Unveiling the Role of IGF-I in Fertility: Effect of Long-Acting Bovine Somatotropin (bST) on Terminal Follicular Development and Fertility during an Annual Reproductive Cycle in Sheep
Abstract
Simple Summary
Abstract
1. Introduction
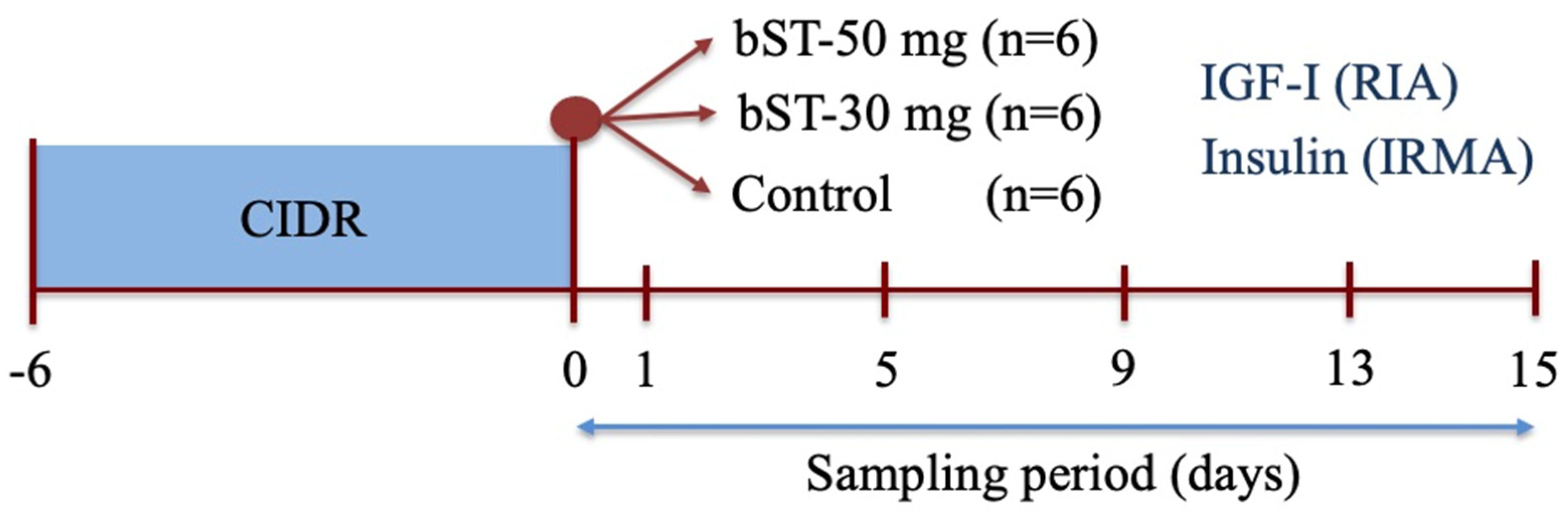
2. Materials and Methods
2.1. Animals and General Management
2.2. Estrous Detection
2.3. Blood Sampling and Endocrine Measures
2.4. Ovarian Ultrasound Measures
2.5. General Definitions
2.6. Experiments
2.6.1. Experiment 1: The Effect of bST Administration on Plasmatic Concentrations of IGF-I and Insulin in Sheep
2.6.2. Experiment 2: The Effect of bST Administration on Follicular Performance, Estrous Presentation, Ovulation Performance, Luteal Development, and Fertility after Mating in Sheep Treated for Estrous Synchronization during the Breeding Season
2.6.3. Experiment 3: The Effect of bST Administration on Follicular Performance, Estrous Presentation, Ovulation Performance, Luteal Development, and Fertility after Mating in Sheep Treated for Induction and Synchronization of Estrus during the Anestrous Season
2.7. Statistical Analysis
3. Results
3.1. Experiment 1. The Effect of bST on Plasma Concentrations of IGF-I and Insulin in Sheep
3.2. Experiment 2: The Effect of bST Administration on Follicular Performance, Estrous Presentation, Ovulation Performance, Luteal Development, and Fertility after Mating in Sheep Treated for Estrous Synchronization during the Breeding Season
3.3. Experiment 3: The Effect of bST Administration on Follicular Performance, Estrous Presentation, Ovulation Performance, Luteal Development, and Fertility after Mating in Sheep Treated for Estrous Synchronization during the Anestrous Season
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meikle, A.; de Brun, V.; Carriquiry, M.; Soca, P.; Sosa, C.; Adrien, M.L.; Chilibroste, P.; Abecia, J.A. Influences of nutrition and metabolism on reproduction of the female ruminant. Anim. Reprod. 2018, 15 (Suppl. S1), 899–911. [Google Scholar] [CrossRef]
- Merkley, C.M.; Shuping, S.L.; Nestor, C.C. Neuronal networks that regulate gonadotropin-releasing hormone/luteinizing hormone secretion during undernutrition: Evidence from sheep. Domest. Anim. Endocrinol. 2020, 2020, 106469. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.J.; Ashworth, C.J.; Rooke, J.A.; Mitchell, L.M.; McEvoy, T.G. Nutrition and fertility in ruminant livestock. Anim. Feed. Sci. Tech. 2006, 126, 259–276. [Google Scholar] [CrossRef]
- Scaramuzzi, R.J.; Campbell, B.K.; Downing, J.A.; Kendall, N.R.; Khalid, M.; Muñoz-Gutiérrez, M.; Somchit, A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and mechanisms that regulate folliculogenesis and ovulation rate. Reprod. Nutr. Dev. 2006, 46, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Manfredi-Lozano, M.; Roa, J.; Tena-Sempere, M. Connecting metabolism and gonadal function: Novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front. Neuroendocrinol. 2018, 48, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Nestor, C.C.; Merkley, C.M.; Lehman, M.N.; Hileman, S.M.; Goodman, R.L. KNDy neurons as the GnRH pulse generator: Recent studies in ruminants. Peptides 2023, 164, 171005. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.R.; Blache, D.; Kay, J.K.; Miller, D.R.; Sheahan, A.J.; Miller, D.W. Neuroendocrine and physiological regulation of intake with particular reference to domesticated ruminant animals. Nutr. Res. Rev. 2008, 21, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Orisaka, M.; Fukuda, S.; Tajima, K.; Yamazaki, Y.; Mizutani, T.; Yoshida, Y. Luteinizing Hormone facilitates antral follicular maturation and survival via thecal paracrine signaling in cattle. Endocrinology 2018, 159, 2337–2347. [Google Scholar] [CrossRef]
- Sirard, M.-A. The two-step process of ovarian follicular growth and maturation in mammals can be compared to a fruit ripening where quality depends on the second step. Biol. Reprod. 2022, 106, 230–234. [Google Scholar] [CrossRef]
- Landry, D.A.; Sirard, M.-A. Follicle capacitation: A meta-analysis to investigate the transcriptome dynamics following follicle-stimulating hormone decline in bovine granulosa cells. Biol. Reprod. 2018, 99, 877–887. [Google Scholar] [CrossRef]
- Sirard, M.-A. Folliculogenesis and acquisition of oocyte competence in cows. Anim. Reprod. 2019, 16, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Moorey, S.E.; Hessock, E.A.; Edwards, J.L. Preovulatory follicle contributions to oocyte competence in cattle: Importance of the ever-evolving intrafollicular environment leading up to the luteinizing hormone surge. J. Anim. Sci. 2022, 100, skac153. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Miyazaki, Y.; Shirafuji, A.; Tamamura, C.; Tsuyoshi, H.; Tsang, B.K.; Yoshida, Y. The role of pituitary gonadotropins and intraovarian regulators in follicle development: A mini-review. Reprod. Med. Biol. 2021, 20, 169–175. [Google Scholar] [CrossRef]
- Haughian, J.M.; Ginther, O.J.; Kot, K.; Wiltbank, M.C. Relationships between FSH patterns and follicular dynamics and the temporal associations among hormones in natural and GnRH-induced gonadotropin surges in heifers. Reproduction 2004, 127, 23–33. [Google Scholar] [CrossRef]
- McArdle, C.A.; Roberson, M.S. Gonadotropes and Gonadotropin-Releasing Hormone signaling. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: London, UK, 2015; Volume 1, pp. 335–397. [Google Scholar] [CrossRef]
- Seekallu, S.V.; Toosi, B.M.; Ziegler, A.; Reeves, J.J.; Rawling, N.C. Pulsed GnRH secretion and the FSH secretory peaks that initiate ovarian antral follicular wave emergence in anestrous ewes. Anim. Reprod. Sci. 2010, 120, 56–64. [Google Scholar] [CrossRef]
- Casarini, L.; Paradiso, E.; Lazzaretti, C.; D’Alessandro, S.; Roy, N.; Mascolo, E.; Zareba, K.; García-Gasca, A.; Simoni, M. Regulation of antral follicular growth by an interplay between gonadotropins and their receptors. J. Assist. Reprod. Genet. 2022, 39, 893–904. [Google Scholar] [CrossRef]
- Robker, R.L.; Hennebold, J.D.; Russell, D.L. Coordination of Ovulation and Oocyte Maturation: A Good Egg at the Right Time. Endocrinology 2018, 15, 3209–3218. [Google Scholar] [CrossRef]
