The Effect of Radial Extracorporeal Shock Wave Therapy (rESWT) on the Skin Surface Temperature of the Longissimus Dorsi Muscle in Clinically Healthy Racing Thoroughbreds: A Preliminary Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Data Collection
2.2. Radial Extracorporeal Shock Wave Therapy
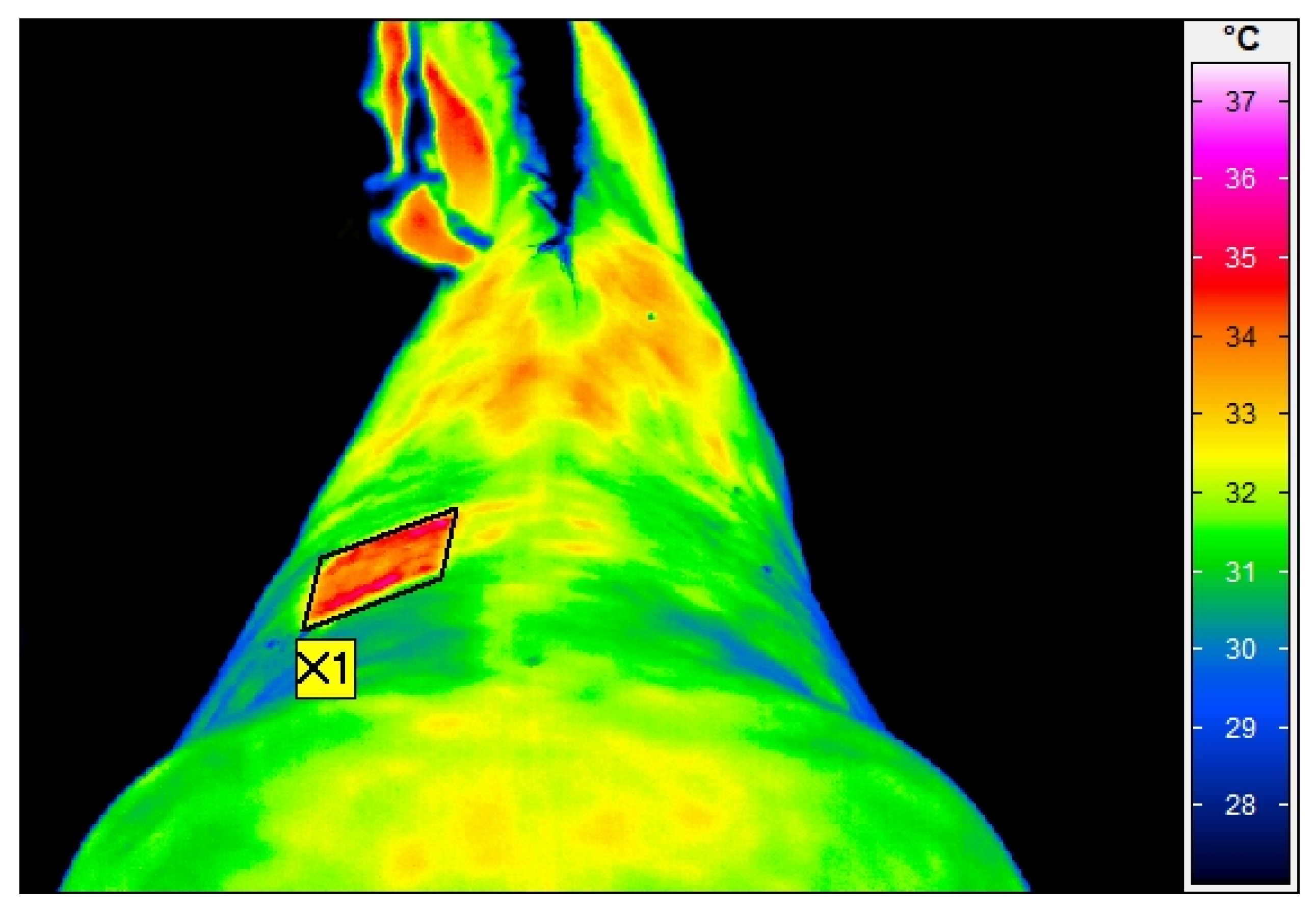
2.3. Thermographic Examination
2.4. Palpation Examination
2.5. Statistical Analysis
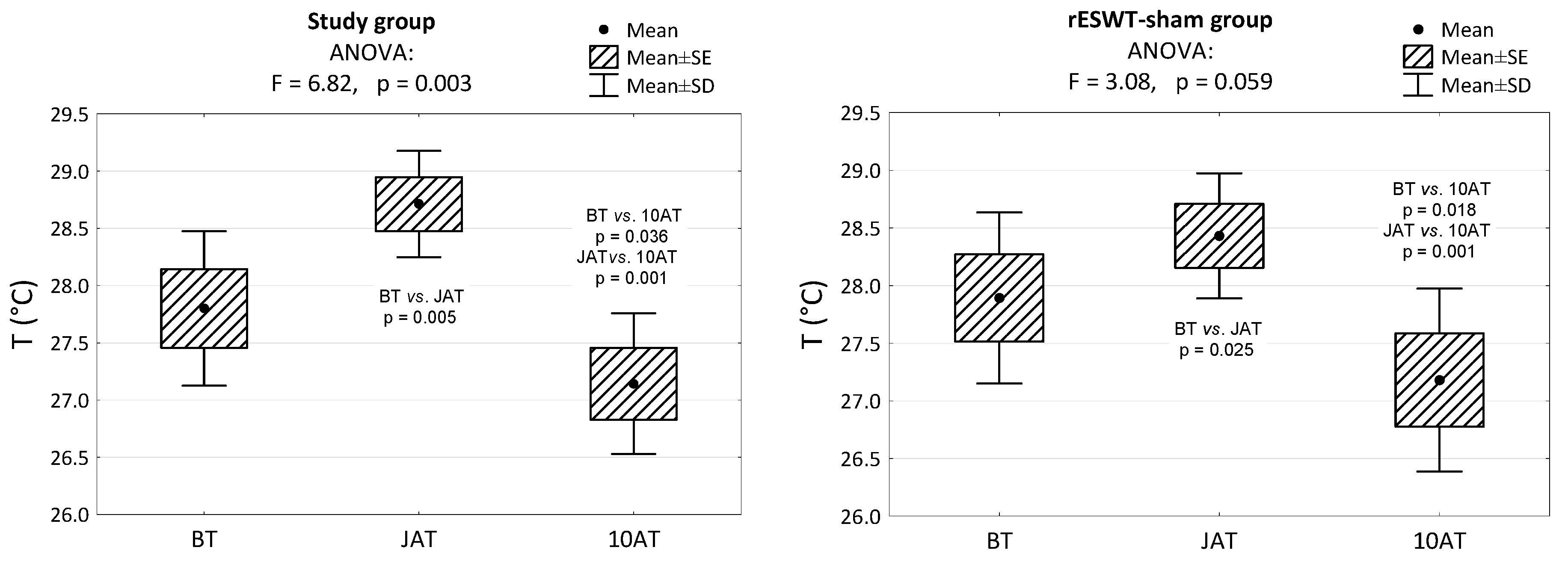
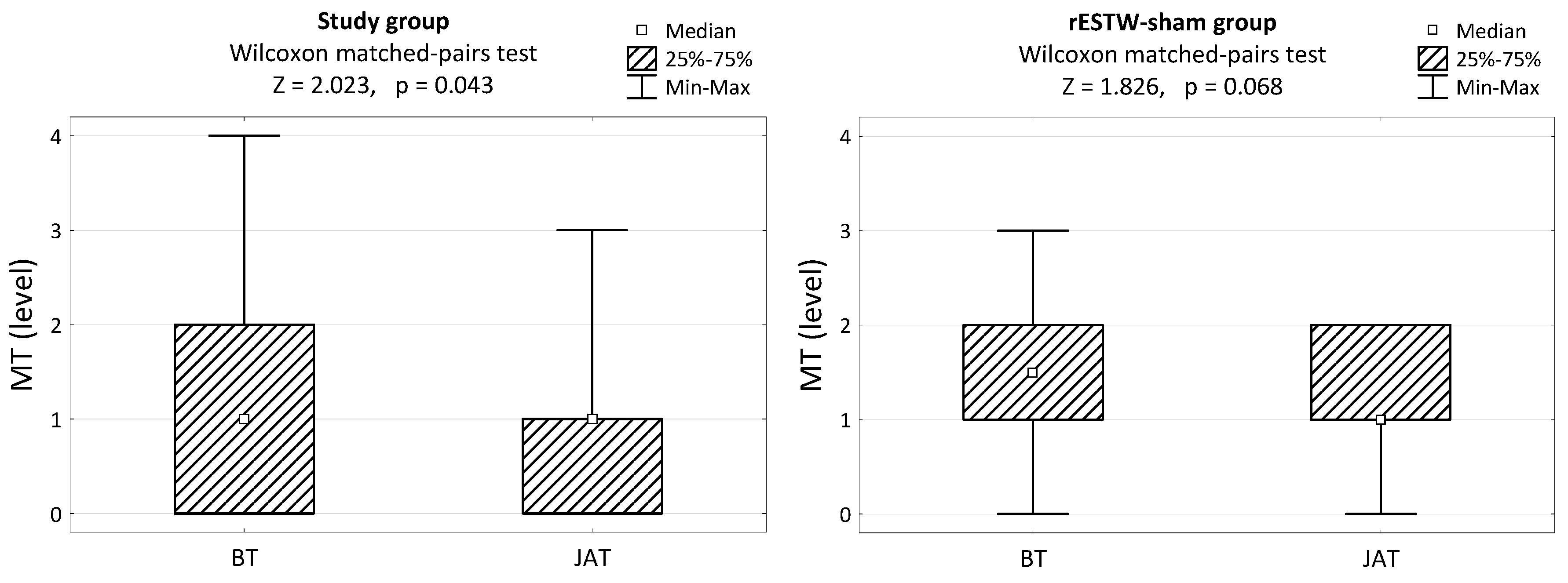
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacKay, A.V.; McOnie, R.C.; Riddell, L.P.; Robinson, K.A. Characterization of the use of shock wave therapy among equine veterinarians. Can. Vet. J. 2020, 61, 990–993. [Google Scholar] [PubMed]
- McClure, S.; Dorfmüller, C. Extracorporeal shock wave therapy: Theory and equipment. Clin. Tech. Equine Pract. 2003, 2, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Dyson, S.; Ross, M.W. Diagnosis and Management of Lameness in the Horse; Elsevier Health Sciences: Amsterdam, The Netherlands, 2003; Volume 1191, pp. 914–919. [Google Scholar]
- Gary, M.; Baxter, G.M. Adams and Stashak’s Lameness in Horses, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; Volume 118, 736, pp. 961–962. [Google Scholar]
- Dahlberg, J.A. Evaluation of the efficacy of extracorporeal shock wave therapy (ESWT) on osteroarthritis and post-ESWT analgesia in animal models. Iowa State Univ. Dig. Repos. 2006, 4, 34–35. [Google Scholar]
- Simplicio, C.L.; Purita, J.; Murrell, W.; Santos, G.S.; Dos Santos, R.G.; Lana, J.F.S.D. Extracorporeal shock wave therapy mechanisms in musculoskeletal regenerative medicine. J. Clin. Orthop. Trauma 2020, 11, S309–S318. [Google Scholar] [CrossRef]
- van der Worp, H.; van den Akker-Scheek, I.; Van Schie, H.; Zwerver, J. ESWT for tendinopathy: Technology and clinical implications. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1451–1458. [Google Scholar] [CrossRef] [Green Version]
- Trebinjac, S.; Mujić-Skikić, E.; Ninković, M.; Karaiković, E. Extracorporeal shock wave therapy in orthopaedic diseases. Bosn. J. Basic Med. 2005, 5, 27. [Google Scholar] [CrossRef]
