Foraging, Fear and Behavioral Variation in a Traplining Hummingbird
Abstract
:Simple Summary
Abstract
1. Introduction
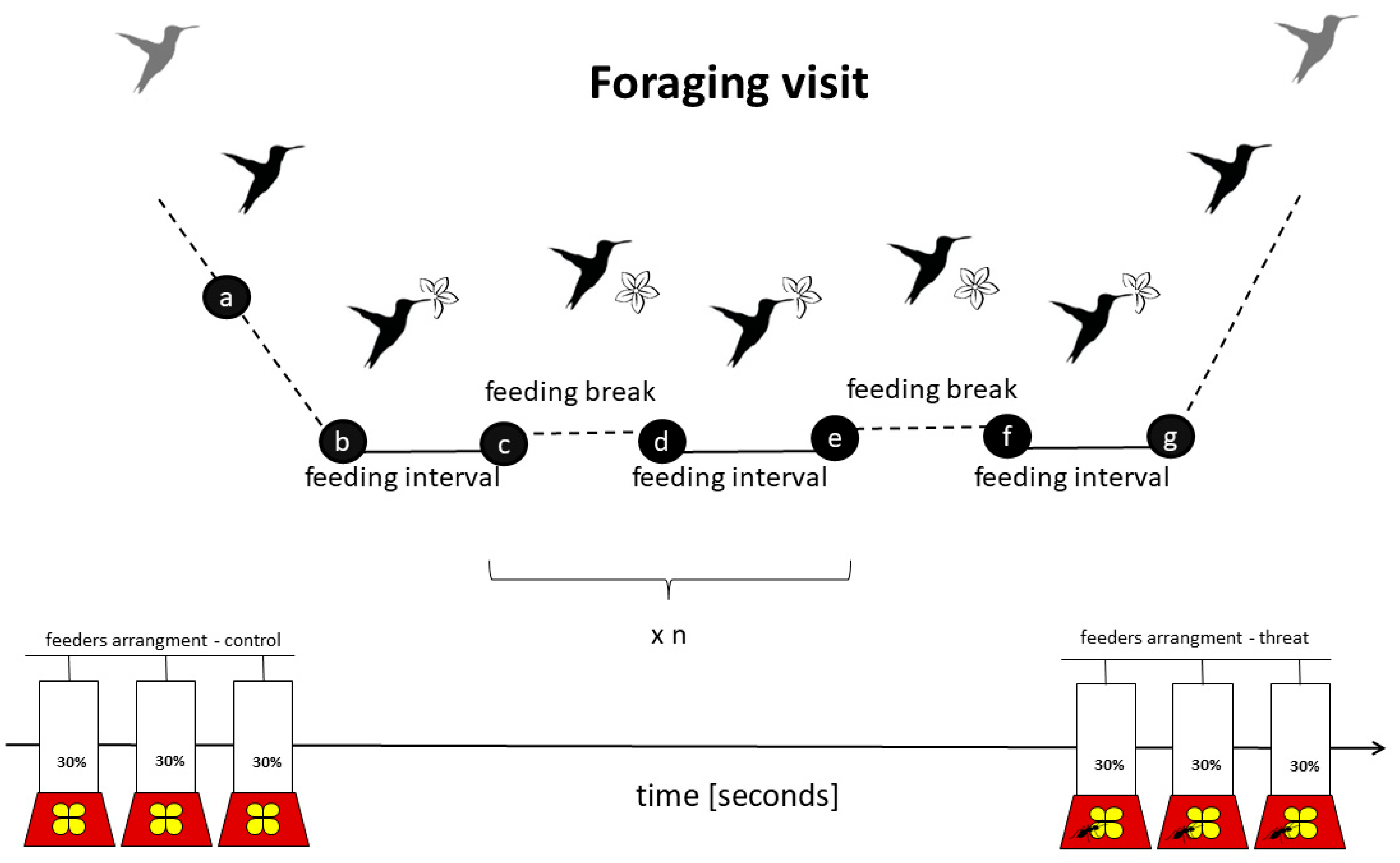
2. Methods
2.1. Fieldwork
2.2. Videos Analysis
2.3. Parameters
2.4. Data Analysis
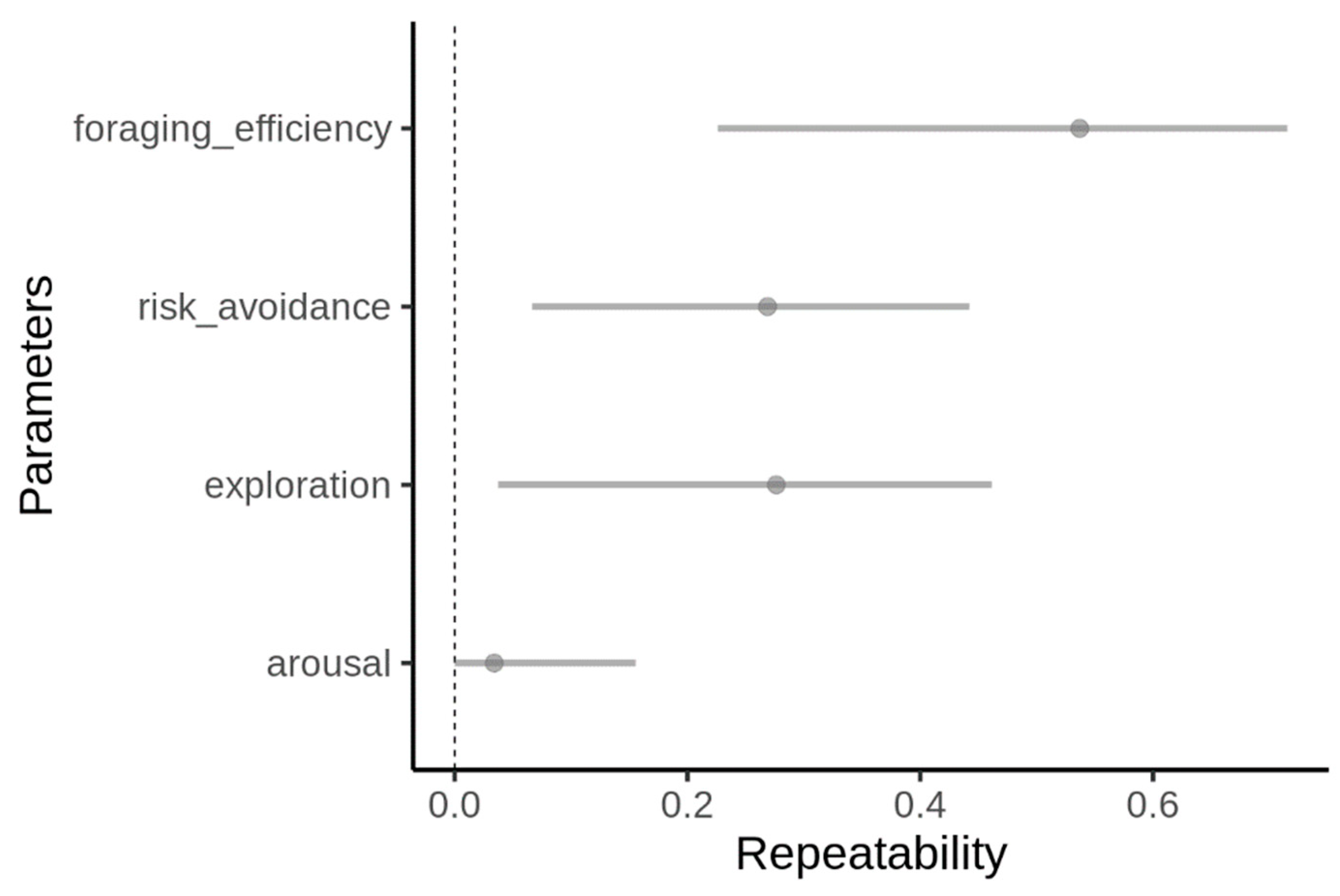
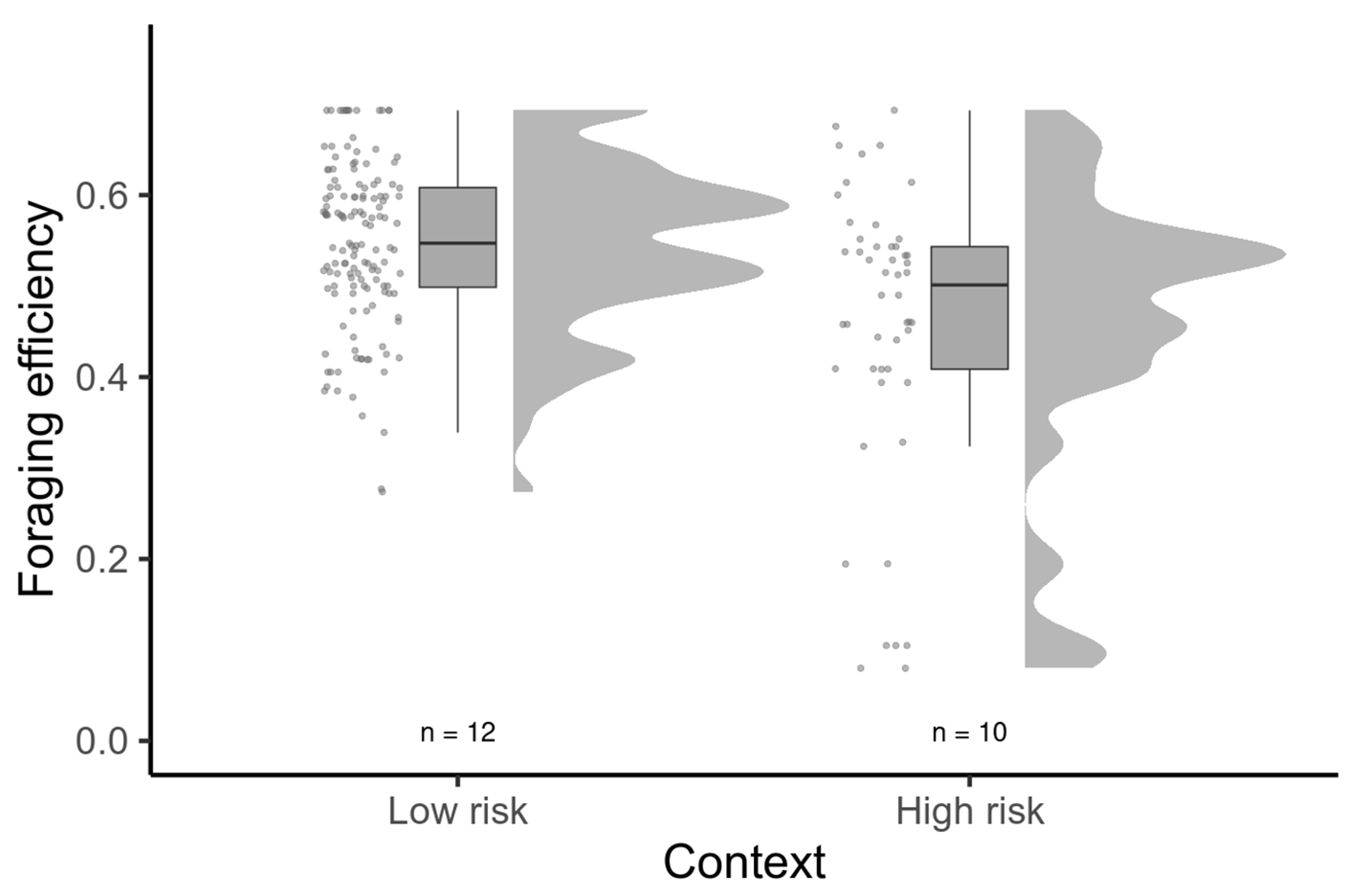
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herborn, K.A.; Heidinger, B.J.; Alexander, L.; Arnold, K.E. Personality Predicts Behavioral Flexibility in a Fluctuating, Natural Environment. Behav. Ecol. 2014, 25, 1374–1379. [Google Scholar] [CrossRef] [Green Version]
- Morrison, M.M.; Raplph, C.J.; Verner, J.; Jehl, J.R.J. Avian Foraging: Theory, Methodology and Applications. Stud. Avian Biol. 1990, 13, 1–515. [Google Scholar]
- Charnov, E.L. Optimal Foraging, the Marginal Value Theorem. Theor. Popul. Biol. 1976, 9, 739–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista, L.M.; Tinbergen, J.; Kacelnik, A. To Walk or to Fly? How Birds Choose among Foraging Modes. Proc. Natl. Acad. Sci. USA 2001, 98, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- Lima, S.L.; Bednekoff, P.A. Temporal Variation in Danger Drives Antipredator Behavior: The Predation Risk Allocation Hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef]
- Houston, A.I.; Mcnamara, J.M.; Hutchinson, J.M.C.; Trans, P.; Lond, R.S. General Results Concerning the Trade-off between Gaining Energy and Avoiding Predation. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1993, 341, 375–397. [Google Scholar] [CrossRef]
- Verdolin, J.L. Meta-Analysis of Foraging and Predation Risk Trade-Offs in Terrestrial Systems. Behav. Ecol. Sociobiol. 2006, 60, 457–464. [Google Scholar] [CrossRef]
- Krebs, J.R. Optimal Foraging, Predation Risk and Territory Defence. Ardea 1980, 68, 83–90. [Google Scholar] [CrossRef]
