The Outcomes of an Opioid-Free Anaesthetic Plan in Fourteen Dogs Undergoing Enucleation Using an Ultrasound-Guided Supra-Temporal Retrobulbar Block: A Retrospective Case Series
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Cases Presentation
2.2. Treatment
| Case 1 12 Years Jack Russell Terrier, FN, 5.6 kg | Case 2 11 Years Jack Russel Terrier, M, 7.4 kg | Case 3 7 Years Alaskan Malamute, FN, 35 kg | Case 4 10 Years Cocker Spaniel, FN, 13.8 kg | Case 5 11 Years Shih Tzu X, FN, 12.6 kg | Case 6 6 Years Chihuahua, M, 4.2 kg | Case 7 7 Years Great Dane, FN, 53 kg | Case 8 13 Years Bearded Collie, FN, 22.9 kg | Case 9 1 Year Bull Mastiff, F, 49.5 kg | Case 10 12 Years English Springer Spaniel, MN, 21.8 kg | Case 11 6 Years Malti-Poo, MN, 7.3 kg | Case 12 8 Years Bassett, FN, 29.3 kg | Case 13 8 Years Maltese Terrier X, FN, 7.6 kg | Case 14 7 Years Springer X, M, 27.2 kg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procedure | Enucleation OD | Enucleation OS | Enucleation OD | Enucleation OS | Enucleation OS | Enucleation OD | Enucleation OS | Enucleation OS | Enucleation OS | Enucleation OS | Enucleation OD | Enucleation OS | Enucleation OD | Enucleation OD |
| Current medication | Omeprazole | Brinzolamide/Timolol Omeprazole | Paracetamol (10 mg/kg) PO Omeprazole | Omeprazole | Omeprazole | Omeprazole | Dorzolamide/ Timolol Omeprazole | Meloxicam PO Maropitant (1 mg/kg) IV Omeprazole | Omeprazole | Meloxicam PO Omeprazole | Omeprazole | Omeprazole | Phenobarbitone Levetiracetam Omeprazole | Meloxicam PO Omeprazole |
| Premedication | Medetomidine (0.01 mg/kg) IM Acepromazine (0.005 mg/kg) IM | Medetomidine (0.008 mg/kg) IM Acepromazine (0.005 mg/kg) IM | Acepromazine (0.005 mg/kg) IM Medetomidine (0.006 mg/kg) IM | Dexmedetomidine (0.001 mg/kg) IV Acepromazine (0.02 mg/kg) IV | Dexmedetomidine (0.001 mg/kg) IV Acepromazine (0.005 mg/kg) IV | Medetomidine (0.006 mg/kg) IM | Dexmedetomidine (0.001 mg/kg) IV | Acepromazine (0.005 mg/kg) IV | Dexmedetomidine (0.005 mg/kg) IM | Acepromazine (0.015 mg/kg) IV | Dexmedetomidine (0.005 mg/kg) IM Acepromazine (0.01 mg/kg) IM | Dexmedetomidine (0.005 mg/kg) IM Followed by another top up of (0.005 mg/kg) Dexmedetomidine IM | Dexmedetomidine (0.005 mg/kg) IM Acepromazine (0.01 mg/kg) IM | Medetomidine (0.01 mg/kg) IM Acepromazine (0.01 mg/kg) IM |
| Induction | Alfaxalone (1 mg/kg) IV | Alfaxalone (1.5 mg/kg) IV | Propofol (2 mg/kg) IV Ketamine (1 mg/kg) IV | Alfaxalone (1.5 mg/kg) IV | Alfaxalone (1.5 mg/kg) IV | Alfaxalone (1.5 mg/kg) IV | Propofol (2 mg/kg) IV Ketamine (1 mg/kg) IV | Propofol (2 mg/kg) IV Ketamine (1 mg/kg) IV | Propofol (1.5 mg/kg) IV Ketamine (1 mg/kg) IV | Alfaxalone (1 mg/kg) IV Midazolam (0.3 mg/kg) IV | Alfaxalone (1 mg/kg) IV | Propofol (1 mg/kg) IV Ketamine (1 mg/kg) IV | Propofol (2 mg/kg) IV | Alfaxalone (1 mg/kg) IV |
| Maintenance | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen | Sevoflurane delivered in 100% oxygen |
