Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 40 Non-Lame Thoroughbred Yearlings
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
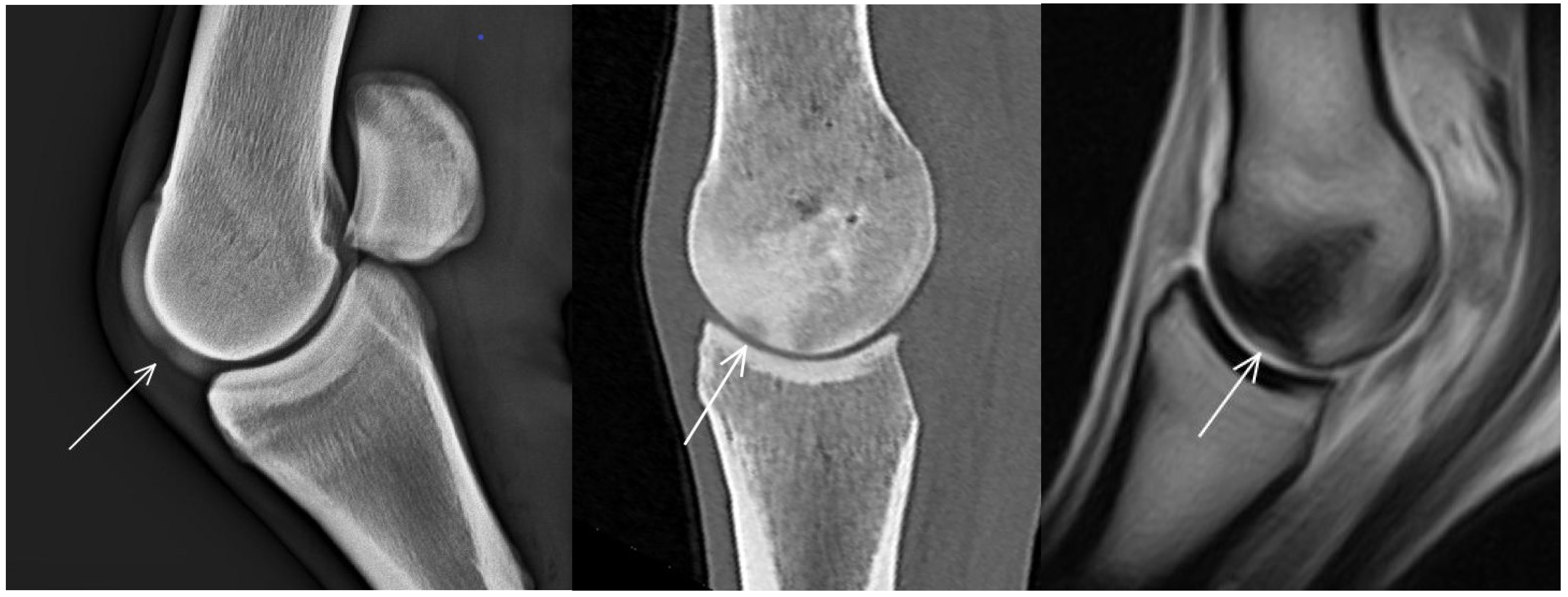
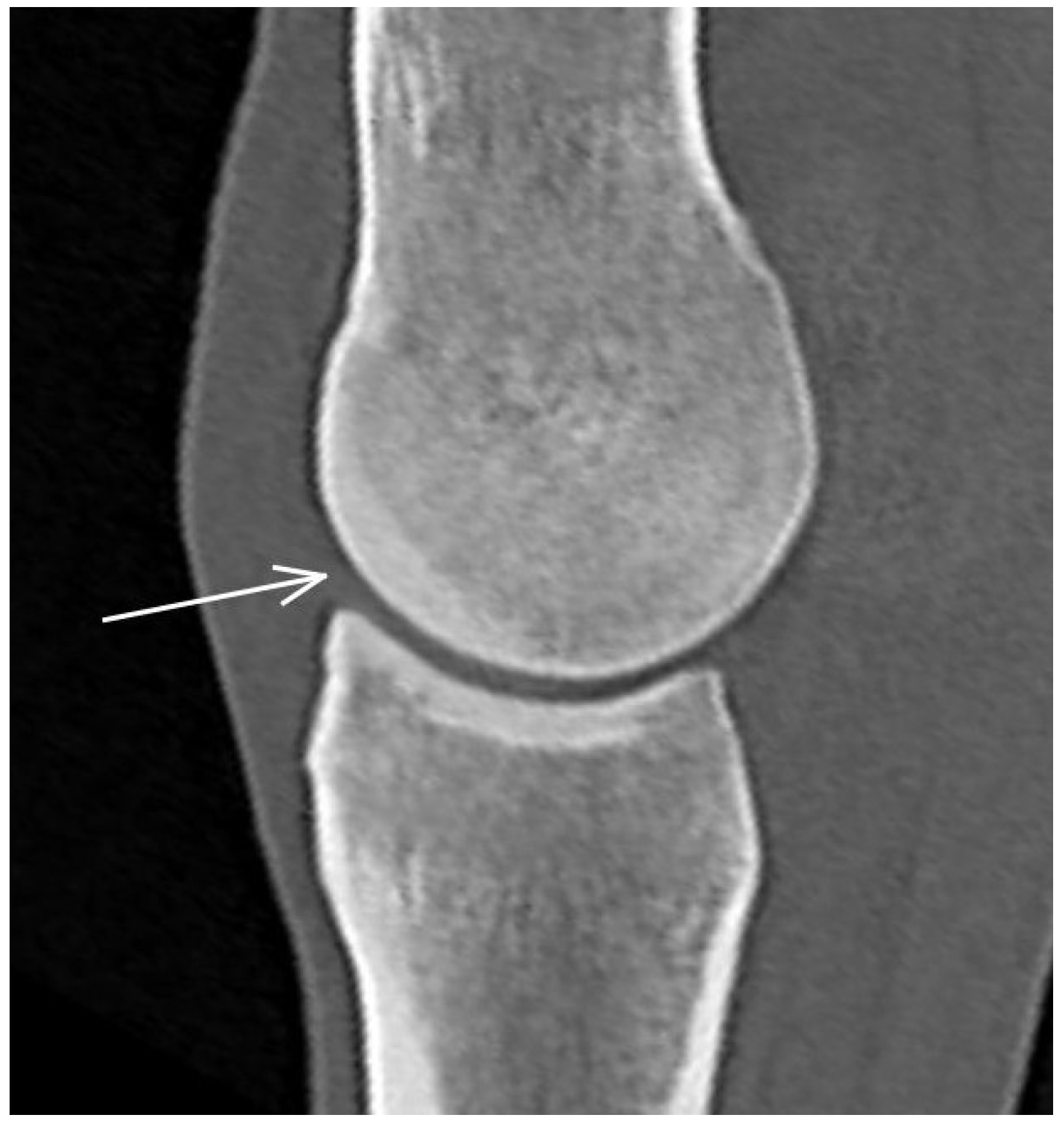
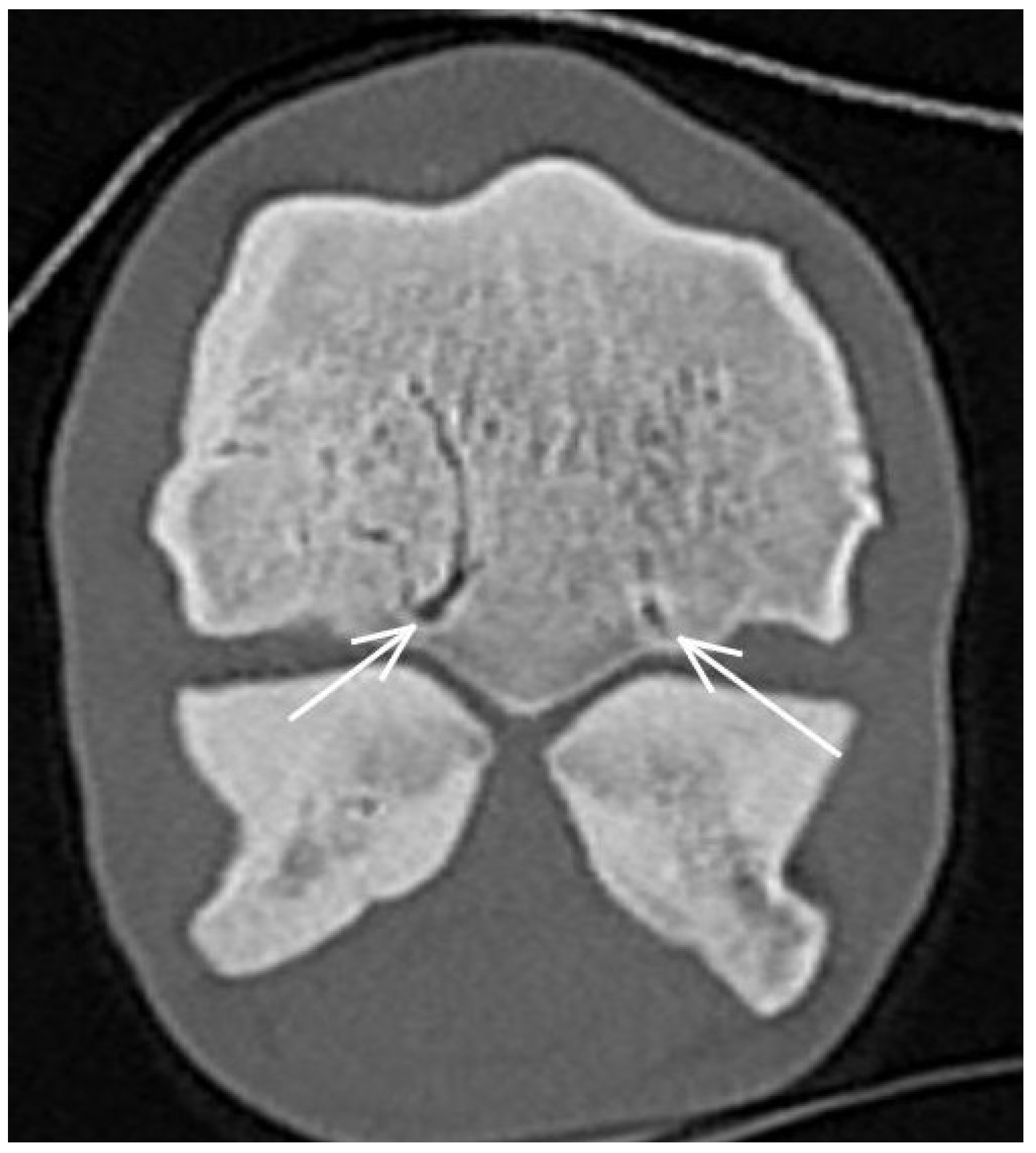
3.1. The Third Metacarpal Bone (McIII)
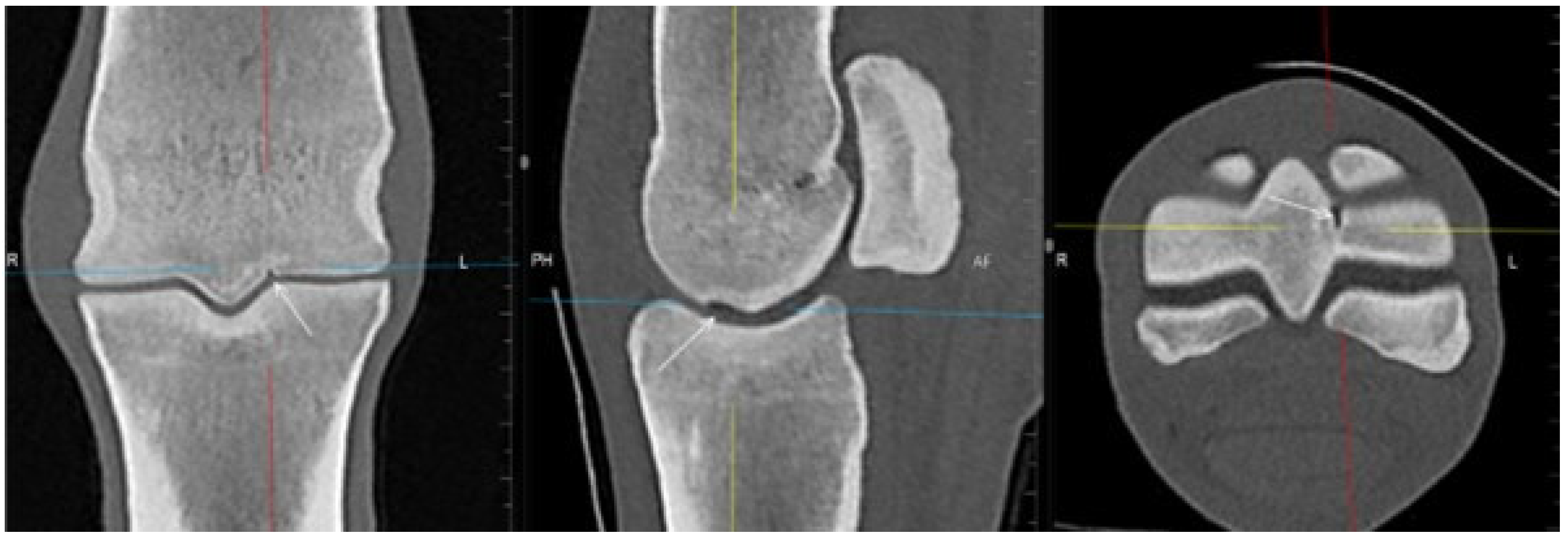
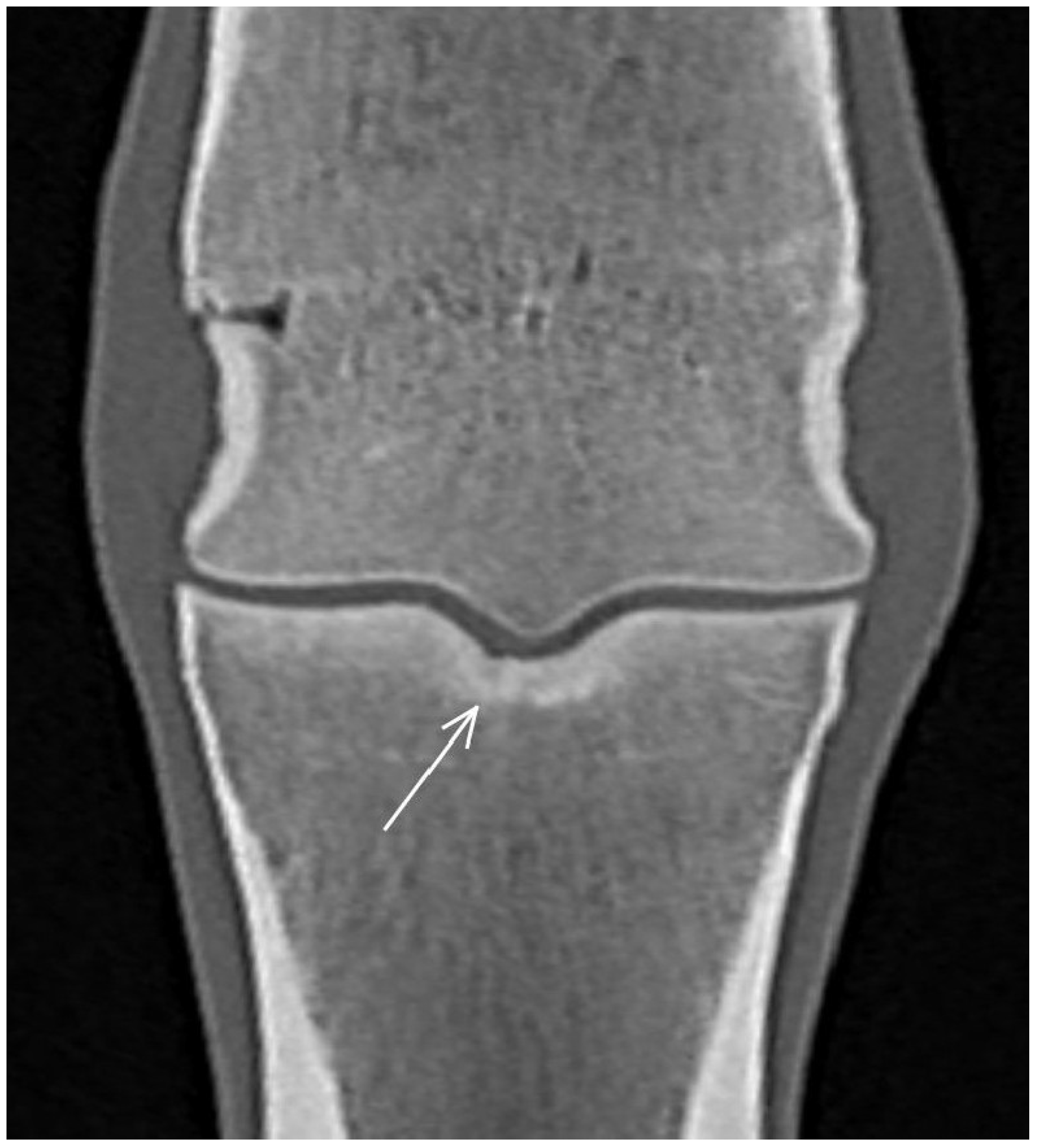
3.2. The Proximal Phalanx
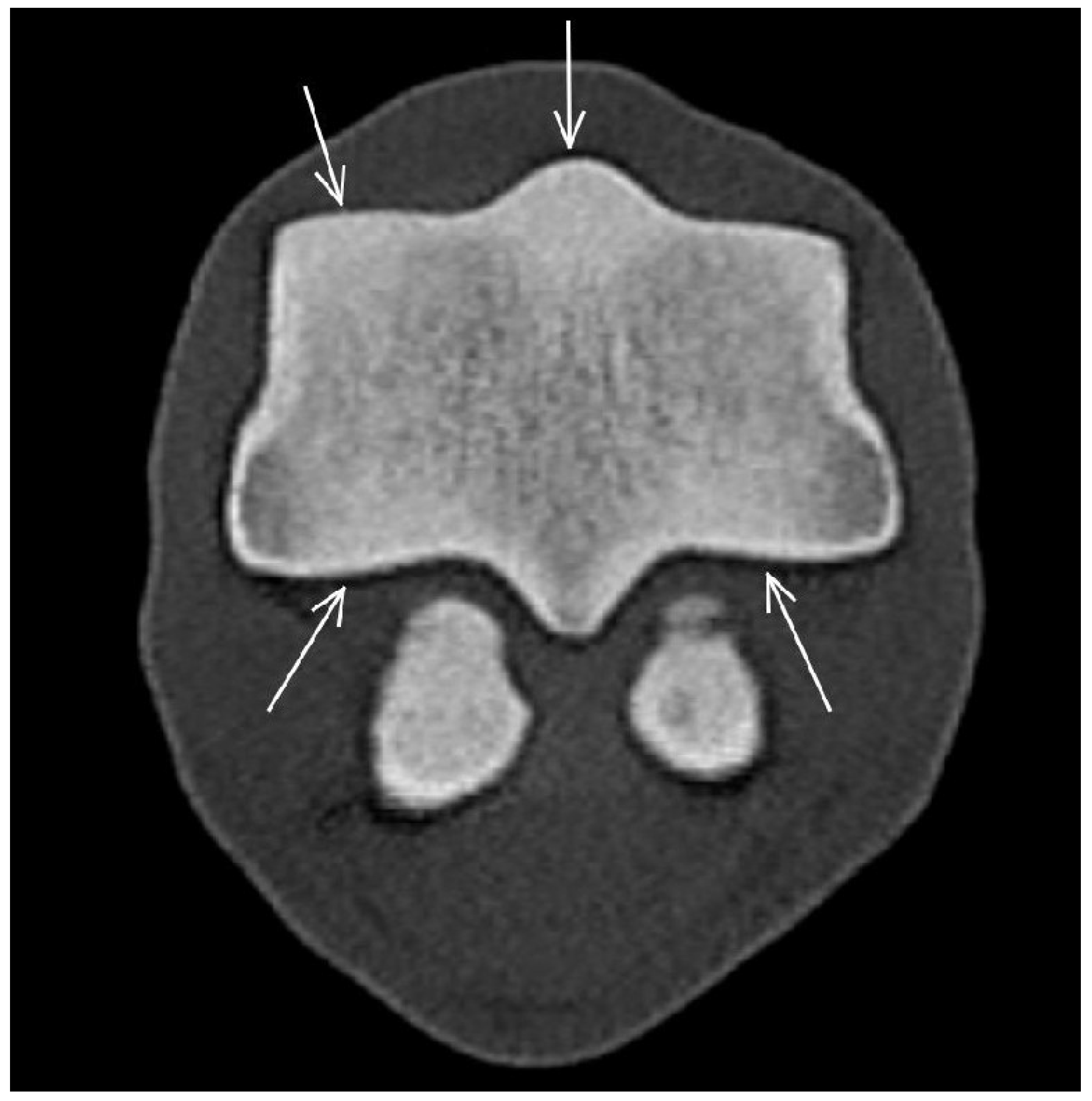
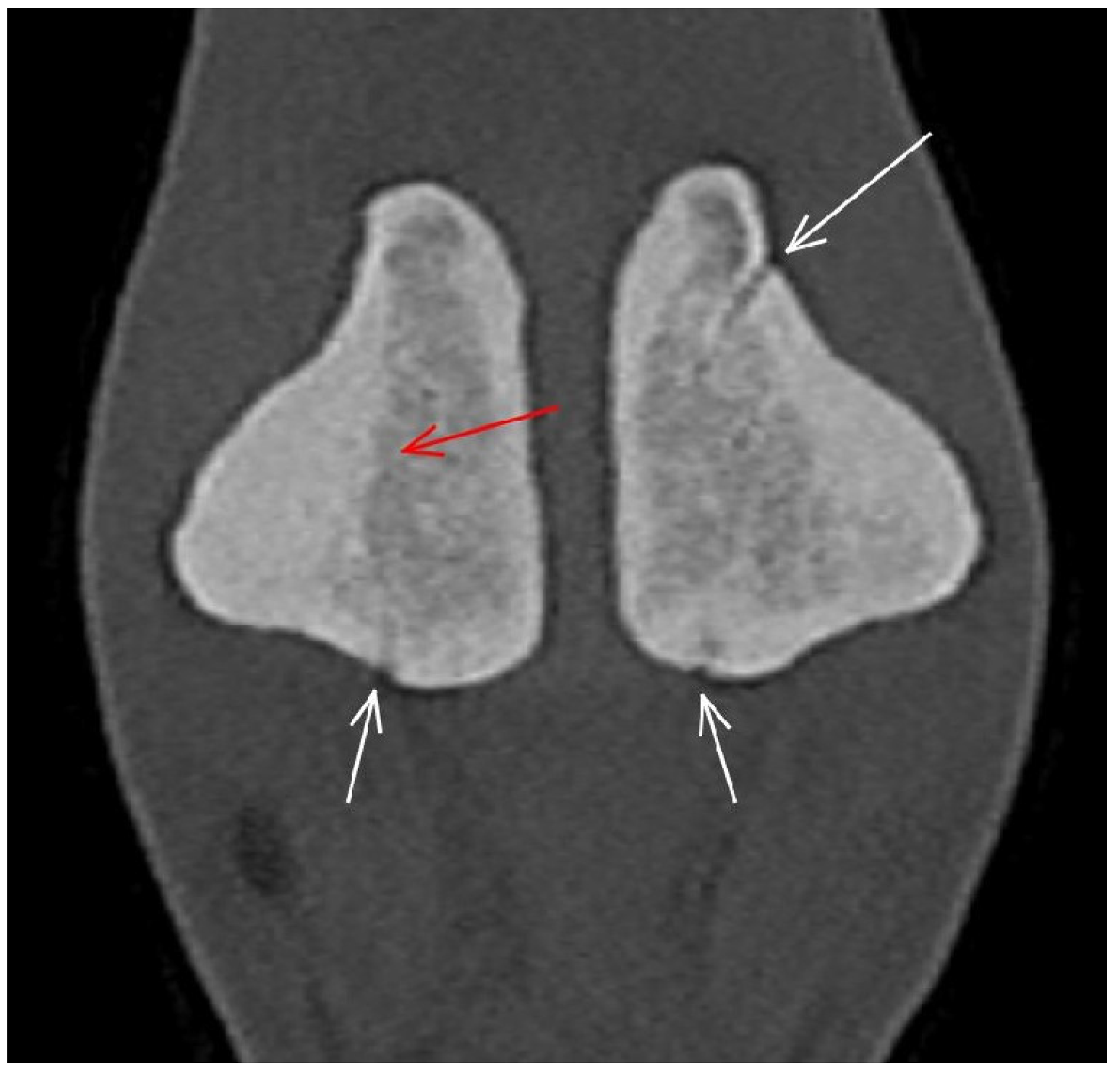
3.3. The Proximal Sesamoid Bones (PSBs)
3.4. Soft Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, D.W.; Dyson, S.J. The Metacarpophalangeal Joint. In Diagnosis and Management of Lameness in the Horse; Ross, M.W., Dyson, S.J., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 394–410. [Google Scholar]
- Denoix, J.-M.; Coudry, V. Clinical insights: Imaging of the equine fetlock in Thoroughbred racehorses: Identification of imaging changes to predict catastrophic injury. Equine Vet. J. 2020, 52, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Stover, S.M.; Daft, B.M.; Kinde, H.; Read, D.H.; Bar, B.C.; Anderson, M.; Moore, J.; Woods, J.; Stoltz, J.; et al. Causes of death in racehorses over a 2 year-period. Equine Vet. J. 1994, 26, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Parkin, T.D.H.; French, N.P.; Riggs, C.M.; Morgan, K.L.; Clegg, P.D.; Proudman, C.J.; Singer, E.L.; Webbon, P.M. Risk of fatal distal limb fractures among Thoroughbreds involved in the five types of racing in the United Kingdom. Vet. Rec. 2004, 154, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, K.L.P.; Wood, J.L.N. Descriptive epidemiology of fractures occurring in British Thoroughbred racehorses in training. Equine Vet. J. 2004, 36, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Whitton, R.C.; Trope, G.D.; Ghasem-Zadeh, A.; Anderson, G.A.; Parkin, T.D.; Mackie, E.J.; Seeman, E. Third metacarpal condylar fatigue fractures in equine athletes occur within previously modelled subchondral bone. Bone 2010, 47, 826–831. [Google Scholar] [CrossRef]
- Loughridge, A.B.; Mess, A.M.; Parkin, T.D.; Kawcak, C.E. Qualitative assessment of bone density at the distal articulating surface of the third metacarpal in Thoroughbred racehorses with and without condylar fracture. Equine Vet. J. 2017, 49, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Tranquille, C.A.; Murray, R.C.; Parkin, T.D.H. Can we use subchondral bone thickness on high-field magnetic resonance images to identify Thoroughbred racehorses at risk of catastrophic lateral condylar fracture? Equine Vet. J. 2017, 49, 167–171. [Google Scholar] [CrossRef]
- Peloso, J.G.; Cohen, N.D.; Vogler, J.B.; Marquis, P.A.; Hilt, L. Association of catastrophic condylar fracture with bony changes of the third metacarpal bone identified by use of standing magnetic resonance imaging in forelimbs from cadavers of Thoroughbred racehorses in the United States. Am. J. Vet. Res. 2019, 80, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, E.N.; Ruspi, B.D.; Wollman, C.W.; Peal, B.T.; Deng, S.; Toler, A.B.; McDonough, S.P.; Palmer, S.E.; Reesink, H.L. Determination of correlation of proximal sesamoid bone osteoarthritis with high-speed furlong exercise and catastrophic sesamoid bone fracture in Thoroughbred racehorses. Am. J. Vet. Res. 2021, 82, 467–477. [Google Scholar] [CrossRef]
- Shaffer, S.K.; Celeste, T.; Garcia, T.C.; Fyhrie, D.P.; Uzal, F.A.; Stover, S.M. Subchondral focal osteopenia with proximal sesamoid bone fracture in Thoroughbred racehorses. Equine Vet. J. 2021, 53, 294–305. [Google Scholar] [CrossRef]
