trematoda; Jones, A., Bray, R.A., Gibson, D.I., Eds.; CAB International and The Natural History Museum: London, UK, 2005; Volume 2, pp. 443–531. [Google Scholar]
- Curran, S.S.; Tkach, V.V.; Overstreet, R.M. A review of Polylekithum Arnold, 1934 and its familial affinities using morphological and molecular data, with description of Polylekithum catahoulensis sp. nov. Acta Parasitol. 2006, 51, 238–248. [Google Scholar] [CrossRef]
- Littlewood, D.T.J.; Bray, R.A.; Waeschenbach, A. Phylogenetic patterns of diversity in cestodes and trematodes. In Parasite Diversity and Diversification: Evolutionary Ecology Meets Phylogenetics; Morand, S., Krasnov, B.R., Littlewood, D.T.J., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 304–319. [Google Scholar]
- Pérez-Ponce de León, G.; Hernández-Mena, D.I. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. J. Helminthol. 2019, 93, 260–276. [Google Scholar] [CrossRef] [Green Version]
- Curran, S.S.; Overstreet, R.M.; Tkach, V.V. Phylogenetic affinities of Plagiocirrus van Cleave and Mueller, 1932 with the description of a new species from the Pascagoula river, Mississippi. J. Parasitol. 2007, 93, 1452–1458. [Google Scholar] [CrossRef]
- Andres, M.J.; Ray, C.L.; Pulis, E.E.; Curran, S.S.; Overstreet, R.M. Molecular characterization of two opecoelid trematodes from fishes in the Gulf of Mexico, with a description of a new species of Helicometra. Acta Parasitol. 2014, 59, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Shedko, M.B.; Sokolov, S.G.; Atopkin, D.M. The first record of Dimerosaccus oncorhynchi (Trematoda: Opecoelidae) in fishes from rivers of Primorsky Territory, Russia, with a discussion on its taxonomic position using morphological and molecular data. Parazitologiya 2015, 49, 171–189. [Google Scholar]
- Martin, S.B.; Crouch, K.; Cutmore, S.C.; Cribb, T.H. Expansion of the concept of the Opistholebetinae Fukui, 1929 (Digenea: Opecoelidae Ozaki, 1925), with Magnaosimum brooksae n. g., n. sp. from Tripodichthys angustifrons (Hollard) (Tetraodontiformes: Triacanthidae) in Moreton Bay, Australia. Syst. Parasitol. 2018, 95, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.B.; Sasal, P.; Cutmore, S.C.; Ward, S.; Aeby, G.S.; Cribb, T.H. Intermediate host switches drive diversification among the largest trematode family: Evidence from the Polypipapiliotrematinae n. subf. (Opecoelidae), parasites transmitted to butterflyfishes via predation of coral polyps. Int. J. Parasitol. 2018, 48, 1107–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.B.; Huston, D.C.; Cutmore, S.C.; Cribb, T.H. A new classification for deep-sea opecoelid trematodes based on the phylogenetic position of some unusual taxa from shallow-water, herbivorous fishes off south-west Australia. Zool. J. Linn. Soc. 2019, 186, 385–413. [Google Scholar] [CrossRef]
- Karar, Y.F.M.; Blend, C.K.; Mohamadain, H.S.; Hassan, H.A.-S.; Khalifa, R.M.A.; Dronen, N.O. Unusual opecoelids from Red Sea triggerfishes with special reference to characteristic concepts of the Opistholebetinae Fukui, 1929 (Digenea: Opecoelidae). Zootaxa 2020, 4834, 001–032. [Google Scholar] [CrossRef]
- Martin, S.B.; Cutmore, S.C.; Cribb, T.H. The Pseudoplagioporinae, a new subfamily in the Opecoelidae Ozaki, 1925 (Trematoda) for a small clade parasitizing mainly lethrinid fishes, with three new species. J. Zool. Syst. Evol. Res. 2020, 58, 79–113. [Google Scholar] [CrossRef]