| Locoregional anaesthesia | US Guided RBB Ropivacaine 0.1 mL × Skull Length = 0.9 mL Eq to 0.16 mL/kg | US Guided RBB Ropivacaine 0.1 mL × Skull Length = 0.85 mL Eq to 0.11 mL/kg | US Guided RBB Ropivacaine 0.1 mL × Skull Length = 1.1 mL Eq to 0.03 mL/kg | US Guided RBB Ropivacaine 0.1 mL × Skull Length = 1 mL Eq to 0.07 mL/kg | US Guided RBB Ropivacaine 0.1 mL × Skull Length = 0.67 mL Eq to 0.05 mL/kg | US Guided RBB Ropivacaine 0.1 mL × Skull Length = 0.65 mL Eq to 0.15 mL/kg | US Guided RBB Ropivacaine 0.1 mL × Skull Length = 1.4 mL Eq to 0.02 mL/kg | US Guided RBB Ropivacaine 0.1 mL × Skull Length = 1 mL Eq to 0.04 mL/kg | US Guided RBB Ropivacaine 0.1 mL/kg = 4 mL | US Guided RBB Ropivacaine 0.1 mL/kg = 2.1 mL | US Guided RBB Ropivacaine 0.1 mL/kg = 0.73 mL | US Guided RBB Ropivacaine 0.1 mL/kg = 3 mL | US Guided RBB Ropivacaine 0.1 mL/kg = 0.76 mL | US Guided RBB Ropivacaine 0.1 mL/kg =2.7 mL |
| Intra-op medication | None | None | None | None | None | Fentanyl (2 mcg/kg) IV Ketamine (0.5 mg/kg) IV | None | Medetomidine (0.002 mg/kg) IV | None | None | Ketamine (0.5 mg/kg) IV | None | None | None |
| Post-op medication | Meloxicam IV Cefalexin PO | Meloxicam IV Cefalexin PO | Meloxicam IV Cefalexin PO | Meloxicam IV Cefalexin PO | Meloxicam IV Cefalexin PO | Meloxicam IV Cefalexin PO | Meloxicam IV Cefalexin PO | Meloxicam IV Cefalexin PO | Meloxicam IV Cefalexin PO | Meloxicam IV Cefalexin PO | Maropitant IV Meloxicam IV Cefalexine PO | Meloxicam IV Cefalexin PO | Maropitant IV Meloxicam IV Cefalexine PO | Meloxicam PO Cefalexine PO Brinzolamide drops OS |
| Pain score 1 h post recovery | 4/24 | 4/24 | 1/24 | 4/24 | 1/24 | 2/24 | 2/24 | 3/24 | 3/24 | 2/24 | 3/24 | 0/24 | 1/24 | 1/24 |
| Pain score 6 h post RBB | 2/24 | 3/24 | 1/24 | 4/24 | 4/24 | 3/24 | 3/24 | 2/24 | 2/24 | 2/24 | 3/24 | 0/24 | 3/24 | 3/24 |
| Pain score at discharge | 2/24 | 3/24 | 1/24 | / | 3/24 | / | 2/24 | 2/24 | / | 1/24 | 3/24 | 0/24 | / | / |
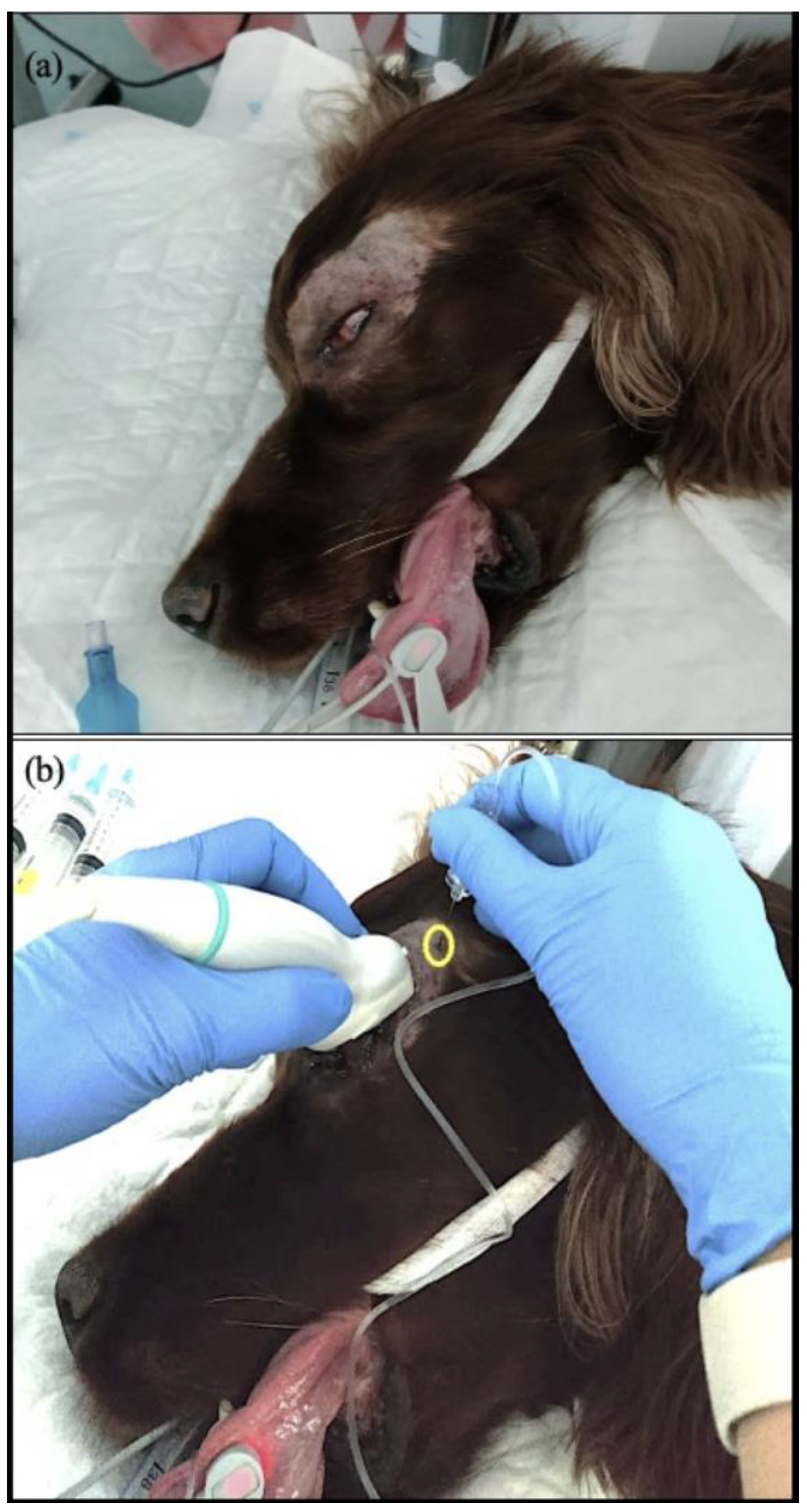
2.3. Retrobulbar Block
2.4. Surgery
2.5. Monitoring
2.6. Recovery and Postoperative Plan
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siu, E.Y.; Moon, T.S. Opioid-free and opioid-sparing anesthesia. Int. Anesth. Clin. 2020, 58, 34–41. [Google Scholar] [CrossRef]
- Ellis, T.A.; Narr, B.J.; Bacon, D.R. Developing a specialty: J.S. Lundy’s three major contributions to anesthesiology. J. Clin. Anesth. 2004, 16, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Baratta, J.L.; Heitz, J.W.; Schwenk, E.S.; Vaghari, B.; Viscusi, E.R. Acute pain management in the postanesthesia care unit. Anesth. Clin. 2012, 30, E1–E15. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, P.; Rodan, I.; Brunt, J.; Downing, R.; Hagedorn, J.; Robertson, S. AAHA/AAFP pain management guidelines for dogs and cats. J. Fel. Med. Surg. 2015, 51, 67–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, B.T.; Steagall, P.V. The present and future of opioid analgesics in small animal practice. J. Vet. Pharm. Ther. 2017, 40, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Torrecilla, M.; Marker, C.L.; Cintora, S.C.; Stoffel, M.; Williams, J.T.; Wickman, K. G-Protein-Gated Potassium Channels Containing Kir3.2 and Kir3.3 Subunits Mediate the Acute Inhibitory Effects of Opioids on Locus Ceruleus neurons. J. Neurosci. 2002, 22, 4328–4334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muir, W.W.; Wiese, A.J.; March, P.A. Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Am. J. Vet. Res. 2003, 64, 1155–1160. [Google Scholar] [CrossRef]
- Kona-Boun, J.-J.; Cuvelliez, S.; Troncy, E. Evaluation of epidural administration of morphine or morphine and bupivacaine for postoperative analgesia after premedication with an opioid analgesic and orthopedic surgery in dogs. J. Am. Vet. Med. Assoc. 2006, 229, 1103–1112. [Google Scholar] [CrossRef]
- Steagall, P.V.M.; Neto, F.J.T.; Minto, B.W.; Campagnol, D.; Correa, M. Evaluation of the isoflurane-sparing effects of lidocaine and fentanyl during surgery in dogs. J. Am. Vet. Med. Assoc. 2006, 229, 1103–1112. [Google Scholar] [CrossRef]
- White, D.M.; Mair, A.R.; Martinez-Taboada, F. Opioid-free anaesthesia in three dogs. Open. Vet. J. 2017, 7, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Ramsin, B.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.; Vallejo, R. Opioid Complications and Side Effects. Pain Phys. 2008, 11, 105–120. [Google Scholar]
- Kogan, L.; Hellyer, P.; Rishniw, M.; Schoenfeld-Tacher, R. The US opioid epidemic and its impact on us general practice veterinarian. Front. Vet. Sci. 2019, 6, 222. [Google Scholar] [CrossRef]
- Richman, J.M.; Liu, S.S.; Courpas, G.; Wong, R.; Rowlingson, A.J.; McGready, J.; Cohen, S.; Wu, C. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth. Analg. 2006, 102, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; Kirkby, S.K. An extended release local anaesthetic: Potential for future use in veterinary surgical patients? Vet. Med. Sci. 2016, 2, 229–238. [Google Scholar] [CrossRef] [PubMed]
- McAllister, A.S. A clinical review of orbital anatomy and its relevance to retrobulbar anaesthesia. Cureus 2013, 5, e97. [Google Scholar] [CrossRef] [Green Version]
- Chiavaccini, L.; Micieli, F.; Meomartino, L.; Duffee, L.R.; Vesce, G. A novel supra-temporal approach to retrobulbar anaesthesia in dogs: Preliminary study in cadavers. Vet. J. 2017, 223, 68–70. [Google Scholar] [CrossRef]
- Klaumann, P.R.; Moreno, J.C.D.; Montiani-Ferreira, F. A morphometric study of the canine skull and periorbita and its implications for regional ocular anesthesia. Vet. Ophthal. 2018, 21, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Reid, J.; Nolan, A.; Hughes, J.; Lascelles, D.; Pawson, P.; Scott, E. Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Anim. Welf. 2007, 16, 97–104. [Google Scholar] [CrossRef]