- Kane, A.J.; Park, R.D.; McIlwraith, C.W.; Rantanen, N.W.; Morehead, J.P.; Bramlage, L.R. Radiographic changes in Thoroughbred yearlings. Part 1: Prevalence at the time of the yearling sales. Equine Vet. J. 2003, 35, 354–365. [Google Scholar] [CrossRef]
- Howard, B.A.; Embertson, R.M.; Rantanen, N.W.; Bramlage, L.R. Survey radiographic findings in Thoroughbred sale yearlings. In Proceedings of the Annual Convention of the American Association of Equine Practitioners, Orlando, FL, USA, 18–22 November 1992; Volume 38, pp. 397–402. [Google Scholar]
- Peat, F.; Kawcak, C.; McIwraith, W.; Berk, J.; Keenon, J.; Mork, D. Radiological findings in the proximal sesamoid bones of yearlings and 2-year-old Thoroughbred sales horses. In Proceedings of the American Association of Equine Practitioners, San Antonio, TX, USA, 18–22 November 2022; Volume 68, pp. 426–427. [Google Scholar]
- Ramzan, P.; Powell, S. Clinical and imaging features of suspected prodromal fracture of the proximal phalanx in three Thoroughbred racehorses. Equine Vet. J. 2010, 42, 164–169. [Google Scholar] [CrossRef]
- Ramzan, P.H.; Palmer, L.; Powell, S.E. Unicortical condylar fracture of the Thoroughbred fetlock: 45 cases (2006–2013). Equine Vet. J. 2014, 47, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, A.L.; Ortved, K.F.; Dallap-Schaer, B.; Gouzeev, S.; Stefanovski, D.; Richardson, D.W.; Wulster, K.B. Validation of standing cone beam computed tomography for diagnosing subchondral fetlock pathology in the Thoroughbred racehorse. Equine Vet. J. 2021, 53, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, E.N.; McDonough, S.P.; Palmer, S.E.; Hernandez, C.J.; Reesink, H.L. Can quantitative computed tomography detect bone morphological changes associated with