- Patrick, S.C.; Bearhop, S.; Grémillet, D.; Lescroël, A.; Grecian, W.J.; Bodey, T.W.; Hamer, K.C.; Wakefield, E.; Le Nuz, M.; Votier, S.C. Individual Differences in Searching Behaviour and Spatial Foraging Consistency in a Central Place Marine Predator. Oikos 2014, 123, 33–40. [Google Scholar] [CrossRef]
- Camprasse, E.C.M.; Cherel, Y.; Bustamante, P.; Arnould, J.P.Y.; Bost, C.A. Intra-and Inter-Individual Variation in the Foraging Ecology of a Generalist Subantarctic Seabird, the Gentoo Penguin. Mar. Ecol. Prog. Ser. 2017, 578, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Moldoff, D.E.; Westneat, D.F. Foraging Sparrows Exhibit Individual Differences but Not a Syndrome When Responding to Multiple Kinds of Novelty. Behav. Ecol. 2017, 28, 732–743. [Google Scholar] [CrossRef]
- Dingemanse, N.J.; Kazem, A.J.N.; Reale, D.; Wright, J. Behavioural Reaction Norms: Animal Personality Meets Individual Plasticity. Trends Ecol. Evol. 2009, 25, 81–89. [Google Scholar] [CrossRef]
- Alonzo, S.H. Integrating the How and Why of within-Individual and among-Individual Variation and Plasticity in Behavior. Curr. Opin. Behav. Sci. 2015, 6, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Toscano, B.J.; Gownaris, N.J.; Heerhartz, S.M.; Monaco, C.J. Personality, Foraging Behavior and Specialization: Integrating Behavioral and Food Web Ecology at the Individual Level. Oecologia 2016, 182, 55–69. [Google Scholar] [CrossRef]
- Bell, A.M.; Hankison, S.J.; Laskowski, K.L. The Repeatability of Behaviour: A Meta-Analysis. Anim. Behav. 2009, 77, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Nussey, D.H.; Wilson, A.J.; Brommer, J.E. The Evolutionary Ecology of Individual Phenotypic Plasticity in Wild Populations. J. Evol. Biol. 2007, 20, 831–844. [Google Scholar] [CrossRef]