- Lee, B.H.; Kumar, K.K.; Wu, E.; Wu, C. Role of regional anesthesia and analgesia in the opioid epidemic. Reg. Anesth. Pain Med. 2019, 44, 492–493. [Google Scholar] [CrossRef]
- Martínez, M.I.G.; Fernández, M.Á.M. Opioid-free anaesthesia for the surgical correction of abnormalities associated with brachycephalic obstructive airway syndrome in five dogs. Comp. Anim. 2021, 26, 57–61. [Google Scholar] [CrossRef]
- Viscasillas, J.; Sanchis-Mora, S.; Burillo, P.; Esteve, V.; del Romero, A.; Lafuente, P.; Redondo, J. Evaluation of quadratus lumborum block as part of an opioid-free anaesthesia for canine ovariohysterectomy. Animals 2021, 11, 3424. [Google Scholar] [CrossRef] [PubMed]
- Ngoc Diep, T.; Monteiro, B.P.; Evangelista, M.C.; Balleydier, A.; Watanabe, R.; Ruel, H.L.; Doodnaught, G.; Le Quang, T.; Steagall, P. Article Anesthetic and analgesic effects of an opioid-free, injectable protocol in cats undergoing ovariohysterectomy: A prospective, blinded, randomized clinical trial. Can. Vet. J. 2020, 61, 621. [Google Scholar]
- Garbin, M.; Bertolizio, G.; Portela, D.A. Thoracic paravertebral block for an opioid-free thoracotomy in a dog. Vet. Anaesth. Analg. 2021, 48, 622–623. [Google Scholar] [CrossRef]
- Gomes, V.H.; Peixoto, A.J.; Edos, S.L.; Fernandes, M.; Oliveira, L.C.; Coelho, C.M.; Fada Silva, M. Effects of dissociative anesthesia opioid-free protocols combined with local anesthesia, with or without flumazenil or atipamezole postoperatively, for orchiectomy in cats. Vet. Anaesth. Analg. 2022, 49, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Geddes, A.T.; Stathopoulou, T.; Viscasillas, J.; Lafuente, P. Opioid-free anaesthesia (OFA) in a springer spaniel sustaining a lateral humeral condylar fracture undergoing surgical repair. Vet. Rec. Case Rep. 2019, 7, e000681. [Google Scholar] [CrossRef]
- Zannin, D.; Isaka, L.J.; Pereira, R.H.; Mencalha, R. Opioid-free total intravenous anesthesia with bilateral ultrasound-guided erector spinae plane block for perioperative pain control in a dog undergoing dorsal hemilaminectomy. Vet. Anaesth. Analg. 2020, 47, 728–731. [Google Scholar] [CrossRef]
- Libera, N.; McFadzean, W. An opioid-free anaesthesia (OFA) technique for dorsal laminectomy in a dog subsequent to severe bradycardia and hypothermia after previous subcutaneous methadone administration. Vet. Rec. Case Rep. 2022, 10, e287. [Google Scholar] [CrossRef]
- Bini, G.; Vettorato, E.; de Gennaro, C.; Corletto, F. A retrospective comparison of two analgesic strategies after uncomplicated tibial plateau levelling osteotomy in dogs. Vet. Anaesth. Analg. 2018, 45, 557–565. [Google Scholar] [CrossRef]
- Ryan, A.C.; Murrell, J.C.; Gurney, M.A. Post-operative nausea and vomiting (PONV) observed in a clinical study designed to assess the analgesic effects of intravenous and subcutaneous methadone in dogs. Vet. J. 2022, 287, 105876. [Google Scholar] [CrossRef]