catastrophic proximal sesamoid bone fracture in Thoroughbred racehorses? Equine Vet. J. 2019, 51, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mageed, M. Standing computed tomography of the equine limb using a multi-slice helical scanner: Technique and feasibility study. Equine Vet. Educ. 2022, 34, 77–83. [Google Scholar] [CrossRef]
- Brounts, S.H.; Lund, J.R.; Whitton, R.C.; Ergun, D.L.; Muir, P. Use of a novel helical fan beam imaging system for computed tomography of the distal limb in sedated standing horses: 167 cases (2019–2020). J. Am. Vet. Med. Assoc. 2022, 22, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- McKnight, A.L. Digital radiography in equine practice. Clin. Tech. Equine Pract. 2004, 3, 352–360. [Google Scholar] [CrossRef]
- Hopper, B.J.; Steel, C.; Richardson, J.L.; Alexander, G.R.; Robertson, I.D. Radiographic evaluation of sclerosis of the third carpal bone associated with exercise and the development of lameness in Standardbred racehorses. Equine Vet. J. 2004, 34, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Firth, E.C.; Rogers, C.W. Musculoskeletal responses of 2-year-old Thoroughbred horses to early training. 7. Bone and articular cartilage response in the carpus. N. Z. Vet. J. 2005, 53, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dykgraaf, S.; Firth, E.C.; Rogers, C.W.; Kawcak, C.E. Effects of exercise on chondrocyte viability and subchondral bone sclerosis in the distal third metacarpal and metatarsal bone of young horses. Vet. J. 2008, 178, 53–61. [Google Scholar] [CrossRef]
- Noordwijk, K.J.; Chen, L.; Ruspi, B.D.; Schurer, S.; Papa, B.; Fasanello, D.C.; McDonough, S.P.; Palmer, S.E.; Porter, I.R.; Basran, P.S.; et al. Metacarpophalangeal joint pathology and bone mineral density increase with exercise but not with incidence of proximal sesamoid bone fracture in Thoroughbred racehorses. Animals 2023, 13, 827. [Google Scholar] [CrossRef]