- Carere, C.; Maestripieri, D. Animal Personalities; The University of Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- Smith, B.R.; Blumstein, D.T. Fitness Consequences of Personality: A Meta-Analysis. Behav. Ecol. 2008, 19, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating Animal Temperament within Ecology and Evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef] [Green Version]
- Quinn, J.L.; Cole, E.F.; Bates, J.; Payne, R.W.; Cresswell, W. Personality Predicts Individual Responsiveness to the Risks of Starvation and Predation. Proc. R. Soc. B 2012, 279, 1919–1926. [Google Scholar] [CrossRef] [Green Version]
- Dall, S.R.X.; Houston, A.I.; McNamara, J.M. The Behavioural Ecology of Personality: Consistent Individual Differences from an Adaptive Perspective. Ecol. Lett. 2004, 7, 734–739. [Google Scholar] [CrossRef]
- Dingemanse, N.J.; Réale, D. Natural Selection and Animal Personality. Behaviour 2005, 142, 1165–1190. [Google Scholar] [CrossRef]
- Bergeron, P.; Montiglio, P.O.; Réale, D.; Humphries, M.M.; Gimenez, O.; Garant, D. Disruptive Viability Selection on Adult Exploratory Behaviour in Eastern Chipmunks. J. Evol. Biol. 2013, 26, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.M.; Dingemanse, N.J.; Nakagawa, S.; McDonald, G.C.; Løvlie, H.; Robledo-Ruiz, D.A.; Pizzari, T. Sexual Selection and Personality: Individual and Group-Level Effects on Mating Behaviour in Red Junglefowl. J. Anim. Ecol. 2021, 90, 1288–1306. [Google Scholar] [CrossRef] [PubMed]
- Le Cœur, C.; Thibault, M.; Pisanu, B.; Thibault, S.; Chapuis, J.L.; Baudry, E. Temporally Fluctuating Selection on a Personality Trait in a Wild Rodent Population. Behav. Ecol. 2015, 26, 1285–1291. [Google Scholar] [CrossRef] [Green Version]