- Martin, S.B.; Downie, A.J.; Cribb, T.H. A new subfamily for a clade of opecoelids (Trematoda: Digenea) exploiting marine fishes as second-intermediate hosts, with the first report of opecoelid metacercariae from an elasmobranch. Zool. J. Linn. Soc. 2020, 188, 455–472. [Google Scholar] [CrossRef]
- Sokolov, S.G.; Shchenkov, S.V.; Frolov, E.V.; Gordeev, I.I. A Phylogenetic Re-Evaluation of the Stenakrine Opecoelids (Trematoda, Digenea: Opecoeloidea) with Some Taxonomic Novelties. Diversity 2022, 14, 949. [Google Scholar] [CrossRef]
- Martin, S.B.; Cutmore, S.C.; Cribb, T.H. Revision of Neolebouria Gibson, 1976 (Digenea: Opecoelidae), with Trilobovarium n. g., for species infecting tropical and subtropical shallow-water fishes. Syst. Parasitol. 2017, 94, 307–338. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, S.G.; Shchenkov, S.V.; Gordeev, I.I. Phylogenetic position of deep-sea opecoelid digenean Tellervotrema beringi (Mamaev, 1965) (Trematoda: Opecoelidae) based on novel genetic data. Syst. Parasitol. 2020, 97, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Justine, J.-L. Phylogeny of parasitic Platyhelminthes: A critical study of synapomorphies proposed on the basis of the ultrastructure of spermiogenesis and spermatozoa. Can. J. Zool. 1991, 69, 1421–1440. [Google Scholar] [CrossRef]
- Justine, J.-L. Spermatozoal ultrastructure and phylogeny of the parasitic Platyhelminthes. Mém. Mus. Natn. Hist. Nat. 1995, 166, 55–86. [Google Scholar]
- Justine, J.-L. Spermatozoa as phylogenetic characters for the Eucestoda. J. Parasitol. 1998, 84, 385–408. [Google Scholar] [CrossRef]
- Justine, J.-L. Spermatozoa as phylogenetic characters for the Platyhelminthes. In Interrelationships of the Platyhelminthes; Littlewood, D.T.J., Bray, R.A., Eds.; Taylor and Francis: London, UK, 2001; pp. 231–238. [Google Scholar]
- Quilichini, Y.; Foata, J.; Marchand, B. Ultrastructural study of the spermatozoon of Pronoprymna ventricosa (Digenea, Baccigerinae), parasite of the twaite shad Alosa fallax Lacepede (Pisces, Teleostei). Parasitol. Res. 2007, 101, 1125–1130. [Google Scholar] [CrossRef]
- Quilichini, Y.; Foata, J.; Justine, J.-L.; Bray, R.A.; Marchand, B. Ultrastructural study of the spermatozoon of Heterolebes maculosus (Digenea, Opistholebetidae), a parasite of the porcupinefish Diodon hystrix (Pisces, Teleostei). Parasitol. Int. 2010, 59, 427–434. [Google Scholar] [CrossRef]
- Quilichini, Y.; Foata, J.; Justine, J.-L.; Bray, R.A.; Marchand, B. Spermatozoon ultrastructure of Gyliauchen sp. (Digenea: Gyliauchenidae), an intestinal parasite of Siganus fuscescens (Pisces: Teleostei). Biol. Bull. 2011, 221, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Levron, C.; Miquel, J.; Oros, M.; Scholz, T. Spermatozoa of tapeworms (Platyhelminthes, Eucestoda): Advances in ultrastructural and phylogenetic studies. Biol. Rev. 2010, 85, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, A.J.S.; Miquel, J.; Ndiaye, P.I.; Justine, J.-L.; Falchi, A.; Bâ, C.T.; Marchand, B.; Quilichini, Y. Advances in spermatological characters in the Digenea: Review and proposal of spermatozoa models and their phylogenetic importance. Adv. Parasitol. 2017, 98, 111–165. [Google Scholar] [PubMed]
- Justine, J.-L.; Poddubnaya, L.G. Spermiogenesis and spermatozoon ultrastructure in basal polyopisthocotylean monogeneans, Hexabothriidae and Chimaericolidae, and their significance for the phylogeny of the Monogenea. Parasite 2018, 25, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhoum, A.J.S.; Kacem, H.; Neifar, L.; Miquel, J. The Opecoelidae sperm model and its contribution to phylogeny: Spermatozoon ultrastructural particularities of Allopodocotyle pedicellata (Plagioporinae, Digenea, Platyhelminthes). Zool. Anz. 2017, 266, 28–34. [Google Scholar] [CrossRef]