- Firth, E.C.; Rogers, C.W.; van Weeren, R.P.; Barneveld, A.; McIlwraith, C.W.; Kawcak, C.E.; Goodship, A.E.; Smith, R.K.W. Mild exercise early in life produces changes in bone size and strength but not in density in the proximal phalangeal, third metacarpal and third carpal bones of foals. Vet. J. 2011, 190, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Martig, S.; Hitchens, P.L.; Stevenson, M.A.; Whitton, R.C. Subchondral bone morphology in the metacarpus of racehorses in training changes with distance from the articular surface but not with age. J. Anat. 2018, 232, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Lipreri, G.; Bladon, B.M.; Giorio, M.E.; Singer, E.R. Conservative versus surgical treatment of 21 sports horses with osseous trauma in the proximal phalangeal sagittal groove diagnosed by low-field MRI. Vet. Surg. 2018, 47, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-T.; Foote, A.K.; Bolas, N.M.; Peter, V.G.; Pokora, R.; Patrick, H.; Sargan, D.R.; Murray, R.C. Three-dimensional imaging and histopathological features of third metacarpal/tarsal parasagittal groove and proximal phalanx sagittal groove fissures in Thoroughbred horses. Animals 2023, 13, 2912. [Google Scholar] [CrossRef] [PubMed]
- Olive, J.; D’Anjou, J.O.; Girard, C.; Laverty, S.; Theoret, C.L. Imaging and histological features of central subchondral osteophytes in racehorses with metacarpophalangeal joint osteoarthritis. Equine Vet. J. 2009, 41, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Spike, D.; Bramlage, L.R.; Howard, B.A.; Embertson, R.M.; Hance, S.R. Radiographic proximal sesamoiditis in Thoroughbred Sales Yearlings. In Proceedings of the American Association of Equine Practitioners, Phoenix, TX, USA, 7–10 December 1997; Volume 43, pp. 132–133. [Google Scholar]
