Role of Macrophages in the Pathogenesis of Genotype VII Newcastle Disease Virus in Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses and Plasmid
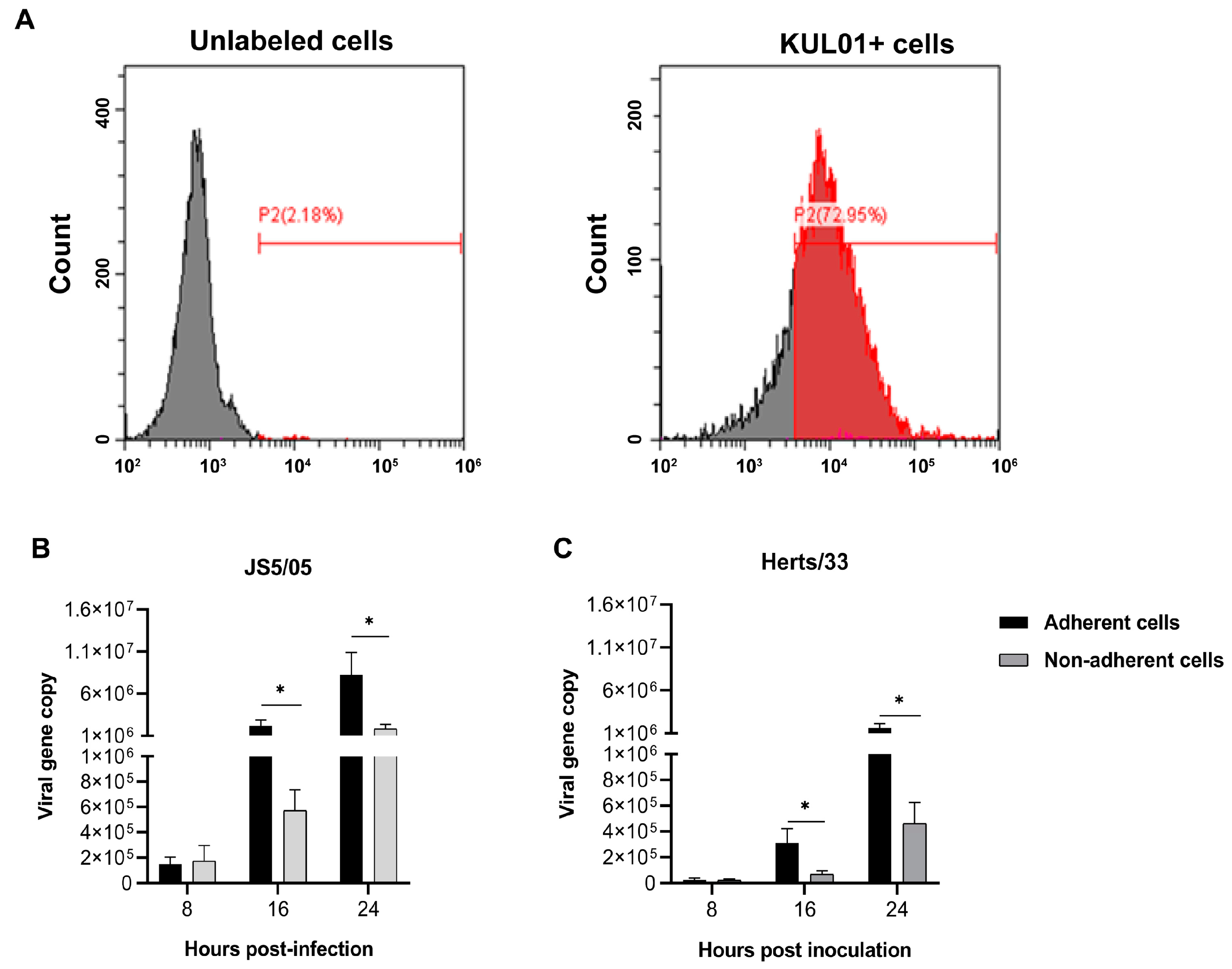
2.2. Isolation and Identification of Macrophages from PBMCs
