The Impacts of Supplemental Protein during Development on Amino Acid Concentrations in the Uterus and Pregnancy Outcomes of Angus Heifers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
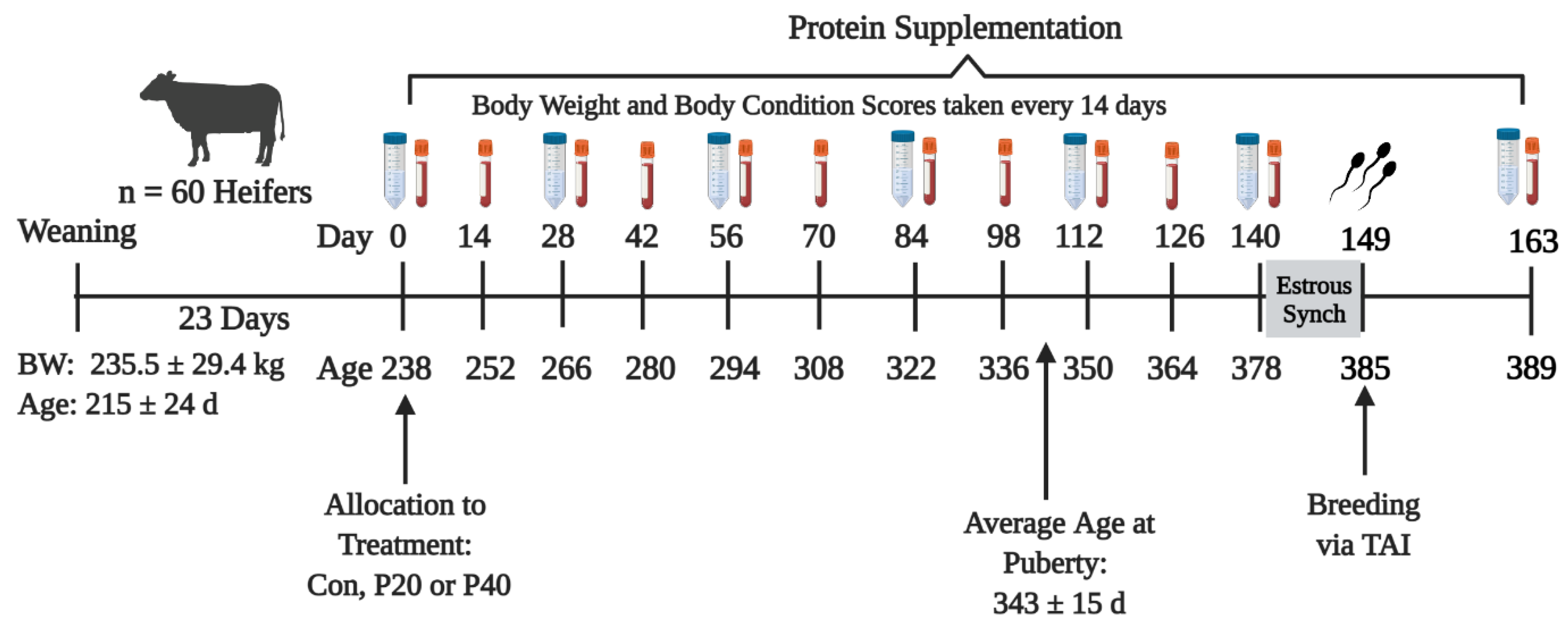
2.1. Animal Management and Experimental Design
2.2. Uterine Fluid Collection
2.3. Progesterone and Puberty
2.4. Amino Acid Analysis
2.5. Statistical Analyses
3. Results
3.1. Body Weight and Body Condition Score
3.2. Age and Plasma Progesterone at Puberty
3.3. Uterine pH
3.4. Amino Acids during Development
3.5. Amino Acids after Insemination
3.6. Correlation between Development and Pregnancy
3.7. Consequential Impacts of Protein on Pregnancy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA-NASS. Cattle Inventory; USDA APHIS:VS:CEAH; United States Department of Agriculture: Fort Collins, CO, USA, 2019; ISSN 1948-9099.