- Ginther, O.J. The theory of follicle selection in cattle. Domest. Anim. Endocrinol. 2016, 57, 85–99. [Google Scholar] [CrossRef]
- Gong, J.G.; Campbell, B.K.; Webb, R. Defining the gonadotrophin requirement for the selection of a single dominant follicle in cattle. Reprod. Fertil. Dev. 2020, 32, 322–334. [Google Scholar] [CrossRef]
- Spicer, L.J. Proteolytic Degradation of Insulin-Like Growth Factor Binding Proteins by Ovarian Follicles: A Control Mechanism for Selection of Dominant Follicles. Biol. Reprod. 2004, 70, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.P.; Friggens, N.C.; Lucy, M.; Roche, J.R. Milk production and fertility in cattle. Annu. Rev. Anim. Biosci. 2016, 4, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.T. Nutritional management to optimize fertility of dairy cows in pasture-based systems. Animal 2014, 8 (Suppl. S1), 15–26. [Google Scholar] [CrossRef]
- Walsh, S.W.; Mattews, D.; Browne, J.A.; Crowe, M.A.; Mihm, M.; Diskin, M.; Evans, A.C.O. Acute dietary restriction in heifers alters expression of genes regulating exposure and response to gonadotrophins and IGF in dominant follicles. Anim. Reprod. Sci. 2012, 133, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.C. Growth hormone regulation of follicular growth. Reprod. Fertil. Dev. 2012, 24, 19–28. [Google Scholar] [CrossRef]
- Hull, K.L.; Harvey, S. Growth Hormone and Reproduction: A Review of Endocrine and Autocrine/Paracrine Interactions. Int. J. Endocrinol. 2014, 2014, 234014. [Google Scholar] [CrossRef]
- Monte, A.P.O.; Barros, V.R.P.; Santos, J.M.; Menezes, V.G.; Cavalcante, A.Y.P.; Gouveia, B.B.; Bezerra, M.E.S.; Macedo, T.J.S.; Matos, M.H.T. Immunohistochemical localization of insulin-like growth factor-1 (IGF-1) in the sheep ovary and the synergistic effect of IGF-1 and FSH on follicular development in vitro and LH receptor immunostaining. Theriogenology 2019, 129, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, S.C.; Armouti, M.; Ko, C.; Stocco, C. IGF1R Expression in Ovarian Granulosa Cells Is Essential for Steroidogenesis, Follicle Survival, and Fertility in Female Mice. Endocrinology 2017, 158, 2309–2318. [Google Scholar] [CrossRef]
- Le Roith, D. The insulin-like growth factor system. Exp. Diab. Res. 2003, 4, 205–2012. [Google Scholar] [CrossRef]
- Nyegaard, M.; Overgaard, M.T.; Su, Y.-Q.; Hamilton, A.E.; Kwintkiewicz, J.; Hsieh, M.; Nayak, N.R.; Conti, M.; Conover, C.A.; Giudice, L.C. Lack of Functional Pregnancy-Associated Plasma Protein-A (PAPPA) Compromises Mouse Ovarian Steroidogenesis and Female Fertility. Biol. Reprod. 2010, 82, 1129–1138. [Google Scholar] [CrossRef]
- Cox, J.F.; Navarrete, F.; Carrasco, A.; Dorado, J.; Saravia, F. Effect of bST administration on plasma concentrations of IGF-I and follicular dynamics and ovulation during the interovulatory cycle of sheep and goats. Theriogenology 2019, 123, 159–166. [Google Scholar] [CrossRef]
- Renaville, R.; Hammadi, M.; Portetelle, D. Role of the somatotropic axis in the mammalian metabolism. Domest. Anim. Endocrinol. 2002, 23, 351–360. [Google Scholar] [CrossRef]