- Ramzan, P.; Palmer, L.; Dallas, R.; Shepherd, M. Subclinical ultrasonographic abnormalities of the suspensory ligament branch of the athletic horse: A survey of 60 Thoroughbred racehorses. Equine Vet. J. 2013, 45, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Fairburn, A.; Busschers, V.; Barr, A. Subclinical ultrasonographic abnormalities of the suspensory ligament branches in National Hunt horses. Equine Vet. J. 2017, 49, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Dyson, S.J.; Murray, R.C. Magnetic resonance imaging of distal sesamoidean ligament injury. Vet. Radiol. Ultrasound 2008, 49, 516–528. [Google Scholar] [CrossRef]



| Pulse Sequence | TE (ms) | TR (ms) | Flip Angle (°) | Slice Thickness (mm) | Slice Gap (mm) | Matrix Size (FE × PE) | FOV (mm) |
|---|---|---|---|---|---|---|---|
| Pilot | 7 | 66 | 45 | 7 | 0 | 150 × 120 | 220 |
| Pilot of a pilot | 7 | 66 | 45 | 7 | 0 | 150 × 120 | 220 |
| STIR TEST | 22 | 2100 | 50/60/85/110 | 4 | 0.8 | 256 × 144 | 200 |
| T1W GRE MI | 8 | 50 | 55 | 5 | 1 | 170 × 130 | 170 |
| T2*W GRE MI | 13 | 68 | 25 | 5 | 1 | 340 × 160 | 170 |
| T2W FSE FAST | 88 | 1544 | 90 | 5 | 1 | 168 × 168 | 170 |
| STIR FSE FAST | 22 | 2336 | 95 | 5 | 1 | 168 × 168 | 170 |
| Region | CT | MRI | Radiography |
|---|---|---|---|
| McIII Sagittal ridge | |||
| Hypoattenuating lesion in the dorsal subchondral bone Subchondral bone thickening Dorsal/palmar half Increased attenuation in the trabecular bone Dorsal/palmar half Cone shaped/patchy Focal separated hyperattenuation | Hyperintense signal in the dorsal subchondral bone Subchondral bone thickening Dorsal/palmar half Decreased signal intensity in the trabecular bone Dorsal/palmar half Cone shaped/patchy Focal separated intermediate/low signal intensity | Radiolucent lesion Subchondral bone thickening Dorsal Increased trabecular bone opacity | |