- Patterson, D.J.; Perry, R.C.; Kiracofe, G.H.; Bellows, R.A.; Staigmiller, R.B.; Corah, L.R. Management considerations in heifer development and puberty. J. Anim. Sci. 1992, 70, 4018–4035. [Google Scholar] [CrossRef]
- Funston, R.N.; Martin, J.L.; Larson, D.M.; Roberts, A.J. Physiology and endocrinology symposium: Nutritional aspects of developing replacement heifers. J. Anim. Sci. 2012, 90, 1166–1171. [Google Scholar] [CrossRef]
- Wiltbank, J.N.; Kasson, C.W.; Ingalls, J.E. Puberty in crossbred and straightbred beef heifers on two levels of feed. J. Anim. Sci. 1969, 29, 602–605. [Google Scholar] [CrossRef]
- Nelsen, T.C.; Long, C.R.; Cartwright, T.C. Postinflection Growth in straightbred and Crossbred Cattle, II, Relationships among Weight, Height and Pubertal Characters. J. Anim. Sci. 1982, 55, 293–304. [Google Scholar] [CrossRef]
- Oyedipe, E.O.; Osori, D.I.; Akerejola, O.; Saror, D. Effect of level of nutrition on onset of puberty and conception rates of Zebu heifers. Theriogenology 1982, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Day, M.L.; Nogueira, G.P. Management of age at puberty in beef heifers to optimize efficiency of beef production. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Utter, S.D.; Houghton, P.L.; Corah, L.R.; Simms, D.D.; Spire, M.F.; Butine, M.D. Factors Influencing First-Service Conception and Overall Pregnancy Rates in Commercial Beef Heifers; Kansas State University Research Report; Kansas State University: Manhattan, KS, USA, 1994; pp. 107–110. [Google Scholar]
- Buonaiuto, G.; Lopez-Villalobos, N.; Costa, A.; Niero, G.; Degano, L.M.; Mammi, L.E.; Cavallin, D.; Palmonari, A.; Formigoni, A.; Visentin, G. Stayability in Simmental cattle as affected by muscularity and body condition score between calvings. Front. Vet. Sci. 2023, 10, 1141286. [Google Scholar] [CrossRef] [PubMed]
- Short, R.E.; Bellows, R.A. Relationships among weight gains, age at puberty and reproductive performance in heifers. J. Anim. Sci. 1971, 32, 127–131. [Google Scholar] [CrossRef]
- Ferrell, C.L. Effects of postweaning rate of gain on onset of puberty and productive performance of heifers of different breeds. J. Anim. Sci. 1982, 55, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Elrod, C.C.; Butler, W.R. Reduction of fertility and alteration of uterine pH in heifers fed excess ruminally degradable protein. J. Anim. Sci. 1993, 71, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Lalman, D.L.; Petersen, M.K.; Ansotegui, R.P.; Tess, M.W.; Clark, C.K.; Wiley, J.S. The effects of ruminally undegradable protein, propionic acid, and monensin on puberty and pregnancy in beef heifers. J. Anim Sci. 1993, 71, 2843–2852. [Google Scholar] [CrossRef]