- Sartori, R.; Consentini, C.E.C.; Alves, R.L.O.R.; Silva, L.O.; Wiltbank, M.C. Review: Manipulation of follicle development to improve fertility of cattle in timed-artificial insemination programs. Animal 2023, 17, 100769. [Google Scholar] [CrossRef]
- Wathes, D.C. Mechanisms linking metabolic status and disease with reproductive outcome in the dairy cow. Reprod. Domest. Anim. 2012, 47 (Suppl. S4), 304–312. [Google Scholar] [CrossRef]
- Cox, J.F.; Jeria, E.; Bocic, A.; Soto-Saravia, R.; Dorado, J.; Saravia, F. Characterization of the productive performance of Highlander sheep in Southern Chile. Female Reprod. Traits. Small Rumin. Res. 2015, 130, 183–188. [Google Scholar] [CrossRef]
- Romero, O. Evaluación de la Condición Corporal y Edad de los Ovinos. Informativo INIA Carillanca Nº79. 2015. Available online: https://hdl.handle.net/20.500.14001/4553 (accessed on 22 February 2024).
- McNatty, K.P.; Heath, D.A.; Hudson, N.L.; Reader, K.L.; Quirke, L.; Lun, S.; Juengel, J.L. The conflict between hierarchical ovarian follicular development and superovulation treatment. Reproduction 2010, 140, 287–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kirby, C.J.; Smith, M.F.; Keisler, D.H.; Lucy, M.C. Follicular Function in Lactating Dairy Cows Treated with Sustained-Release Bovine Somatotropin. J. Dairy Sci. 1997, 80, 273–285. [Google Scholar] [CrossRef]
- Silva, P.R.B.; Weber, W.J.; Crooker, B.A.; Collier, R.J.; Thatcher, W.W.; Chebel, R.C. Hepatic mRNA expression for genes related to somatotropic axis, glucose and lipid metabolisms, and inflammatory response of periparturient dairy cows treated with recombinant bovine somatotropin. J. Dairy Sci. 2017, 100, 3983–3999. [Google Scholar] [CrossRef]
- Fenwick, M.A.; Fitzpatrick, R.; Kenny, D.A.; Diskin, M.G.; Patton, J.; Murphy, J.J.; Wathes, D.C. Interrelationships between negative energy balance (NEB) and IGF regulation in liver of lactating dairy cows. Domest. Anim. Endocrinol. 2008, 34, 31–44. [Google Scholar] [CrossRef]
- Mazerbourg, S.; Monget, P. Insulin-Like Growth Factor Binding Proteins and IGFBP Proteases: A Dynamic System Regulating the Ovarian Folliculogenesis-Review. Front Endocrinol. 2018, 9, 134. [Google Scholar] [CrossRef]
- Silva, J.R.V.; Figueiredo, J.R.; van den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009, 71, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, M.A.; Zaraza, J.; Oropeza, A.; Webb, R.; Niemann, H. The role of IGF1 in the in vivo production of bovine embryos from superovulated donors. Reproduction 2009, 137, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J. How ultrasound technologies have expanded and revolutionized research in reproduction in large animals. Theriogenology 2014, 81, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Inskeep, E.K. Control of the ovarian cycle of the sheep. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: London, UK, 2015; Volume 2, pp. 1259–1305. [Google Scholar] [CrossRef]
- Kleemann, D.O.; Walker, S.K. Fertility in South Australian commercial Merino flocks: Relationships between reproductive traits and environmental cues. Theriogenology 2005, 63, 2416–2433. [Google Scholar] [CrossRef]