- Downing, F.; Gibson, S. Anaesthesia of brachycephalic dogs. J. Small Anim. Pract. 2018, 59, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, E.R.; Figueroa, C.D.N.; Choma, J.C.; Campagnol, D.; Bettini, C.M. Effects of methadone, alone or in combination with acepromazine or xylazine, on sedation and physiologic values in dogs. Vet. Anaesth. Analg. 2008, 35, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Maiante, A.A.; Teixeira Neto, F.J.; Beier, S.L.; Corrente, J.E.; Pedroso, C.E.B.P. Comparison of the cardio-respiratory effects of methadone and morphine in conscious dogs. J. Vet. Pharm. Ther. 2009, 32, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Menegheti, T.M.; Wagatsuma, J.T.; Pacheco, A.D.; Perez, B.; Pacheco, C.M.; Abimussi, C.J.; dos Santos, P.; de Souza Oliva, V. Electrocardiographic evaluation of the degree of sedation and the isolated use of methadone in healthy dogs. Vet. Anaesth. Analg. 2014, 41, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.V.; Evans, A.T.; Miller, R.A. Effects of preanesthetic administration of morphine on gastroesophageal reflux and regurgitation during anesthesia in dogs. Am. J. Vet. Res. 2005, 66, 386–390. [Google Scholar] [CrossRef]
- Whitehead, K.; Cortes, Y.; Eirmann, L. Gastrointestinal dysmotility disorders in critically ill dogs and cats. J. Vet. Emerg. Crit. Care 2016, 26, 234–253. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.T.; Brown, D.C.; Silverstein, D.C. Early nutritional support is associated with decreased length of hospitalization in dogs with septic peritonitis: A retrospective study of 45 cases (2000-2009). J. Vet. Emerg. Crit. Care 2012, 22, 453–459. [Google Scholar] [CrossRef]
- Peterson, N.W.; Bergman, N.; Buote, P. Effect of epidural analgesia with opioids on the prevalence of urinary retention in dogs undergoing surgery for cranial cruciate ligament rupture. J. Am. Vet. Med. Ass. 2014, 244, 940–943. [Google Scholar] [CrossRef]
- Vasquez, E.J.; Kendall, A.; Musulin, S.; Vaden, S.L. Three-dimensional bladder ultrasound to measure daily urinary bladder volume in hospitalized dogs. J. Vet. Intern. Med. 2021, 35, 2256–2262. [Google Scholar] [CrossRef]
- Mayordomo-Febrer, A.; Rubio, M.; Martínez-Gassent, M.; López-Murcia, M.M. Effects of morphine-alfaxalone-midazolam premedication, alfaxalone induction and sevoflurane maintenance on intraocular pressure and tear production in dogs. Vet. Rec. 2017, 180, 474. [Google Scholar] [CrossRef]
- Romano, M.; Portela, D.A.; Breghi, G.; Otero, P.E. Stress-related biomarkers in dogs administered regional anesthesia or fentanyl for analgesia during stifle surgery. Vet. Anaesth. Analg. 2016, 43, 44–54. [Google Scholar] [CrossRef]