- Wu, S.S.; Ericson, K.J.; Shoskes, D.A. Retrospective comparison of focused shockwave therapy and radial wave therapy for men with erectile dysfunction. Transl. Androl. Urol. 2020, 9, 2122. [Google Scholar] [CrossRef] [PubMed]
- Walewicz, K.; Taradaj, J.; Rajfur, K.; Ptaszkowski, K.; Kuszewski, M.T.; Sopel, M.; Dymarek, R. The effectiveness of radial extracorporeal shock wave therapy in patients with chronic low back pain: A prospective, randomized, single-blinded pilot study. Clin. Interv. Aging 2019, 14, 1859–1869. [Google Scholar] [CrossRef] [Green Version]
- Tabra, S.A.A.; Zaghloul, M.I.; Alashkar, D.S. Extracorporeal shock wave as adjuvant therapy for wrist and hand spasticity in post-stroke patients: A randomized controlled trial. Egypt. Rheumatol. 2021, 48, 21. [Google Scholar] [CrossRef]
- Auersperg, V.; Trieb, K. Extracorporeal shock wave therapy: An update. EFORT Open Rev. 2020, 5, 584. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.L.; Yang, F.; Zhang, Q.; Su, X.Z.; Li, J.; Zhu, H. Radial shockwave treatment promotes human mesenchymal stem cell self-renewal and enhances cartilage healing. Stem Cell Res. Ther. 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, S.; Zaman, F.; Madhuri, V.; Sävendahl, L. Radial extracorporeal shock wave treatment promotes bone growth and chondrogenesis in cultured fetal rat metatarsal bones. Clin. Orthop. Relat. Res. 2020, 478, 668. [Google Scholar] [CrossRef] [PubMed]
- Mattyasovszky, S.G.; Langendorf, E.K.; Ritz, U.; Schmitz, C.; Schmidtmann, I.; Nowak, T.E.; Drees, P. Exposure to radial extracorporeal shock waves modulates viability and gene expression of human skeletal muscle cells: A controlled in vitro study. J. Orthop. Surg. Res. 2018, 13, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, B.M.; Byron, C.R.; Pondenis, H.; Stewart, A.A. The effects of radial shock waves on the metabolism of equine cartilage explants in vitro. N. Z. Vet. J. 2007, 55, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Mori, L.; Solaro, C.; Uccelli, A.; Pelosin, E.; Currà, A.; Trompetto, C. Effect of radial shock wave therapy on pain and muscle hypertonia: A double-blind study in patients with multiple sclerosis. Mult. Scler. J. 2015, 21, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Vidal, X.; Morral, A.; Costa, L.; Tur, M. Radial extracorporeal shock wave therapy (rESWT) in the treatment of spasticity in cerebral palsy: A randomized, placebo-controlled clinical trial. Neurorehabilitation 2011, 29, 413–419. [Google Scholar] [CrossRef]
- Gonkova, M.I.; Ilieva, E.M.; Ferriero, G.; Chavdarov, I. Effect of radial shock wave therapy on muscle spasticity in children with cerebral palsy. Int. J. Rehabil. Res. 2013, 36, 284–290. [Google Scholar] [CrossRef]
- Sohn, M.K.; Cho, K.H.; Kim, Y.J.; Hwang, S.L. Spasticity and electrophysiologic changes after extracorporeal shock wave therapy on gastrocnemius. Ann. Rehabil. Med. 2011, 35, 599–604. [Google Scholar] [CrossRef]
- Wang, C.J.; Huang, H.Y.; Pai, C.H. Shock wave-enhanced neovascularization at the tendon-bone junction: An experiment in dogs. J. Foot Ankle Surg. 2002, 41, 16–22. [Google Scholar] [CrossRef]
- Kang, N.; Zhang, J.; Yu, X.; Ma, Y. Radial extracorporeal shock wave therapy improves cerebral blood flow and neurological function in a rat model of cerebral ischemia. Am. J. Transl. Res. 2017, 9, 2000. [Google Scholar]
- Contaldo, C.; Högger, D.C.; Borozadi, M.K.; Stotz, M.; Platz, U.; Forster, N.; Giovanoli, P. Radial pressure waves mediate apoptosis and functional angiogenesis during wound repair in ApoE deficient mice. Micr. Res. 2012, 84, 24–33. [Google Scholar] [CrossRef]
- Silveira, A.; Koenig, J.B.; Arroyo, L.G.; Trout, D.; Moens, N.M.; LaMarre, J.; Brooks, A. Effects of unfocused extracorporeal shock wave therapy on healing of wounds of the distal portion of the forelimb in horses. Am. J. Vet. Res. 2010, 71, 229–234. [Google Scholar] [CrossRef]