- Carrillo, F.; Hernández-Cerón, J.; Orozco, V.; Hernández, J.A.; Gutiérrez, C.G. A single dose of bovine somatotropin 5 days before the end of progestin-based estrous synchronization increases prolificacy in sheep. Anim. Reprod. Sci. 2007, 102, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Ramón, J.P.; Cocero, M.J.; Alabart, J.L.; Beckers, J.F. Exogenous growth hormone improves the number of transferable embryos in superovulated ewes. Theriogenology 2001, 55, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Montero-Pardo, A.; Hernández-Cerón, J.; Rojas-Maya, S.; Valencia, J.; Rodríguez-Cortez, A.; Gutiérrez, C.G. Increased cleavage and blastocyst rate in ewes treated with bovine somatotropin 5 days before the end of progestin-based estrous synchronization. Anim. Reprod. Sci. 2011, 2011, 69–73. [Google Scholar] [CrossRef]
- Seekallu, S.; Toosi, B.; Grazul-Bilska, A.T.; Rawlings, N.C. Markers of ovarian antral follicular development in sheep: Comparison of follicles destined to ovulate from the final or penultimate follicular wave of the estrous cycle. Reproduction 2010, 140, 559–568. [Google Scholar] [CrossRef][Green Version]
- Mihm, M.; Baguisi, A.; Boland, M.P.; Roche, J.F. Association between the duration of dominance of the ovulatory follicle and pregnancy rate in beef heifers. J. Reprod. Fertil. 1994, 102, 123–130. [Google Scholar] [CrossRef]
- Mihm, M.; Curran, N.; Hyttel, P.; Knight, P.G.; Boland, M.P.; Roche, J.F. Effect of dominant follicle persistence on follicular fluid oestradiol and inhibin and on oocyte maturation in heifers. J. Reprod. Fertil. 1999, 116, 293–304. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Souza, A.H.; Carvalho, P.D.; Cunha, A.P.; Giordano, J.O.; Fricke, P.M.; Baez, G.M.; Diskin, M.G. Physiological and practical effects of progesterone on reproduction in dairy cattle. Animal 2014, 8 (Suppl. S1), 70–81. [Google Scholar] [CrossRef]
- Eckery, D.C.; Moeller, C.L.; Nett, T.M.; Sawyer, H.R. Localization and quantification of binding sites for Follicle-Stimulating Hormone, Luteinizing Hormone, Growth Hormone and Insulin-like Growth Factor I in sheep ovarian follicles. Biol. Reprod. 1997, 57, 507–513. [Google Scholar] [CrossRef]
- Kölle, S.; Sinowatz, F.; Boie, G.; Lincoln, D. Developmental Changes in the Expression of the Growth Hormone receptor messenger ribonucleic acid and protein in the bovine ovary. Biol. Reprod. 1998, 59, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Izadyar, F.; Zhao, J.; Van Tol, H.T.A.; Colenbrander, B.; Bevers, M.M. Messenger RNA expression and protein localization of Growth Hormone in bovine ovarian tissue and in cumulus oocyte complexes (COCs) during in vitro maturation. Mol. Reprod. Dev. 1999, 53, 398–407. [Google Scholar] [CrossRef]
- Schams, D.; Berisha, B.; Kosmann, M.; Amselgruber, W.M. Expression and localization of IGF family members in bovine antral follicles during final growth and in luteal tissue during different stages of estrous cycle and pregnancy. Domest. Anim. Endocrinol. 2002, 22, 51–72. [Google Scholar] [CrossRef]