- Mouchet, A.; Cole, E.F.; Matthysen, E.; Nicolaus, M.; Quinn, J.L.; Roth, A.M.; Tinbergen, J.M.; van Oers, K.; van Overveld, T.; Dingemanse, N.J. Heterogeneous Selection on Exploration Behavior within and among West European Populations of a Passerine Bird. Proc. Natl. Acad. Sci. USA 2021, 118, e2024994118. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.K. Hummingbird Flight: Sustaining the Highest Mass-Specific Metabolic Rates among Vertebrates. Experientia 1992, 48, 565–570. [Google Scholar] [CrossRef]
- Stiles, F.G.; Wolf, L.L. Ecology and Evolution of Lek Mating Behavior in the Long-Tailed Hermit Hummingbird. Ornithol. Monogr. 1979, 27, 78. [Google Scholar] [CrossRef]
- Araya-Salas, M.; Gonzalez-Gomez, P.; Wojczulanis-Jakubas, K.; López, V.; Wright, T.F. Spatial Memory Is as Important as Weapon and Body Size for Territorial Ownership in a Lekking Hummingbird. Sci. Rep. 2018, 8, 2001. [Google Scholar] [CrossRef] [Green Version]
- Stiles, F.G. Possible Specialization for Hummingbird-Hunting in the Tiny Hawk. Auk 1978, 95, 550–553. [Google Scholar] [CrossRef]
- Nyffeler, M.; Maxwell, M.R.; Remsen, J.V. Bird Predation by Praying Mantises: A Global Perspective. Wilson J. Ornithol. 2017, 129, 331–344. [Google Scholar] [CrossRef]
- Owen, J.L.; Cokendolpher, J.C. Tailless Whipscorpion (Phrynus longipes) Feeds on Antillean Crested Hummingbird (Orthorhyncus cristatus). Wilson J. Ornithol. 2006, 118, 422–423. [Google Scholar] [CrossRef]
- Sazima, I. Lightning Predator: The Ferruginous Pygmy Owl Snatches Flower-Visiting Hummingbirds in Southwestern Brazil. Rev. Bras. Ornitol. 2015, 23, 12–14. [Google Scholar] [CrossRef]
- Zenzal, T.J.; Fish, A.C.; Jones, T.M.; Ospina, E.A.; Moore, F.R. Observations of Predation and Anti-Predator Behavior of Rubythroated Hummingbirds During Migratory Stopover. Southeast. Nat. 2013, 12, N21–N25. [Google Scholar] [CrossRef]
- Lorenz, S. Carolina Mantind (Stagmomantis carolica) Captures and Feeds on a Broad-Tailed Hummingbird (Selasphorus platycercus). Bull. Texas Ornithol. Soc. 2007, 40, 1–40. [Google Scholar]