2.3. In Vitro Infectivity of NDV in the Adherent and Non-Adherent Cells Derived from PBMC
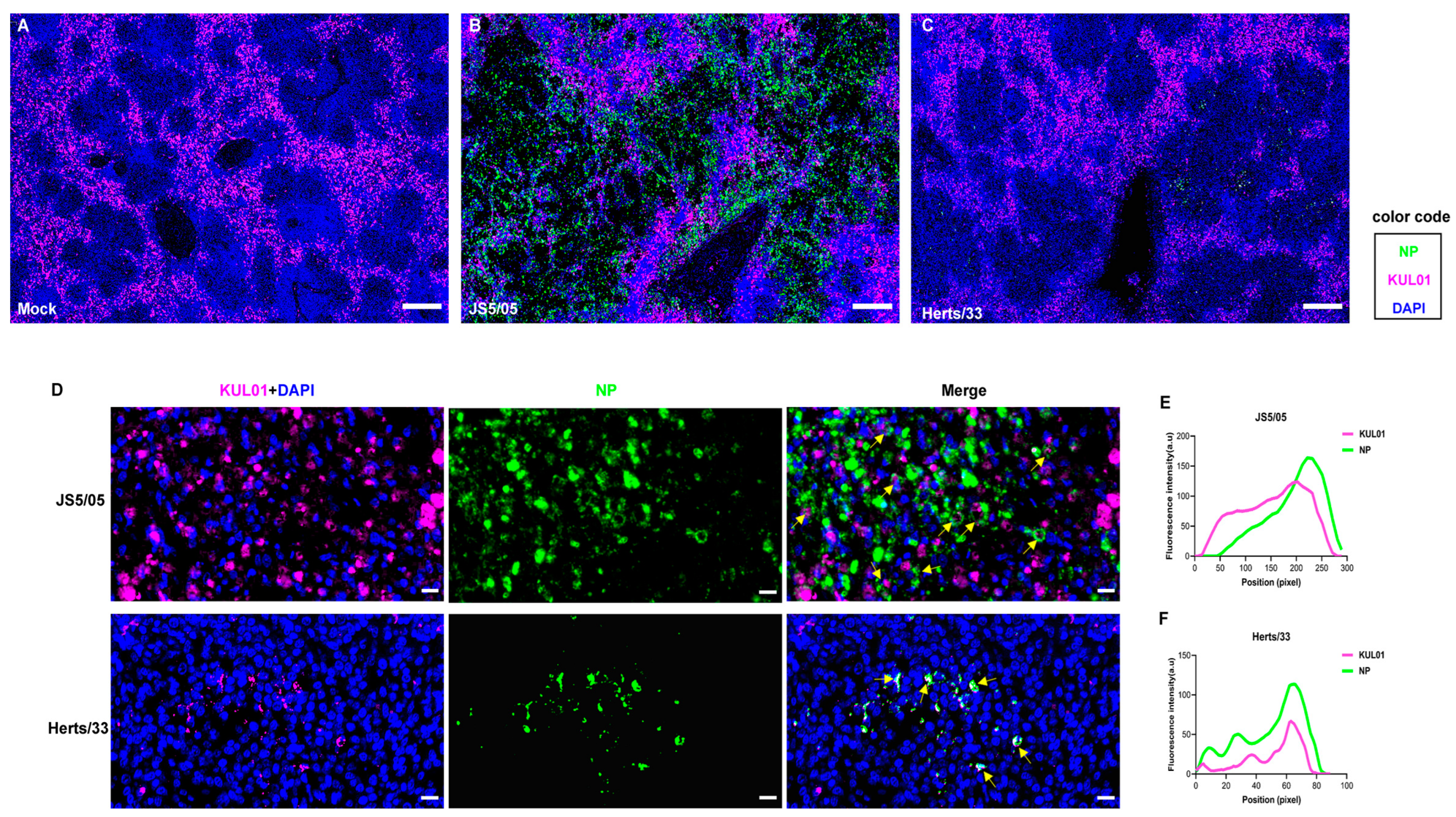
2.4. In Vivo Infectivity of NDV in Macrophages
2.5. Macrophage Depletion in Chickens
2.6. Chicken Infection Study
2.7. Histopathological Analysis