- Kane, K.K.; Hawkins, D.E.; Pulsipher, G.D.; Denniston, D.J.; Krehbiel, C.R.; Thomas, M.G.; Petersen, M.K.; Hallford, D.M.; Remmenga, M.D.; Roberts, A.J.; et al. Effect of increasing levels of undegradable intake protein on metabolic and endocrine factors in estrous cycling beef heifers. J. Anim. Sci. 2004, 82, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Crouse, M.S.; Greseth, N.P.; McLean, K.J.; Crosswhite, M.R.; Pereira, N.N.; Ward, A.K.; Reynolds, L.P.; Dahlen, C.R.; Neville, B.W.; Borowicz, P.P.; et al. Maternal nutrition and stage of early pregnancy in beef heifers: Impacts on hexose and AA concentrations in maternal and fetal fluids. J. Anim. Sci. 2019, 97, 1296–1316. [Google Scholar] [CrossRef] [Green Version]
- Forde, N.; Simintiras, C.A.; Sturmey, R.; Mamo, S.; Kelly, A.K.; Spencer, T.E.; Bazer, F.W.; Lonergan, P. Amino acids in the uterine luminal fluid reflects the temporal changes in transporter expression in the endometrium and conceptus during early pregnancy in cattle. PLoS ONE 2014, 9, e100010. [Google Scholar] [CrossRef]
- Bridges, G.; Lake, S.; Kruse, S.; Bird, S.; Funnell, B.; Arias, R.; Walker, J.; Grant, J.; Perry, G. Comparison of three CIDR-based fixed-time AI protocols in beef heifers. J. Anim. Sci. 2014, 92, 3127–3133. [Google Scholar] [CrossRef]
- Dunne, L.D.; Diskin, M.G.; Sreenan, J.M. Embryo and foetal loss in beef heifers between day 14 of gestation and full term. Anim. Reprod. Sci. 2000, 58, 39–44. [Google Scholar] [CrossRef]
- Bazer, F.W. Uterine Protein Secretions: Relationship to Development of the Conceptus. J. Anim. Sci. 1975, 41, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Hugentobler, S.A.; Diskin, M.G.; Leese, H.J.; Humpherson, P.G.; Watson, T.; Sreenan, J.M.; Morris, D.G. Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol. Reprod. Dev. 2007, 74, 445–454. [Google Scholar] [CrossRef]
- Groebner, A.E.; Rubio-Aliaga, I.; Schulke, K.; Reichenbach, H.D.; Daniel, H.; Wolf, E.; Meyer, H.H.; Ulbrich, S.E. Increase of essential amino acids in the bovine uterine lumen during preimplantation development. Reproduction 2011, 141, 685–695. [Google Scholar] [CrossRef]
- Meier, S.; Mitchell, M.D.; Walker, C.G.; Roche, J.R.; Verkerk, G.A. Amino acid concentrations in uterine fluid during early pregnancy differ in fertile and subfertile dairy cow strains. J. Dairy Sci. 2014, 97, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Marston, T.T.; Lusby, K.S.; Wettemann, R.P. Effects of postweaning diet on age and weight at puberty and milk production of heifers. J. Anim. Sci. 1995, 73, 63–68. [Google Scholar] [CrossRef]
- Wagner, J.J.; Lusby, K.; Oltjen, J.; Rakestraw, J.; Wettemann, R.; Walters, L.E. Carcass composition in mature Hereford cows: Estimation and effect on daily metabolizable energy requirement during winter. J. Anim. Sci. 1988, 66, 603–612. [Google Scholar] [CrossRef]