- Couchoux, C.; Cresswell, W. Personality Constraints versus Flexible Antipredation Behaviors: How Important Is Boldness in Risk Management of Redshanks (Tringa totanus) Foraging in a Natural System? Behav. Ecol. 2012, 23, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Carr, J.M.; Golinski, J.E. Vigilance Behaviors of Ruby-Throated Hummingbirds (Archilochus colubris) Reflect Elevated Risk of Competitive Interactions with Vespine Wasps. Wilson J. Ornithol. 2020, 132, 295–305. [Google Scholar] [CrossRef]
- Sih, A.; Chyng, H.J.; Neylan Isabelle Ortiz-Jimenez, C.; Sakai, O.; Szeligowski, R. Fear Generalization and Behavioral Responses to Multiple Dangers. Trends Ecol. Evol. 2023, 38, 369–380. [Google Scholar] [CrossRef]
- Pastell, M. CowLog—Cross-Platform Application for Coding Behaviours from Video. J. Open Res. Softw. 2016, 4, e15. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Stoffel, M.A.; Nakagawa, S.; Schielzeth, H. RptR: Repeatability Estimation and Variance Decomposition by Generalized Linear Mixed-Effects Models. Methods Ecol. Evol. 2017, 8, 1639–1644. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R. Deviance Information Criterion (DIC). Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons Ltd.: Hoboken, NY, USA, 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Hadfield, J.D. MCMCglmm: MCMC Methods for Multi-Response GLMMs in R. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Schielzeth, H. Repeatability for Gaussian and Non-Gaussian Data: A Practical Guide for Biologists. Biol. Rev. 2010, 85, 935–956. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.C.O.; Sih, A.; Chivers, D.P. The Paradox of Risk Allocation: A Review and Prospectus. Anim. Behav. 2009, 78, 579–585. [Google Scholar] [CrossRef]