| Medial/lateral condyle | |||
| Subchondral bone thickening Dorsal/palmar half Increased attenuation in the trabecular bone Dorsal/palmar half Hypoattenuating lesion in the subchondral bone Location | Subchondral bone thickening Dorsal/palmar half Decreased signal intensity in the trabecular bone Dorsal/palmar half Increased signal intensity in the subchondral bone Location | Increased opacity in the trabecular bone Lucent lesion in the subchondral bone Location | |
| Proximal phalanx Sagittal groove | |||
| Subchondral bone thickening Dorsal/middle/palmar third Increased attenuation in the trabecular bone Hypoattenuating lesion in the subchondral bone Location | Subchondral bone thickening Dorsal/middle/palmar third Decreased signal intensity in the trabecular bone Increased signal intensity in the subchondral bone Location | Subchondral bone thickening Increased opacity in the trabecular bone Lucent lesion in the subchondral bone Location | |
| Medial/lateral glenoid | |||
| Subchondral bone thickening Dorsal/middle/palmar third Increased attenuation in the trabecular bone Hypoattenuating lesion in the subchondral bone Location Periarticular modelling | Subchondral bone thickening Dorsal/middle/palmar third Decreased signal intensity in the trabecular bone Increased signal intensity in the subchondral bone Location Periarticular modelling | Subchondral bone thickening Increased opacity in the trabecular bone Lucent lesion in the subchondral bone Location Periarticular modelling | |
| Proximal sesamoid bone Medial/Lateral | |||