- Yan, X.; Zeng, B.; Chai, Y.; Luo, C.; Li, X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann. Plast. Surg. 2008, 61, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Boening, K.; Loeffeld, S.; Weitkamp, K.; Matuschek, S. Radial extracorporeal shock wave therapy for chronic insertion desmopathy of the proximal suspensory ligament. Proc. Am. Assoc. Equine Pract. 2000, 46, 203–207. [Google Scholar]
- Crowe, O.M.; Dyson, S.J.; Wright, I.M.; Schramme, M.C.; Smith, R.K.W. Treatment of chronic or recurrent proximal suspensory desmitis using radial pressure wave therapy in the horse. Equine Vet. J. 2004, 36, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; Nickels, F.A.; Caron, J.P.; Mullineaux, D.R.; Clayton, H.M. Investigation of the immediate analgesic effects of extracorporeal shock wave therapy for treatment of navicular disease in horses. Vet. Surg. 2005, 34, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.E. Treatment of dorsal metacarpal disease in the Thoroughbred racehorse with radial extracorporeal shock wave therapy. Proc. Am. Assoc. Equine Pract. 2002, 48, 318–321. [Google Scholar]
- Da Costa Gómez, T.M.; Radtke, C.L.; Kalscheur, V.L.; Swain, C.A.; Scollay, M.C.; Edwards, R.B.; Muir, P. Effect of focused and radial extracorporeal shock wave therapy on equine bone microdamage. Vet. Surg. 2004, 33, 49–55. [Google Scholar] [CrossRef]
- Soroko, M.; Davies-Morel, M.C.G.; Howell, K. The application of infrared thermography in equestrian sport. In Application of Infrared Thermography in Sports Science; Quesada, J.I.P., Ed.; Springer: Cham, Switzerland, 2017; pp. 265–296. [Google Scholar]
- Tunley, B.V.; Henson, F.M.D. Reliability and repeatability of thermographic examination and the normal thermographic image of the thoracolumbar region in the horse. Equine Vet. J. 2004, 36, 306–312. [Google Scholar] [CrossRef]
- Autio, E.; Neste, R.; Airaksinen, S.; Heiskanen, M.L. Measuring the Heat Loss in Horses in Different Seasons by Infrared Thermography. J. Appl. Anim. Welf. Sci. 2006, 9, 211–221. [Google Scholar] [CrossRef]