- Shimizu, T.; Murayama, C.; Sudo, N.; Kawashima, C.; Tetsuka, M.; Miyamoto, A. Involvement of insulin and growth hormone (GH) during follicular development in the bovine ovary. Anim. Reprod. Sci. 2008, 106, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of folliculogenesis and the determination of ovulationrate in ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.; Garnsworthy, P.C.; Gong, J.-G.; Armstrong, D.G. Control of follicular growth: Local interactions and nutritional influences. J. Anim. Sci. 2004, 82 (Suppl. S13), E63–E74. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Shimizu, T.; Kawashima, C.; Kaneko, E.; Tetsuka, M.; Miyamoto, A. Insulin-like growth factor-I (IGF-I) system during follicle development in the bovine ovary: Relationship among IGF-I, type 1 IGF receptor (IGFR-1) and pregnancy-associated plasma protein-A (PAPP-A). Mol. Cell. Endocrinol. 2007, 264, 197–203. [Google Scholar] [CrossRef]
- Mani, A.M.; Fenwick, M.A.; Cheng, Z.; Sharma, M.K.; Singh, D.; Wathes, D.C. IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction 2010, 139, 139–151. [Google Scholar] [CrossRef]
- Dardente, H.; Lomet, D.; Robert, V.; Decourt, C.; Beltramo, M.; Pellicer-Rubio, M.-T. Seasonal breeding in mammals: From basic science to applications and back. Theriogenology 2016, 86, 324–332. [Google Scholar] [CrossRef]
- McCosh, R.B.; Szeligo, B.M.; Bedenbaugh, M.N.; Lopez, J.A.; Hardy, S.L.; Hileman, S.M.; Lehman, M.N.; Goodman, R.L. Evidence that endogenous somatostatin inhibits episodic, but not surge, secretion of LH in female sheep. Endocrinology 2017, 158, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Tsuchida, H.; Ieda, N.; Ikegami, K.; Inoue, I.; Uenoyama, Y.; Tsukamura, H. Somatostatin-Somatostatin Receptor 2 Signaling Mediates LH Pulse Suppression in Lactating Rats. Endocrinology 2019, 160, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.K.; Kendall, N.R.; Baird, D.T. The effect of the presence and pattern of Luteinizing Hormone stimulation on ovulatory follicle development in sheep. Biol. Reprod. 2007, 76, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Uenoyama, Y.; Okamoto, S.; Tsuchida, H.; Ikegami, K.; Goto, T.; Majarune, S.; Nakamura, S.; Sanbo, M.; Hirabayashi, M.; et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc. Natl. Acad. Sci. USA 2021, 118, e2009156118. [Google Scholar] [CrossRef]


| Parameters | bST | Control | p Value |
|---|---|---|---|
| Number of sheep (replicates) | 46 (5) | 46 (5) | |
| Body weight at day 0 (kg) | 66.6 ± 2.24 | 69.2 ± 2.09 | 0.411 |
| Body weight at day 15 (kg) | 67.4 ± 2.25 | 69.5 ± 2.08 | 0.483 |
| Follicles ≥ 3.5 at day 0: | |||
| Number (n) | 2.0 ± 0.18 | 2.2 ± 0.18 | 0.530 |
| Diameter (mm) | 4.7 ± 0.09 | 4.7 ± 0.09 | 0.738 |
| Follicles ≥ 4.3 at day 2: | |||
| Number (n) | 2.1 ± 0.11 | 2.1 ± 0.10 | 0.771 |
| Diameter (mm) | 5.6 ± 0.08 | 5.6 ± 0.08 | 0.822 |
| Estrous presentation (%) | 100 (46/46) | 97.8 (45/46) | >0.999 |
| Interval CIDR-estrus (h) | 37.0 ± 1.45 | 35.4 ± 1.61 | 0.451 |
| Ovulated ewes (%) | 100 (46/46) | 100 (46/46) | 1.000 |
| Ovulation efficiency (%) | 86.5 (45/52) | 81.1 (43/53) | 0.598 |