- Kacem, H.; Diagne, P.M.; Miquel, J. Ultrastructural organisation of the spermatozoon of Allopodocotyle tunisiensis Derbel and Neifar, 2009 (Digenea, Opecoelidae), an intestinal parasite of Solea aegyptiaca Chabanaud, 1927 (Teleostei, Soleidae). Tissue Cell 2019, 57, 1–7. [Google Scholar] [CrossRef]
- Diagne, P.M.; Bâ, C.T.; Ndiaye, P.I.; Bray, R.A.; Marchand, B.; Quilichini, Y. Sperm ultrastructure of Podocotyloides magnatestis (Digenea, Opecoeloidea, Opecoelidae) a parasite of Parapristipoma octolineatum (Pisces, Teleostei). Zool. Anz. 2016, 264, 56–63. [Google Scholar] [CrossRef]
- Quilichini, Y.; Foata, J.; Justine, J.-L.; Bray, R.A.; Marchand, B. Sperm ultrastructure of Helicometra epinepheli (Platyhelminthes, Digenea, Opecoelidae), parasite of Epinephelus fasciatus (Pisces, Teleostei). Histol. Histopathol. 2011, 26, 1019–1028. [Google Scholar]
- Levron, C.; Ternengo, S.; Marchand, B. Ultrastructure of spermiogenesis and the spermatozoon of Helicometra fasciata (Digenea, Opecoelidae), a parasite of Labrus merula (Pisces, Teleostei). Acta Parasitol. 2003, 48, 255–264. [Google Scholar]
- Bâ, A.; Marchand, B.; Ndiaye, P.I.; Bâ, C.T.; Bakhoum, A.J.S.; Bray, R.A.; Quilichini, Y. Ultrastructure of the spermatozoon of Labracetabulum gephyroberici (Digenea, Opecoelidae) intestinal parasite of Gephyroberyx darwinii (Teleostei, Trachichtyidae) in Senegal). Zool. Anz. 2020, 286, 58–63. [Google Scholar] [CrossRef]
- Miquel, J.; Nourrisson, C.; Marchand, B. Ultrastructure of spermiogenesis and the spermatozoon of Opecoeloides furcatus (Trematoda, Digenea, Opecoelidae), a parasite of Mullus barbatus (Pisces, Teleostei). Parasitol. Res. 2000, 86, 301–310. [Google Scholar] [CrossRef]
- Levron, C.; Ternengo, S.; Marchand, B. Spermiogenesis and sperm ultrastructure of Poracanthium furcatum (Digenea, Opecoelidae), a parasite of Mullus surmuletus (Pisces, Teleostei). Acta Parasitol. 2004, 49, 190–200. [Google Scholar]
- Kacem, H.; Quilichini, Y.; Neifar, L.; Torres, J.; Miquel, J. Ultrastructure of the spermatozoon of Macvicaria obovata (Digenea, Opecoelidae), a parasite of Sparus aurata (Pisces, Teleostei) from the Gulf of Gabès, Mediterranean Sea. Acta Parasitol. 2017, 62, 520–528. [Google Scholar] [CrossRef]
- Quilichini, Y.; Foata, J.; Marchand, B. Ultrastructural study of the spermatozoon of Nicolla testiobliquum (Digenea, Opecoelidae) parasite of brown trout Salmo trutta (Pisces, Teleostei). Parasitol. Res. 2007, 101, 1295–1301. [Google Scholar] [CrossRef]
- Quilichini, Y.; Foata, J.; Orsini, A.; Marchand, B. Spermiogenesis and spermatozoon ultrastructure of Nicolla wisniewskii (Digenea: Opecoelidae), an intestinal parasite of brown trout Salmo trutta (Pisces: Teleostei). J. Parasitol. 2007, 93, 469–478. [Google Scholar] [CrossRef]
- Bartoli, P.; Gibson, D.I.; Bray, R.A. The Opecoelidae (Digenea) of sparid fishes of the western Mediterranean. IV. Peracreadium Nicoll, 1909 and Cainocreadium Nicoll, 1909. Syst. Parasitol. 1989, 14, 53–67. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electronopaque stain in electronmicroscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiéry, J.P. Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J. Microsc. (Paris) 1967, 6, 987–1018. [Google Scholar]