- Geary, T.; Pas, J.W.; Thrift, F.; Dolezal, S. Effects of a timed insemination following synchronization of ovulation using the Ovsynch or CO-Synch protocol in beef cows. Prof. Anim. Sci. 1998, 14, 217–220. [Google Scholar] [CrossRef]
- Pohler, K.G.; Pereira, M.H.C.; Lopes, F.R.; Lawrence, J.C.; Keisler, D.H.; Smith, M.F.; Vasconcelos, J.L.M.; Green, J.A. Circulating concentrations of bovine pregnancy-associated glycoproteins and late embryonic mortality in lactating dairy herds. J. Dairy Sci. 2015, 99, 1584–1594. [Google Scholar] [CrossRef]
- Ciccioli, N.H.; Charles-Edwards, S.L.; Floyd, C.; Wettemann, R.P.; Purvis, H.T.; Lusby, K.S.; Horn, G.W.; Lalman, D.L. Incidence of puberty in beef heifers fed high- or low-starch diets for different periods before breeding. J. Anim. Sci. 2005, 83, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.; Larimore, E.; Bridges, G.; Cushman, R. Management strategies for improving lifetime reproductive success in beef heifers. Proc. Appl. Reprod. Strateg. Beef Cattle 2012, 30, 249–266. [Google Scholar]
- Byerley, D.J.; Staigmiller, R.B.; Berardinelli, J.G.; Short, R.E. Pregnancy rates of beef heifers bred either on puberal or third estrus. J. Anim. Sci. 1987, 65, 645–650. [Google Scholar] [CrossRef]
- Engelken, T.J. Developing replacement beef heifers. Theriogenology 2008, 70, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Selk, G.E.; Wettemann, R.P.; Lusby, K.S.; Oltjen, J.W.; Mobley, S.L.; Rasby, R.J.; Garmendia, J.C. Relationships among weight change, body condition and reproductive performance of range beef cows. J. Anim. Sci. 1988, 66, 3153–3159. [Google Scholar] [CrossRef] [PubMed]
- Gunn, P.J.; Schoonmaker, J.P.; Lemenager, R.P.; Bridges, G.A. Feeding excess crude protein to gestating and lactating beef heifers: Impact on parturition, milk composition, ovarian function, reproductive efficiency and pre-weaning progeny growth. Livest. Sci. 2014, 167, 435–448. [Google Scholar] [CrossRef]
- Martin, J.L.; Cupp, A.S.; Rasby, R.J.; Hall, Z.C.; Funston, R.N. Utilization of dried distillers grains for developing beef heifers. J. Anim. Sci. 2007, 85, 2298–2303. [Google Scholar] [CrossRef]
- Ocon, O.M.; Hansen, P.J. Disruption of bovine oocytes and preimplantation embryos by urea and acidic pH. J. Dairy Sci. 2003, 86, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.J.; Williams, D.A.; Gardner, D.K. Intracellular pH of the mouse preimplantation embryo: Amino acids act as buffers of intracellular pH. Hum. Reprod. 1998, 13, 3441–3448. [Google Scholar] [CrossRef]
- Lane, M.; Bavister, B.D. Regulation of intracellular pH in bovine oocytes and cleavage stage embryos. Mol. Reprod. Dev. 1999, 54, 396–401. [Google Scholar] [CrossRef]