| Corpora lutea at day 7: | |||
| Number (n) | 2.0 ± 0.10 | 1.9 ± 0.11 | 0.842 |
| Total luteal area (mm) | 171.5 ± 8.86 | 164.0 ± 9.54 | 0.567 |
| Progesterone (ng/mL) | 7.02 ± 0.76 | 6.93 ± 0.75 | 0.930 |
| Pregnancy rate (%) | 100 (23/23) | 95.8 (23/24) | >0.999 |
| Lambing rate (%) | 100 (23/23) | 91.3 (21/23) | 0.709 |
| Fecundity rate (%) | 182.6 (42/23) | 120.8 (29/24) | 0.012 |
| Reproductive success (%) | 87.5 (42/48) | 54.7 (29/53) | <0.001 |
| Parameters | bST | Control | p Value |
|---|---|---|---|
| Number of sheep (replicates) | 25 (3) | 23 (3) | |
| Body weight at day 0 (kg) | 60.9 ± 1.73 | 61.3 ± 2.16 | 0.876 |
| Body weight at day 15 (kg) | 62.0 ± 1.75 | 61.8 ± 2.23 | 0.947 |
| Follicles ≥ 3.5 at day 0: | |||
| Number (n) | 2.2 ± 0.22 | 2.3 ± 0.19 | 0.734 |
| Diameter (mm) | 4.4 ± 0.08 | 4.4 ± 0.10 | 0.829 |
| Follicles ≥ 4.3 at day 2: | |||
| Number (n) | 2.0 ± 0.15 | 2.3 ± 0.24 | 0.218 |
| Diameter (mm) | 5.1 ± 0.10 | 5.0 ± 0.11 | 0.847 |
| Estrous presentation (%) | 84.0 (21/25) | 95.7 (22/23) | 0.350 |
| Interval CIDR-estrus (h) | 40.4 ± 2.00 | 39.8 ± 2.5 | 0.803 |
| Ovulated ewes (%) | 84.0 (21/25) | 95.7 (22/23) | 0.350 |
| Ovulation efficiency (%) | 69.4 (34/49) | 79.2 (42/53) | 0.267 |
| Corpora lutea at day 7: | |||
| Number (n) | 1.4 ± 0.16 | 1.8 ± 0.16 | 0.078 |
| Total luteal area (mm) | 130.7 ± 13.65 | 138.3 ± 10.08 | 0.458 |
| Progesterone (ng/mL) | 6.1 ± 0.54 | 6.1 ± 0.34 | 0.921 |
| Pregnancy rate (%) | 64.0 (16/25) | 91.3 (21/23) | 0.232 |
| Lambing rate (%) | 93.8 (15/16) | 81.0 (17/21) | 0.364 |
| Fecundity rate (%) | 84.0 (21/25) | 104.3 (24/23) | 0.414 |
| Reproductive success (%) | 42.9 (21/49) | 45.3 (24/53) | 0.844 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, J.F.; Carrasco, A.; Navarrete, F.; Allende, R.; Saravia, F.; Dorado, J. Unveiling the Role of IGF-I in Fertility: Effect of Long-Acting Bovine Somatotropin (bST) on Terminal Follicular Development and Fertility during an Annual Reproductive Cycle in Sheep. Animals 2024, 14, 1097. https://doi.org/10.3390/ani14071097
Cox JF, Carrasco A, Navarrete F, Allende R, Saravia F, Dorado J. Unveiling the Role of IGF-I in Fertility: Effect of Long-Acting Bovine Somatotropin (bST) on Terminal Follicular Development and Fertility during an Annual Reproductive Cycle in Sheep. Animals. 2024; 14(7):1097. https://doi.org/10.3390/ani14071097
Chicago/Turabian StyleCox, José Francisco, Albert Carrasco, Felipe Navarrete, Rodrigo Allende, Fernando Saravia, and Jesús Dorado. 2024. "Unveiling the Role of IGF-I in Fertility: Effect of Long-Acting Bovine Somatotropin (bST) on Terminal Follicular Development and Fertility during an Annual Reproductive Cycle in Sheep" Animals 14, no. 7: 1097. https://doi.org/10.3390/ani14071097
APA StyleCox, J. F., Carrasco, A., Navarrete, F., Allende, R., Saravia, F., & Dorado, J. (2024). Unveiling the Role of IGF-I in Fertility: Effect of Long-Acting Bovine Somatotropin (bST) on Terminal Follicular Development and Fertility during an Annual Reproductive Cycle in Sheep. Animals, 14(7), 1097. https://doi.org/10.3390/ani14071097