- Bâ, C.T.; Ndiaye, P.I.; Dione, A.; Quilichini, Y.; Marchand, B. Ultrastructure of the spermatozoon of Holorchis micracanthum (Digenea: Lepocreadiidae), an intestinal parasite of Plectorhinchus mediterraneus (Pisces, Teleostei) in Senegal. Parasitol. Res. 2011, 109, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Bâ, A.; Ndiaye, P.I.; Bakhoum, A.J.S.; Bray, R.A.; Bâ, C.T.; Marchand, B.; Quilichini, Y. Ultrastructure of the spermatozoon of Aephnidiogenes senegalensis (Digenea, Aephnidiogenidae), an intestinal parasite of Pomadasys jubelini (Teleostei, Haemulidae) off the coast of Dakar (Senegal). Zool. Anz. 2018, 276, 35–41. [Google Scholar] [CrossRef]
- Kacem, H.; Miquel, J. Spermatological characters in the Lepocreadioidea, with first data on Holorchis pycnoporus (Aephnidiogenidae), a parasite of the Striped seabream Lithognathus mormyrus (Sparidae) from the Gulf of Gabes (Tunisia). Tissue Cell 2020, 67, 101409. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, A.J.S.; Quilichini, Y.; Justine, J.-L.; Bray, R.A.; Miquel, J.; Feliu, C.; Bâ, C.T.; Marchand, B. First spermatological study in the Atractotrematidae (Digenea, Haploporoidea): The case of Atractotrema sigani, intestinal parasite of Siganus lineatus. Parasite 2015, 22, 26. [Google Scholar] [CrossRef] [Green Version]
- Bakhoum, A.J.S.; Sène, A.; Ndiaye, P.I.; Bâ, C.T.; Miquel, J. Spermiogenesis and the spermatozoon ultrastructure of Robphildollfusium fractum (Digenea: Gyliauchenidae), an intestinal parasite of Sarpa salpa (Pisces: Teleostei). C. R. Biol. 2012, 335, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Kacem, H.; Bakhoum, A.J.S.; Eira, C.; Neifar, L.; Miquel, J. Ultrastructural characters of the spermatozoon of the digenean Hypocreadium caputvadum Kacem et al, 2011 (Lepocreadioidea: Lepocreadiidae), an intestinal parasite of Balistes capriscus in Tunisia. C. R. Biol. 2012, 335, 637–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhoum, A.J.S.; Quilichini, Y.; Justine, J.-L.; Bray, R.A.; Bâ, C.T.; Marchand, B. Neomultitestis aspidogastriformis Bray and Cribb, 2003 (Digenea, Lepocreadiidae): Mature spermatozoon and sperm morphologies in the Lepocreadioidea. Cell Biol. Int. 2015, 39, 799–807. [Google Scholar] [CrossRef]
- Ndiaye, P.I.; Bakhoum, A.J.S.; Sène, A.; Diagne, P.M.; Miquel, J. The ultrastructural characters of the mature spermatozoon of Opechona bacillaris (Molin, 1859) (Digenea, Lepocreadiidae) a parasite of Scomber colias Gmelin, 1789 (Scombridae) off the coast of Dakar (Senegal). Acta Zool. (Stockh.) 2015, 96, 91–98. [Google Scholar] [CrossRef]
- Quilichini, Y.; Ndiaye, P.I.; Sène, A.; Justine, J.-L.; Bray, R.A.; Tkach, V.V.; Bâ, C.T.; Marchand, B. Ultrastructural characters of the spermatozoa in Digeneans of the genus Bianium Stunkard, 1930 (Digenea, Lepocreadiidae) parasites of fishes: A comparative study of Bianium plicitum and Bianium arabicum. Parasitol. Res. 2015, 114, 3747–3757. [Google Scholar] [CrossRef] [PubMed]
- Kacem, H.; Miquel, J. Sperm ultrastructure of Prodistomum polonii (Digenea, Lepocreadioidea), an intestinal parasite of the Atlantic horse mackerel, Trachurus trachurus (Teleostei, Carangidae), from the Gulf of Gabes, Mediterranean Sea. Zool. Anz. 2020, 286, 100–107. [Google Scholar] [CrossRef]
- Kacem, H.; Miquel, J. Spermatological characteristics of Siphoderina aloysiae (Digenea, Cryptogonimidae), an intestinal parasite of Sciaena umbra (Teleostei: Sciaenidae). Zoomorphology 2019, 138, 185–192. [Google Scholar] [CrossRef]
- Ehlers, U. Phylogenetisches System der Plathelminthes. Verh. Naturwiss. Ver. Hambg. (NF) 1984, 27, 291–294. [Google Scholar]