- Simon, E.L.; Gaughan, E.M.; Epp, T.; Spire, M. Influence of exercise on thermographically determined surface temperatures of thoracic and pelvic limbs in horses. J. Am. Vet. Med. Assoc. 2006, 229, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Rindler, N.; Biermann, N.M.; Westermann, S.; Buchner, H.H.F. The effect of pulsed electromagnetic field therapy on surface temperature of horses’ backs. Wien. Tierärztl. Mon. 2014, 101, 137–141. [Google Scholar]
- Godlewska, M.; Soroko, M.; Zielińska, P. Assessment of vein diameter and body surface temperature after high-intensity laser therapy (HILT) on the tarsal joint in healthy horses. J. Equine Vet. Sci. 2020, 93, 103198. [Google Scholar] [CrossRef]
- Zielińska, P.; Soroko, M.; Howell, K.; Godlewska, M.; Hildebrand, W.; Dudek, K. Comparison of the effect of high-intensity laser therapy (HILT) on skin surface temperature and vein diameter in pigmented and non-pigmented skin in healthy racehorses. Animals 2021, 11, 1965. [Google Scholar] [CrossRef]
- Lubkowska, A.; Radecka, A.; Parchimowicz, M.; Bryczkowska, I.; Chudecka, M. The effects of ultrasound and shockwave treatment on muscle regional oxygen saturation using near-infrared spectroscopy. Pomeranian J. Life Sci. 2018, 64, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Verna, M.; Turner, T.A.; Anderson, K.L. Scintigraphic, radiographic, and thermographic appearance of the metacarpal and metatarsal regions of adult healthy horses treated with nonfocused extracorporeal shock wave therapy—A pilot study. Vet. Ther. 2005, 6, 268–276. [Google Scholar]
- Ringer, S.K.; Lischer, C.J.; Ueltschi, G. Assessment of scintigraphic and thermographic changes after focused extracorporeal shock wave therapy on the origin of the suspensory ligament and the fourth metatarsal bone in horses without lameness. Am. Am. J. Vet. Res. 2005, 66, 1836–1842. [Google Scholar] [CrossRef]
- Schauer, A.L. Effect of Age, Breed and Sex on Longissimus Dorsi Muscle Area Subcutaneous Fat Depth in Horses. Honors Scholar Theses, University of Connecticut, Department of Animal Science, Storrs, CT, USA. 2015. Available online: https://opencommons.uconn.edu/srhonors_theses/444 (accessed on 22 March 2023).