- Montiglio, P.; Sih, A.; Mathot, K.J.; Wolf, M.; Dingemanse, N.J. Animal Personality and State—Behaviour Feedbacks: A Review and Guide for Empiricists. Trends Ecol. Evol. 2015, 30, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Dingemanse, N.J.; Kazem, A.J.N.; Wright, J.; Biologiques, S. Evolutionary and Ecological Approaches to the Study of Personality. Philosoph. Trans. R. Soc. B Biol. Sci. 2010, 365, 3937–3946. [Google Scholar] [CrossRef] [Green Version]
- Nácarová, J.; Veselý, P.; Fuchs, R. Effect of the Exploratory Behaviour on a Bird’s Ability to Categorize a Predator. Behav. Processes 2018, 151, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Exnerová, A.; Svádová, K.H.; Fučíková, E.; Drent, P.; Štys, P. Personality Matters: Individual Variation in Reactions of Naive Bird Predators to Aposematic Prey. Proc. R. Soc. B Biol. Sci. 2010, 277, 723–728. [Google Scholar] [CrossRef] [Green Version]
- Tello-Ramos, M.C.; Hurly, T.A.; Healy, S.D. Traplining in Hummingbirds: Flying Shortdistance Sequences among Several Locations. Behav. Ecol. 2015, 26, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, K.; Thomson, J.D. Efficient Harvesting of Renewing Resources. Behav. Ecol. 2005, 16, 592–605. [Google Scholar] [CrossRef] [Green Version]
- Gill, F.B. Trapline Foraging by Hermit Hummingbirds: Competition for an Undefended, Renewable Resource. Ecology 1988, 69, 1933–1942. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral Syndromes: An Ecological and Evolutionary Overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Cleasby, I.R.; Nakagawa, S.; Schielzeth, H. Quantifying the Predictability of Behaviour: Statistical Approaches for the Study of between-Individual Variation in the within-Individual Variance. Methods Ecol. Evol. 2015, 6, 27–37. [Google Scholar] [CrossRef]

| Predictors | df | DIC | ΔDIC | Weight DIC | AIC | ΔAIC | Weight AIC |