| Prominent vascular channels Thickening of axial/abaxial compact bone Increased attenuation in trabecular bone Modelling | Thickening of axial/abaxial compact bone Decreased signal intensity in trabecular bone Modelling | Prominent vascular channels Modelling | |
| Suspensory ligament branches | Asymmetry in size between medial and lateral branches Increased attenuation Enlargement of cross-sectional area | N/A | N/A |
| Oblique sesamoidean ligaments | Asymmetry in size between medial and lateral ligaments Increased attenuation Enlargement of cross-sectional area | Asymmetry in size between medial and lateral ligaments Increased signal intensity Enlargement of cross-sectional area | N/A |
| Other soft tissues abnormality | Increased attenuation Enlargement of cross-sectional area Abnormal shape | Increased signal intensity Enlargement of cross-sectional area Abnormal shape | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, A.; Boros, K.; Dyson, S. Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 40 Non-Lame Thoroughbred Yearlings. Animals 2023, 13, 3466. https://doi.org/10.3390/ani13223466
Nagy A, Boros K, Dyson S. Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 40 Non-Lame Thoroughbred Yearlings. Animals. 2023; 13(22):3466. https://doi.org/10.3390/ani13223466
Chicago/Turabian StyleNagy, Annamaria, Koppány Boros, and Sue Dyson. 2023. "Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 40 Non-Lame Thoroughbred Yearlings" Animals 13, no. 22: 3466. https://doi.org/10.3390/ani13223466
APA StyleNagy, A., Boros, K., & Dyson, S. (2023). Magnetic Resonance Imaging, Computed Tomographic and Radiographic Findings in the Metacarpophalangeal Joints of 40 Non-Lame Thoroughbred Yearlings. Animals, 13(22), 3466. https://doi.org/10.3390/ani13223466