- Perry, G.; Perry, B. Effect of preovulatory concentrations of estradiol and initiation of standing estrus on uterine pH in beef cows. Dom. Anim. Endocrin. 2008, 34, 333–338. [Google Scholar] [CrossRef]
- Perry, G.; Perry, B. Effects of standing estrus and supplemental estradiol on changes in uterine pH during a fixed-time artificial insemination protocol. J. Anim. Sci. 2008, 86, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Dyck, G. The effect of postulating diet intake on embryonic and fetal survival, and litter size in gilts. Can. J. Anim. Sci. 1991, 71, 675–681. [Google Scholar] [CrossRef]
- Wallace, J.M.; Aitken, R.P.; Cheyne, M.A. Effect of post-ovulation nutritional status in ewes on early conceptus survival and growth in vivo and luteotrophic protein secretion in vitro. Reprod. Fert. Dev. 1994, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.R.; Chapman, T.E.; Holtan, D.W.; Swanson, L.V. Relationship of dietary crude protein to composition of uterine secretions and blood in high-producing postpartum dairy cows. J. Dairy Sci. 1983, 66, 1854–1862. [Google Scholar] [CrossRef]
- Grant, J.K.; Steichen, P.L.; Wright, C.L.; Vonnahme, K.A.; Bauer, M.L.; Jennings, J.S.; Perry, G.A. Influence of nitrogen and sulfur intake on bovine uterine pH throughout the luteal phase. J. Anim. Sci. 2013, 91, 1186–1192. [Google Scholar] [CrossRef]
- Hugentobler, S.A.; Sreenan, J.M.; Humpherson, P.G.; Leese, H.J.; Diskin, M.G.; Morris, D.G. Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood. Reprod. Fertil. Dev. 2010, 22, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Geppert, T.; Meyer, A.; Perry, G.; Gunn, P. Effects of excess metabolizable protein on ovarian function and circulating amino acids of beef cows: 1. Excessive supply from corn gluten meal or soybean meal. Animal 2017, 11, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Spencer, T.E.; Bazer, F.W.; Wu, G. Developmental changes of amino acids in ovine fetal fluids. Biol. Reprod. 2003, 68, 1813–1820. [Google Scholar] [CrossRef]
- Gao, H.; Wu, G.; Spencer, T.E.; Johnson, G.A.; Li, X.; Bazer, F.W. Select nutrients in the ovine uterine lumen. i. amino acids, glucose, and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol. Reprod. 2009, 80, 86–93. [Google Scholar] [CrossRef]
- Bell, A.W.; Kennaugh, J.M.; Battaglia, F.C.; Meschia, G. Uptake of amino acids and ammonia at mid-gestation by the fetal lamb. Q. J. Exp. Physiol. 1989, 74, 635–643. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Bazer, F.W. mTOR: The master regulator of conceptus development in response to uterine histotroph during pregnancy in ungulates. In Molecules to Medicine with mTOR; Maiese, K., Ed.; Adacemic Press: Boston, MA, USA, 2016; pp. 23–35. [Google Scholar]