2.8. TUNEL Staining
3. Data analysis
4. Results
4.1. NDV Has Higher Infectivity in Macrophages than Lymphocytes
4.2. Genotype VII NDV Presents an Enhanced Tropism to Macrophages in Chickens
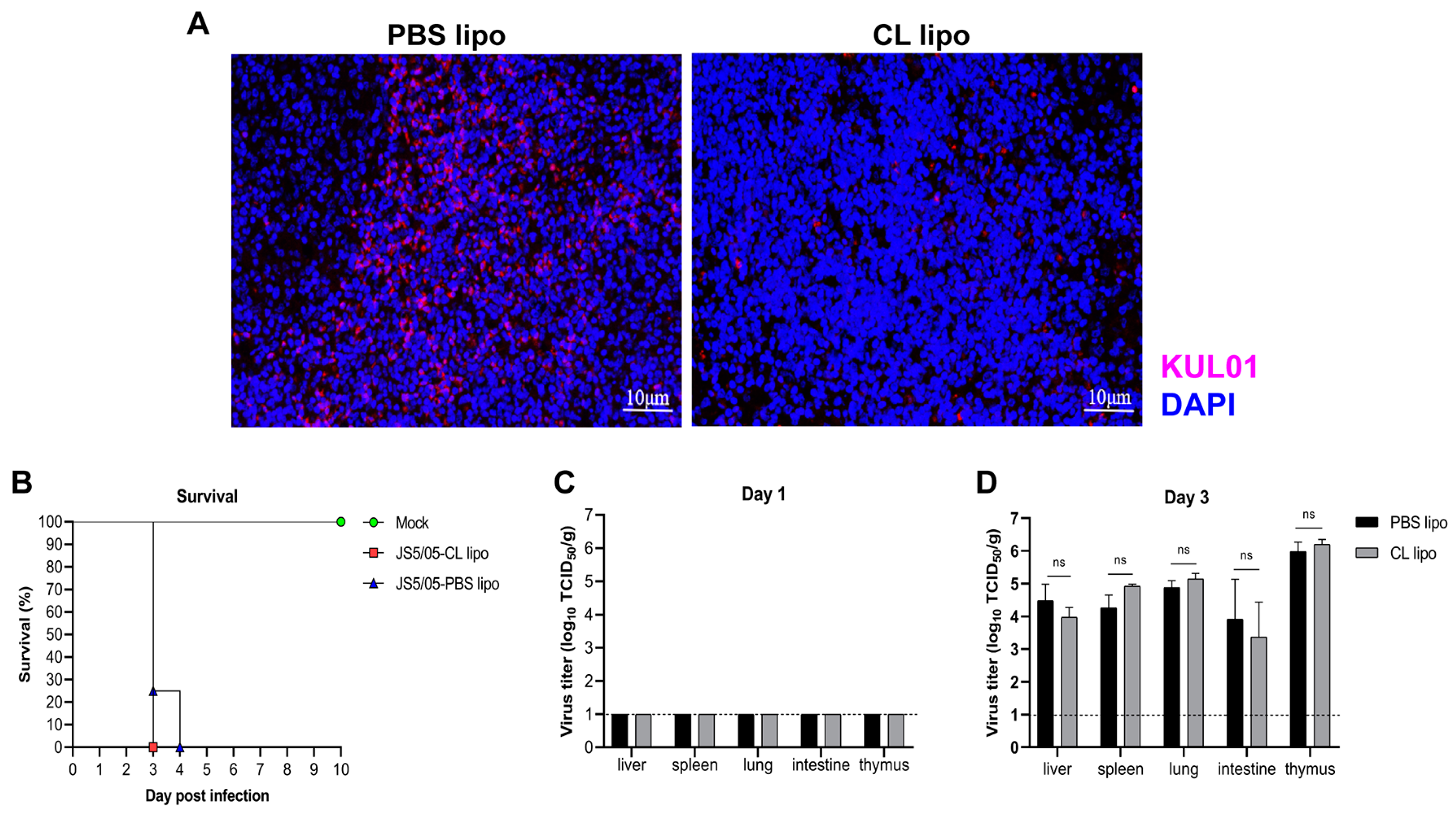