- Stashak, T.S. Examination for lameness. In Adam’s Lameness in Horses; Troy, D., Ed.; Williams and Wilkins: Philadelphia, PA, USA, 2002; pp. 113–183. [Google Scholar]
- Martin, B.B., Jr.; Klide, A.M. Physical examination of horses with back pain. Vet. Clin. N. Am. Equine Pract. 1999, 15, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Soroko, M.; Zaborski, D.; Dudek, K.; Yarnell, K.; Górniak, W.; Vardasca, R. Evaluation of thermal pattern distributions in racehorse saddles using infrared thermography. PLoS ONE 2019, 14, e0221622. [Google Scholar] [CrossRef]
- Soroko, M.; Howell, K. Infrared thermography: Current applications in equine medicine. J. Equine Vet. Sci. 2018, 60, 90–96. [Google Scholar] [CrossRef]
- Zielińska, P.; Soroko-Dubrovina, M.; Dudek, K.; Ruzhanova-Gospodinova, I.S. A preliminary study of the influence of high intensity laser therapy (HILT) on skin surface temperature and longissimus dorsi muscle tone changes in Thoroughbred racehorses with back pain. Animals 2023, 13, 794. [Google Scholar] [CrossRef]
- Howell, K.; Dudek, K.; Soroko, M. Thermal camera performance and image analysis repeatability in equine thermography. Infrared Phys. Technol. 2020, 110, 103447. [Google Scholar] [CrossRef]
- Soroko, M.; Howell, K.; Dudek, K.; Henklewski, R.; Zielińska, P. The influence of breed, age, gender, training level and ambient temperature on forelimb and back temperature in racehorses. Anim. Sci. J. 2017, 88, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Varcoe-Cocks, K.; Sagar, K.N.; Jeffcott, L.B.; McGowan, C.M. Pressure algometry to quantify muscle pain in racehorses with suspected sacroiliac dysfunction. Equine Vet. J. 2006, 38, 558–562. [Google Scholar] [CrossRef]
- Kisch, T.; Wuerfel, W.; Forstmeier, V.; Liodaki, E.; Stang, F.H.; Knobloch, K.; Kraemer, R. Repetitive shock wave therapy improves muscular microcirculation. J. Surg. Res. 2016, 201, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Romeo, P.; Lavanga, V.; Pagani, D.; Sansone, V. Extracorporeal shock wave therapy in musculoskeletal disorders: A review. Med. Princ. Pract. 2014, 23, 7–13. [Google Scholar] [CrossRef]
- Love, T.J. Thermography as an indicator of blood perfusion. Ann. N. Y. Acad. 1980, 335, 429–437. [Google Scholar] [CrossRef]
- Imboden, I.; Waldern, N.M.; Wiestner, T.; Lischer, C.J.; Ueltschi, G.; Weishaupt, M.A. Short term analgesic effect of extracorporeal shock wave therapy in horses with proximal palmar metacarpal/plantar metatarsal pain. Vet. J. 2009, 179, 50–59. [Google Scholar] [CrossRef]
- Park, K.D.; Lee, W.Y.; Park, M.H.; Ahn, J.K.; Park, Y. High-versus low-energy extracorporeal shock-wave therapy for myofascial pain syndrome of upper trapezius: A prospective randomized single blinded pilot study. Medicine 2018, 97, e11432. [Google Scholar] [CrossRef]
- Moon, S.W.; Kim, J.H.; Jung, M.J.; Son, S.; Lee, J.H.; Shin, H.; Oh, M.K. The effect of extracorporeal shock wave therapy on lower limb spasticity in subacute stroke patients. Ann. Rehabil. Med. 2013, 37, 461–470. [Google Scholar] [CrossRef]