- Bazer, F.W.; Wu, G.; Johnson, G.A.; Kim, J.; Song, G. Uterine histotroph and conceptus development: Select nutrients and secreted phosphoprotein 1 affect mechanistic target of rapamycin cell signaling in ewes. Biol. Reprod. 2011, 85, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Diskin, M.; Kenny, D. Optimising reproductive performance of beef cows and replacement heifers. Animal 2014, 8, 27–39. [Google Scholar] [CrossRef]
- Roberts, A.J.; Ketchum, J.N.; Funston, R.N. Developmental and reproductive characteristics of beef heifers classified by number of estrous cycles experienced by start of first breeding. Trans. Anim. Sci. 2019, 3, 541–548. [Google Scholar] [CrossRef]




| Feed | Composition | Dry Matter (%) | Crude Protein (%) | Fat (%) | RUP 1 (%) |
|---|---|---|---|---|---|
| Hay | 100% Grass Hay | 95.6 | 7.6 | 1.97 | --- |
| Con 2 | 100% Cracked Corn | 87.5 | 8.2 | 3.77 | 60.8 |
| P20 3 | 25% Cracked Corn 75% DDG 4 | 91.6 | 23.6 | 7.40 | 75.1 |
| P40 5 | 25% DDG 75% Soybean Meal | 90.2 | 47.1 | 2.80 | 45.9 |
| AAs | Day of Sampling | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 28 | 56 | 84 | 112 | 140 | SEM | Trt 3 | Day | Trt × Day | |
| Arginine (Arg) | 0.65 cd | 0.95 bc | 4.43 a | 3.65 ab | 0.031 d | 2.39 ab | 0.60 | 0.27 | <0.001 | 0.27 |
| Citrulline (Cit) | 0.03 bc | 0.11 a | 0.19 a | 0.14 a | 0.01 c | 0.16 ab | 0.04 | 0.08 | <0.001 | 0.60 |
| Glutamine (Gln) | 0.77 b | 1.50 a | 1.07 ab | 0.97 ab | 0.05 c | 1.32 ab | 0.22 | 0.24 | <0.001 | 0.99 |
| Ornithine (Orn) | 0.03 bc | 0.14 a | 0.09 ab | 0.08 ab | 0.01 c | 0.09 ab | 0.03 | 0.21 | <0.001 | 0.95 |
| Proline (Pro) | 0.21 cd | 0.47 ab | 0.40 bc | 0.69 a | 0.04 d | 0.30 bc | 0.09 | 0.74 | <0.001 | 0.80 |
| AAs | Day of Sampling | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 28 | 56 | 84 | 112 | 140 | SEM | Trt 3 | Day | Trt × Day | |
| Alpha-Aminobutyric Acid (Aaba) 4 | 0.11 c | 0.70 a | 0.30 b | 0.92 a | 0.13 c | 0.45 b | 0.08 | 0.66 | <0.001 | 0.93 |
| Glycine (Gly) | 1.52 b | 1.81 ab | 1.94 ab | 4.46 a | 1.72 b | 11.47 a | 3.56 | 0.99 | 0.001 | 0.64 |
| Isoleucine (Ile) | 0.22 cd | 0.67 a | 0.37 bc | 0.57 ab | 0.07 d | 0.32 c | 0.08 | 0.72 | <0.001 | 0.88 |
| Leucine (Leu) | 0.22 bc | 0.64 a | 0.43 b | 0.46 ab | 0.15 c | 0.39 b | 0.09 | 0.59 | <0.001 | 0.65 |
| 1-Methyl-Histidine (1 MH) | 0.01 bc | 0.02 bc | 0.04 b | 0.08 a | 0.01 c | 0.03 bc | 0.01 | 0.35 | <0.001 | 0.83 |
| Valine (Val) | 0.76 b | 1.35 a | 1.15 ab | 1.24 a | 0.23 c | 1.11 ab | 0.17 | 0.49 | <0.001 | 0.53 |
| AAs | Day of Sampling | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 28 | 56 | 84 | 112 | 140 | SEM | Trt 3 | Day | Trt × Day | |
| Alanine (Ala) | 0.58 c | 1.93 a | 1.29 b | 2.02 a | 0.23 c | 1.40 b | 0.22 | 0.60 | <0.001 | 0.95 |