|---|---|---|---|---|---|---|---|
| md_all_interactions | 10 | −400.0909 | 0.00 | 1 | −396.3073 | 0.00 | 0.99 |
| md_arousal_exploration | 8 | −388.2385 | 11.85 | 0 | −386.2831 | 10.02 | 0.01 |
| md_arousal_risk_avoidance | 8 | −378.9807 | 21.11 | 0 | −376.8184 | 19.49 | 0.00 |
| md_arousal | 6 | −363.3410 | 36.75 | 0 | −363.2509 | 33.06 | 0.00 |
| md_risk_avoidance_exploration | 8 | −350.1568 | 49.93 | 0 | −348.8140 | 47.49 | 0.00 |
| md_exploration | 6 | −345.7716 | 54.32 | 0 | −346.4065 | 49.90 | 0.00 |
| md_risk_avoidance | 6 | −315.2258 | 84.87 | 0 | −315.0929 | 81.21 | 0.00 |
| md_context | 4 | −308.6036 | 91.49 | 0 | −310.7995 | 85.51 | 0.00 |
| md_null | 3 | −296.3098 | 103.78 | 0 | −299.8347 | 96.47 | 0.00 |
| Predictor | Effect Size | CI 2.5% | CI 97.5% | pMCMC |
|---|---|---|---|---|
| contextHigh risk | −0.1409 | −0.2732 | −0.0132 | 0.0322 |
| Arousal | 0.0684 | 0.0275 | 0.1083 | 0.0006 |
| Exploration | 0.3686 | 0.1244 | 0.6167 | 0.0023 |
| risk_avoidance | −0.0327 | −0.0663 | 0.0023 | 0.0641 |
| contextHigh risk:arousal | 0.2445 | 0.1541 | 0.3436 | 0.0001 |
| contextHigh risk:exploration | −0.8355 | −1.1641 | −0.4925 | 0.0001 |
| contextHigh risk:risk_avoidance | −0.0270 | −0.0793 | 0.021 | 0.2918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojczulanis-Jakubas, K.; Araya-Salas, M. Foraging, Fear and Behavioral Variation in a Traplining Hummingbird. Animals 2023, 13, 1997. https://doi.org/10.3390/ani13121997
Wojczulanis-Jakubas K, Araya-Salas M. Foraging, Fear and Behavioral Variation in a Traplining Hummingbird. Animals. 2023; 13(12):1997. https://doi.org/10.3390/ani13121997
Chicago/Turabian StyleWojczulanis-Jakubas, Katarzyna, and Marcelo Araya-Salas. 2023. "Foraging, Fear and Behavioral Variation in a Traplining Hummingbird" Animals 13, no. 12: 1997. https://doi.org/10.3390/ani13121997