- Jamieson, B.G.M.; Justine, J.-L. Spermatozoa, Spermatogenesis and Fertilization in Schistosoma. In Schistosoma: Biology, Pathology and Control; Jamieson, B.G.M., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 300–319. [Google Scholar]
- Justine, J.-L.; Mattei, X. A spermatozoon with two 9 + 0 axonemes in a parasitic flatworm, Didymozoon (Digenea: Didymozoidae). J. Submicrosc. Cytol. 1983, 15, 1101–1105. [Google Scholar]
- Quilichini, Y.; Foata, J.; Justine, J.-L.; Bray, R.A.; Marchand, B. Spermatozoon ultrastructure of Aponurus laguncula (Digenea: Lecithasteridae), a parasite of Aluterus monoceros (Pisces, Teleostei). Parasitol. Int. 2010, 59, 22–28. [Google Scholar] [CrossRef]
- Kacem, H.; Świderski, Z.; Miquel, J. Sperm cell ultrastructure of the haplosplanchnid Haplosplanchnus caudatus (Platyhelminthes: Digenea)-An intestinal parasite of Mugil cephalus, and its potential phylogenetic application. Zool. Anz. 2019, 283, 201–206. [Google Scholar] [CrossRef]
- Pamplona-Basilio, M.C.; Baptista-Farias, M.F.D.; Kohn, A. Spermatogenesis and spermiogenesis in Didymocystis wedli Ariola, 1902 (Didymozoidae, Digenea). Mem. Inst. Oswaldo Cruz 2001, 96, 1153–1159. [Google Scholar] [CrossRef] [Green Version]
- Justine, J.-L.; Lambert, A.; Mattei, X. Spermatozoon ultrastructure and phylogenetic relationships in the monogeneans (Platyhelminthes). Int. J. Parasitol. 1985, 15, 601–608. [Google Scholar] [CrossRef]
- Justine, J.-L. Cladistic study in the Monogenea (Platyhelminthes), based upon a parsimony analysis of spermiogenetic and spermatozoal ultrastructural characters. Int. J. Parasitol. 1991, 21, 821–838. [Google Scholar] [CrossRef]
- Ndiaye, P.I.; Quilichini, Y.; Sène, A.; Bray, R.A.; Bâ, C.T.; Marchand, B. Prosorchis palinurichthi Kurochkin, Parukhin & Korotaeva, 1971 (Digenea, Sclerodistomidae): Ultrastructure of the mature spermatozoon. Zool. Anz. 2013, 252, 404–409. [Google Scholar]
- Ndiaye, P.I.; Quilichini, Y.; Marigo, A.M.; Bâ, C.T.; Tkach, V.V.; Marchand, B. Ultrastructural characteristics of the mature spermatozoon of the digenean Sclerodistomum italicum (Stossich, 1893) (Hemiuroidea, Sclerodistomidae) intestinal parasite of Hypocanthus amia (Teleostei, Carangidae). Tissue Cell 2017, 49, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kacem, H.; Giese, E.G.; Miquel, J. Sperm characters in the Hemiuridae (Digenea): First data on Aphanurus stossichii (Aphanurinae) and Ectenurus lepidus (Dinurinae). Parasitol. Res. 2020, 119, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Burton, P.R. Fine structure of the reproductive system of a frog lung-fluke. III. The spermatozoon and its differentiation. J. Parasitol. 1972, 58, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Bâ, A.; Bakhoum, A.J.S.; Ndiaye, P.I.; Bâ, C.T.; Marchand, B.; Quilichini, Y. Spermatological characteristics of Sclerodistomoides pacificus (Digenea, Sclerodistomoididae) a parasite of the flying fish Cheilopogon pinnatibarbatus (Teleostei, Exocoetidae). Tissue Cell 2020, 62, 101314. [Google Scholar] [CrossRef]
- Foata, J.; Quilichini, Y.; Greani, S.; Marchand, B. Sperm ultrastructure of the digenean Aphallus tubarium (Rudolphi, 1819) Poche, 1926 (Platyhelminthes, Cryptogonimidae) intestinal parasite of Dentex dentex (Pisces, Teleostei). Tissue Cell 2012, 44, 15–21. [Google Scholar] [CrossRef]
- Kacem, H.; Blasco, S.; Foronda, P.; Miquel, J. Sperm characters of Timoniella imbutiforme (Digenea, Opisthorchioidea, Cryptogonimidae), a parasite of the European seabass Dicentrarchus labrax. Zool. Anz. 2017, 271, 49–56. [Google Scholar] [CrossRef]