| Aspartic Acid (Asp) 4 | 9.53 b | 3.67 b | 1.96 b | 110.91 a | 11.36 b | 26.52 a | 21.4 | 0.13 | <0.001 | 0.56 |
| Delta-hydroxylysine (Dhl) | 0.03 d | 0.08 b | 0.07 bc | 0.14 a | 0.03 cd | 0.05 bcd | 0.01 | 0.10 | <0.001 | 0.95 |
| Histidine (His) | 0.09 c | 0.73 a | 0.15 bc | 0.47 ab | 0.03 c | 1.15 ab | 0.24 | 0.50 | <0.001 | 0.83 |
| Lysine (Lys) | 0.21 bcd | 0.42 a | 0.37 ab | 0.19 abc | 0.02 d | 0.12 cd | 0.08 | 0.64 | <0.001 | 0.95 |
| Methionine (Met) | 0.04 bc | 0.07 ab | 0.08 ab | 0.07 ab | 0.01 c | 0.14 a | 0.02 | 0.76 | <0.001 | 0.64 |
| Phenylalanine (Phe) | 0.07 bc | 0.18 a | 0.17 ab | 0.13 abc | 0.05 c | 0.13 abc | 0.03 | 0.47 | <0.001 | 0.50 |
| Sarcosine (Sar) | 0.10 b | 0.15 ab | 0.18 ab | 0.19 a | 0.02 c | 0.08 bc | 0.02 | 0.90 | <0.001 | 0.58 |
| Tryptophan (Trp) | 0.04 bc | 0.09 a | 0.06 ab | 0.07 ab | 0.01 c | 0.04 bc | 0.01 | 0.22 | <0.001 | 0.51 |
| Tyrosine (Tyr) | 0.06 bc | 0.13 ab | 0.14 ab | 0.09 ab | 0.01 c | 0.28 a | 0.05 | 0.54 | <0.001 | 0.45 |
| AAs | Treatment | Semen Exposure | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | P20 | P40 | SEM | Yes | No | SEM | Trt 2 | Exposure 3 | |
| Alanine (Ala; pH) | 0.72 | 1.82 | 1.56 | 0.89 | 1.34 | 1.21 | 0.75 | 0.42 | 0.86 |
| Arginine (Arg; pH) | 1.88 a | 5.82 ab | 18.75 b | 7.74 | 6.36 | 8.77 | 7.02 | 0.03 | 0.69 |
| Citrulline (Cit) | 0.10 | 0.11 | 0.19 | 0.09 | 0.16 | 0.10 | 0.08 | 0.69 | 0.51 |
| Cysteine (CC; BCS) | 0.04 | 0.26 | 0.65 | 0.39 | 0.03 | 0.41 | 0.30 | 0.05 | 0.07 |
| Delta-hydroxylysine (Dhl) | 0.01 | 0.05 | 0.07 | 0.04 | 0.04 | 0.04 | 0.04 | 0.45 | 0.85 |
| Glutamine (Gln) | 1.04 | 3.20 | 2.58 | 1.23 | 3.22 | 1.30 | 1.00 | 0.26 | 0.10 |
| Glycine (Gly; pH) | 6.45 | 22.63 | 9.73 | 9.08 | 17.56 | 7.20 | 7.73 | 0.24 | 0.13 |
| Histidine (His; BCS, pH) | 0.19 | 0.27 | 0.12 | 0.12 | 0.18 | 0.20 | 0.10 | 0.61 | 0.88 |
| Isoleucine (Ile; BCS, pH) | 0.26 | 0.29 | 0.42 | 0.21 | 0.41 | 0.25 | 0.17 | 0.85 | 0.45 |
| Leucine (Leu; BCS, pH) | 0.26 | 0.50 | 0.34 | 0.25 | 0.39 | 0.33 | 0.21 | 0.79 | 0.82 |
| Lysine (Lys; pH) | 0.94 | 1.05 | 2.89 | 1.41 | 0.90 | 1.10 | 1.19 | 0.10 | 0.89 |
| Methionine (Met; pH) | 0.05 | 0.19 | 0.18 | 0.08 | 0.12 | 0.14 | 0.07 | 0.20 | 0.86 |
| 1-Methyl-Histidine (1 MH; BW, BCS, P4) | 0.11 a | 0.08 a | 0.27 b | 0.07 | 0.09 | 0.07 | 0.06 | 0.001 | 0.87 |
| Ornithine (Orn; pH) | 0.16 a | 0.13 ab | 0.43 b | 0.18 | 0.08 | 0.18 | 0.15 | 0.03 | 0.57 |
| Phenylalanine (Phe; pH) | 0.23 | 0.59 | 1.19 | 0.59 | 0.41 | 0.58 | 0.52 | 0.16 | 0.98 |
| Proline (Pro; pH) | 0.18 | 0.67 | 0.58 | 0.28 | 0.40 | 0.46 | 0.23 | 0.23 | 0.80 |
| Sarcosine (Sar; BCS) | 0.14 | 0.14 | 0.23 | 0.11 | 0.17 | 0.17 | 0.10 | 0.61 | 0.99 |
| Serine (Ser) | 0.35 | 0.82 | 0.30 | 0.44 | 0.61 | 0.32 | 0.35 | 0.63 | 0.50 |