- Briley, J.; Chiavaccini, L.; Washington, D.; Posner, L.; Chiavaccini, L. Comparison of a blind and an ultrasound-guided technique for retrobulbar anesthesia in dogs undergoing unilateral subconjunctival enucleation. Vet. Ophthal. 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sites, B.D.; Brull, R. Ultrasound guidance in peripheral regional anesthesia: Philosophy, evidence-based medicine, and techniques. Curr. Opin. Anesth. 2006, 19, 630–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shilo-Benjamini, Y. A review of ophthalmic local and regional anesthesia in dogs and cats. Vet. Anaesth. Analg. 2019, 46, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Accola, P.J.; Bentley, E.; Smith, L.J.; Forrest, L.; Baumel, C. Development of a retrobulbar injection techniquefor ocular surgery and analgesia in dogs. J. Am. Vet. Med. Assoc. 2006, 229, 220–225. [Google Scholar] [CrossRef]
- Foster, A.; Medina-Serra, R.; Sanchis-Mora, S.; Plested, M.; Stathopoulou, T.R.; Viscasillas, J. In-plane ultrasound-guided peribulbar block in the dog: An anatomical cadaver study. Vet. Anaesth. Analg. 2021, 48, 272–276. [Google Scholar] [CrossRef]
- Vézina-Audette, R.; Steagall, P.V.M.; Gianotti, G. Prevalence of and covariates associated with the oculocardiac reflex occurring in dogs during enucleation. J. Am. Vet. Med. Assoc. 2019, 255, 454–458. [Google Scholar] [CrossRef]
- Bartholomew, K.J.; Smith, L.J.; Bentley, E.; Lasarev, M.R. Retrospective analysis of complications associated with retrobulbar bupivacaine in dogs undergoing enucleation surgery. Vet. Anaesth. Analg. 2020, 47, 588–594. [Google Scholar] [CrossRef]
- Wagatsuma, J.T.; Deschk, M.; Floriano, B.P.; Ferreira, J.; Fioravanti, H.; Gasparello, I.; Oliva, V. Comparison of anesthetic efficacy and adverse effects associated with peribulbar injection of ropivacaine performed with and without ultrasound guidance in dogs. Am. J. Vet. Res. 2014, 75, 1040–1048. [Google Scholar] [CrossRef]
- Hernandez-Avalos, I.; Mota-Rojas, D.; Mora-Medina, P.; Martinez-Burnes, J.; Alvarado, A.; Verduzco-Mendoza, A.; Lezama-Garcia, K.; Olmos-Hernandez, A. Review of different methods used for clinical recognition and assessment of pain in dogs and cats. Int. J. Vet. Sci. Med. 2019, 7, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citarella, G.; Corona, D.; Parsons, E.; Giannikaki, S.; Rioja, E. The Outcomes of an Opioid-Free Anaesthetic Plan in Fourteen Dogs Undergoing Enucleation Using an Ultrasound-Guided Supra-Temporal Retrobulbar Block: A Retrospective Case Series. Animals 2023, 13, 2059. https://doi.org/10.3390/ani13132059
Citarella G, Corona D, Parsons E, Giannikaki S, Rioja E. The Outcomes of an Opioid-Free Anaesthetic Plan in Fourteen Dogs Undergoing Enucleation Using an Ultrasound-Guided Supra-Temporal Retrobulbar Block: A Retrospective Case Series. Animals. 2023; 13(13):2059. https://doi.org/10.3390/ani13132059
Chicago/Turabian StyleCitarella, Gerardo, Daniele Corona, Eamonn Parsons, Stamatina Giannikaki, and Eva Rioja. 2023. "The Outcomes of an Opioid-Free Anaesthetic Plan in Fourteen Dogs Undergoing Enucleation Using an Ultrasound-Guided Supra-Temporal Retrobulbar Block: A Retrospective Case Series" Animals 13, no. 13: 2059. https://doi.org/10.3390/ani13132059