- Ndiaye, P.I.; Quilichini, Y.; Sène, A.; Tkach, V.V.; Bâ, C.T.; Marchand, B. Ultrastructural study of the spermatozoon of the digenean Enodiotrema reductum Looss, 1901 (Platyhelminthes, Plagiorchioidea, Plagiorchiidae), parasite of the green turtle Chelonia mydas (Linnaeus, 1758) in Senegal. Parasitol. Res. 2012, 111, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, A.J.S.; Quilichini, Y.; Justine, J.-L.; Bray, R.A.; Bâ, C.T.; Marchand, B. Ultrastructural study of sperm cells in Acanthocolpidae: The case of Stephanostomum murielae and Stephanostomoides tenuis (Digenea). PeerJ 2015, 3, e744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Subfamilies and Species | Principal Characters | Secondary Characters | TSpz | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAx | LE | EO | EO+CM | LEO | BCM | LMCM | M | ADM | SB | PSC | |||
| Hamacreadiinae | |||||||||||||
| Allopodocotyle pedicellata | 9+‘1’ | − | + | + | PostA | 2 | AntS | 2 | + | + | CM | IV | [28] |
| Allopodocotyle tunisiensis | 9+‘1’ | − | + | + | PostA | 2 | AntS | 2 | + | + | CM | IV | [29] |
| Podocotyloides magnatestis | 9+‘1’ | − | + | + | PostA | 2 | MedS | 2 | + | + | N | III | [30] |
| Helicometrinae | |||||||||||||
| Helicometra epinepheli | 9+‘1’ | − | + | + | PostA | 2 | MedS | 2 | + | + | CM | III | [31] |
| Helicometra fasciata | 9+‘1’ | − | − | NA | NA | 2 | MedS | 1 | + | − | CM | III | [32] |
| Opecoelinae | |||||||||||||
| Labracetabulum gephyroberici | 9+‘1’ | − | + | + | PostA | 2 | MedS | 2 | + | + | CM | III | [33] |
| Opecoeloides furcatus | 9+‘1’ | − | + | + | PostA | 2 | MedS | 1 | − | + | CM | III | [34] |
| Poracanthium furcatum | 9+‘1’ | − | + | + | PostA | 2 | MedS | 2 | + | + | CM | III | [35] |
| Opistholebetinae | |||||||||||||
| Heterolebes maculosus | 9+‘1’ | − | + | + | PostA | 2 | MedS? | 2 | − | + | CM | III–IV? | [23] |
| Macvicaria obovata | 9+‘1’ | − | + | + | PostA | 2 | PostS | 2 | + | + | CM | III | [36] |
| Peracreadium characis | 9+‘1’ | − | − | NA | NA | 2 | AntS | 2 | + | − | CM | IV | Present study |
| Plagioporinae | |||||||||||||
| Nicolla testiobliqua | 9+‘1’ | − | + | + | PostA | 2 | MedS | 2 | − | + | CM | III | [37] |
| Nicolla wisniewskii | 9+‘1’ | − | + | + | PostA | 2 | MedS | 2 | − | + | CM | III | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacem, H.; Miquel, J. A Review of Sperm Ultrastructural Characters in the Opecoelidae (Digenea) and Their Phylogenetic Implications, with New Data on Peracreadium characis, a Parasite of Diplodus puntazzo in Tunisia. Animals 2023, 13, 1953. https://doi.org/10.3390/ani13121953
Kacem H, Miquel J. A Review of Sperm Ultrastructural Characters in the Opecoelidae (Digenea) and Their Phylogenetic Implications, with New Data on Peracreadium characis, a Parasite of Diplodus puntazzo in Tunisia. Animals. 2023; 13(12):1953. https://doi.org/10.3390/ani13121953
Chicago/Turabian StyleKacem, Hichem, and Jordi Miquel. 2023. "A Review of Sperm Ultrastructural Characters in the Opecoelidae (Digenea) and Their Phylogenetic Implications, with New Data on Peracreadium characis, a Parasite of Diplodus puntazzo in Tunisia" Animals 13, no. 12: 1953. https://doi.org/10.3390/ani13121953
APA StyleKacem, H., & Miquel, J. (2023). A Review of Sperm Ultrastructural Characters in the Opecoelidae (Digenea) and Their Phylogenetic Implications, with New Data on Peracreadium characis, a Parasite of Diplodus puntazzo in Tunisia. Animals, 13(12), 1953. https://doi.org/10.3390/ani13121953