| Threonine (Thr) | 0.13 | 0.53 | 0.32 | 0.21 | 0.24 | 0.36 | 0.18 | 0.32 | 0.58 |
| Tryptophan (Trp; pH) | 0.01 | 0.07 | 0.12 | 0.05 | 0.07 | 0.05 | 0.05 | 0.25 | 0.64 |
| Tyrosine (Tyr; pH) | 0.31 | 0.58 | 1.16 | 0.65 | 0.45 | 0.64 | 0.48 | 0.19 | 0.81 |
| Valine (Val; pH) | 0.16 | 0.51 | 0.62 | 0.29 | 0.42 | 0.35 | 0.22 | 0.29 | 0.81 |
| AAs 1 | Trt 2 | 0 | 28 | 56 | 84 | 112 | 140 | Avg 3 |
|---|---|---|---|---|---|---|---|---|
| Arginine | Con | --- | --- | 0.42 (0.07) | --- | --- | --- | --- |
| P20 | −0.39 (0.09) 4 | --- | 0.50 (0.03) | --- | --- | --- | --- | |
| P40 | --- | --- | 0.53 (0.02) | --- | --- | --- | --- | |
| Citrulline | Con | --- | --- | 0.62 (<0.01) | --- | --- | --- | 0.49 (0.03) |
| P20 | --- | --- | 0.68 (<0.01) | --- | --- | --- | --- | |
| Cysteine | Con | --- | 0.45 (0.05) | 0.37 (0.10) | --- | 0.59 (<0.01) | --- | 0.41 (0.07) |
| P20 | --- | --- | --- | --- | --- | 0.75 (<0.01) | 0.60 (<0.01) | |
| P40 | --- | 0.92 (<0.01) | 0.51 (0.03) | −0.56 (0.01) | 0.45 (0.05) | 0.48 (0.04) | 0.53 (0.02) | |
| Glutamine | Con | --- | --- | --- | --- | --- | --- | --- |
| P20 | --- | --- | --- | --- | −0.40 (0.08) | --- | --- | |
| P40 | --- | --- | --- | --- | --- | --- | --- | |
| 1-Methyl-Histidine | Con | --- | --- | 0.61 (<0.01) | 0.64 (<0.01) | --- | --- | 0.93 (<0.01) |
| P20 | --- | --- | --- | --- | --- | --- | --- | |
| P40 | --- | --- | --- | --- | --- | --- | --- |
| Frequency Results | CON | P20 | P40 | p-Value |
|---|---|---|---|---|
| AI | 8 | 4 | 4 | 0.13 |
| 1st Cycle | 3 | 4 | 4 | |
| 2nd Cycle | 8 | 4 | 4 | |
| 3rd Cycle | 0 | 3 | 3 | |
| 4th Cycle | 0 | 2 | 0 | |
| Final Open | 0 | 3 | 4 | |
| Time to Conception, d 1 | 24.0 ± 4.5 | 46.1 ± 7.1 | 39.5 ± 5.8 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandt, K.J.; Ault-Seay, T.B.; Payton, R.R.; Schneider, L.G.; Edwards, J.L.; Myer, P.R.; Rhinehart, J.D.; McLean, K.J. The Impacts of Supplemental Protein during Development on Amino Acid Concentrations in the Uterus and Pregnancy Outcomes of Angus Heifers. Animals 2023, 13, 1995. https://doi.org/10.3390/ani13121995
Brandt KJ, Ault-Seay TB, Payton RR, Schneider LG, Edwards JL, Myer PR, Rhinehart JD, McLean KJ. The Impacts of Supplemental Protein during Development on Amino Acid Concentrations in the Uterus and Pregnancy Outcomes of Angus Heifers. Animals. 2023; 13(12):1995. https://doi.org/10.3390/ani13121995
Chicago/Turabian StyleBrandt, Kiernan J., Taylor B. Ault-Seay, Rebecca R. Payton, Liesel G. Schneider, J. Lannett Edwards, Phillip R. Myer, Justin D. Rhinehart, and Kyle J. McLean. 2023. "The Impacts of Supplemental Protein during Development on Amino Acid Concentrations in the Uterus and Pregnancy Outcomes of Angus Heifers" Animals 13, no. 12: 1995. https://doi.org/10.3390/ani13121995