- Park, S.K.; Yang, D.J.; Uhm, Y.H.; Yoon, J.H.; Kim, J.H. Effects of extracorporeal shock wave therapy on upper extremity muscle tone in chronic stroke patients. J. Phys. Ther. Sci. 2018, 30, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Santamato, A.; Beatrice, R.; Micello, M.F.; Fortunato, F.; Panza, F.; Bristogiannis, C.; Ranieri, M. Power doppler ultrasound findings before and after focused extracorporeal shock wave therapy for achilles tendinopathy: A pilot study on pain reduction and neovascularization effect. Ultrasound Med. Biol. 2019, 45, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Di Renzo, A.; Errichi, B.M.; Cacchio, M.; Acerbi, G. Effects of shock waves on microcirculation, perfusion, and pain management in critical limb ischemia. Angiology 2005, 56, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Ranner, W.; Gerhards, H. DiagnostikbeiVerdacht auf Rückenerkrankungenbeim. Pferd. Pferdeheilkunde 2001, 17, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Merrifield-Jones, M.; Tabor, G.; Williams, J. Inter-and intra-rater reliability of soft tissue palpation scoring in the equine thoracic epaxial region. J. Equine Vet. Sci. 2019, 83, 102812. [Google Scholar] [CrossRef] [PubMed]
| Time | Group | |
|---|---|---|
| Study | rESWT-Sham | |
| Before rESWT, rho (95% CI) | 0.427 (−0.194; 0.804) | −0.256 (−0.724; 0.373) |
| After rESWT, rho (95% CI) | 0.467 (−0.145; 0.821) | 0.258 (−0.371; 0.725) |
| Total, rho (95% CI) | 0.350 (−0.062; 0.660) | −0.102 (−0.485; 0.314) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śniegucka, K.; Soroko-Dubrovina, M.; Zielińska, P.; Dudek, K.; Žuffová, K. The Effect of Radial Extracorporeal Shock Wave Therapy (rESWT) on the Skin Surface Temperature of the Longissimus Dorsi Muscle in Clinically Healthy Racing Thoroughbreds: A Preliminary Study. Animals 2023, 13, 2028. https://doi.org/10.3390/ani13122028
Śniegucka K, Soroko-Dubrovina M, Zielińska P, Dudek K, Žuffová K. The Effect of Radial Extracorporeal Shock Wave Therapy (rESWT) on the Skin Surface Temperature of the Longissimus Dorsi Muscle in Clinically Healthy Racing Thoroughbreds: A Preliminary Study. Animals. 2023; 13(12):2028. https://doi.org/10.3390/ani13122028
Chicago/Turabian StyleŚniegucka, Karolina, Maria Soroko-Dubrovina, Paulina Zielińska, Krzysztof Dudek, and Kristína Žuffová. 2023. "The Effect of Radial Extracorporeal Shock Wave Therapy (rESWT) on the Skin Surface Temperature of the Longissimus Dorsi Muscle in Clinically Healthy Racing Thoroughbreds: A Preliminary Study" Animals 13, no. 12: 2028. https://doi.org/10.3390/ani13122028
APA StyleŚniegucka, K., Soroko-Dubrovina, M., Zielińska, P., Dudek, K., & Žuffová, K. (2023). The Effect of Radial Extracorporeal Shock Wave Therapy (rESWT) on the Skin Surface Temperature of the Longissimus Dorsi Muscle in Clinically Healthy Racing Thoroughbreds: A Preliminary Study. Animals, 13(12), 2028. https://doi.org/10.3390/ani13122028