4.3. Clodronate Liposome Treatment Causes Efficient Macrophage Depletion
4.4. Macrophage Depletion Does Not Alter Disease Outcomes and NDV Replication in Chickens
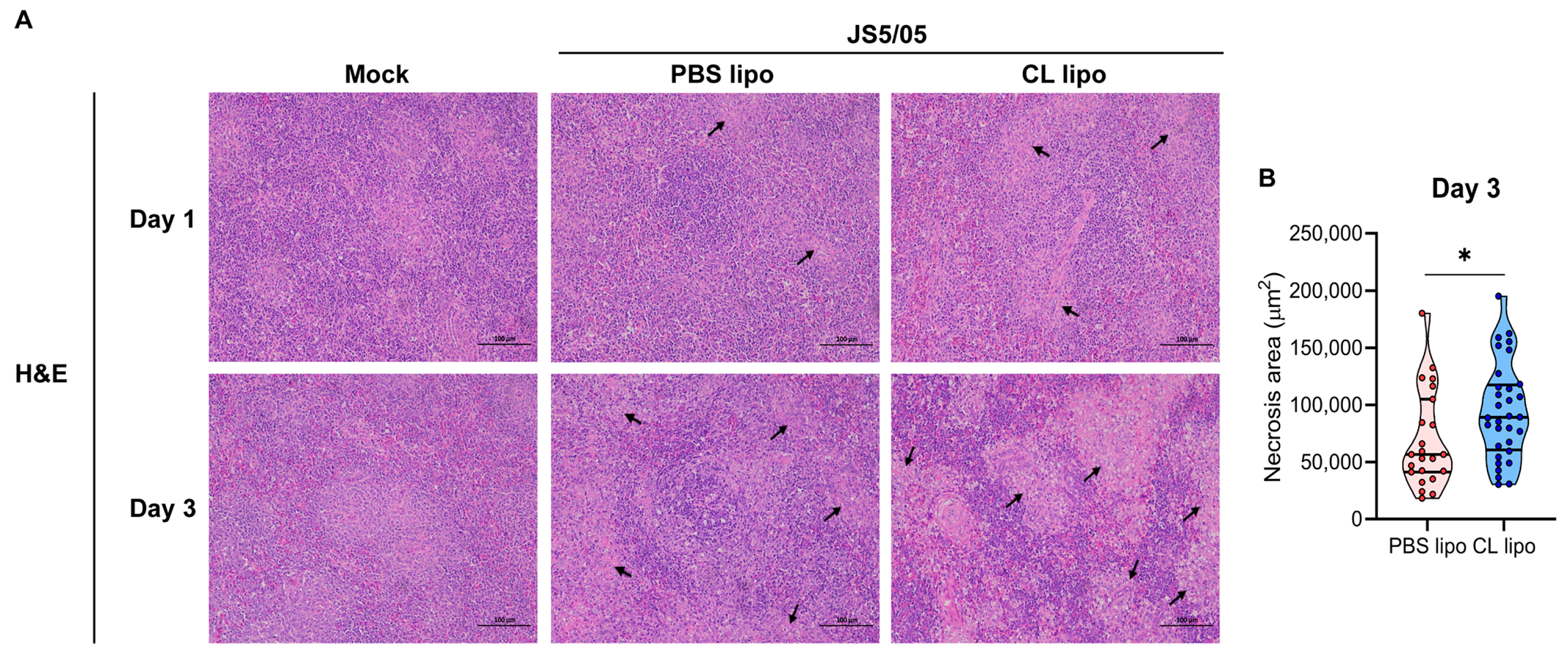
4.5. Macrophage Depletion Exacerbates Tissue Damage Caused by NDV
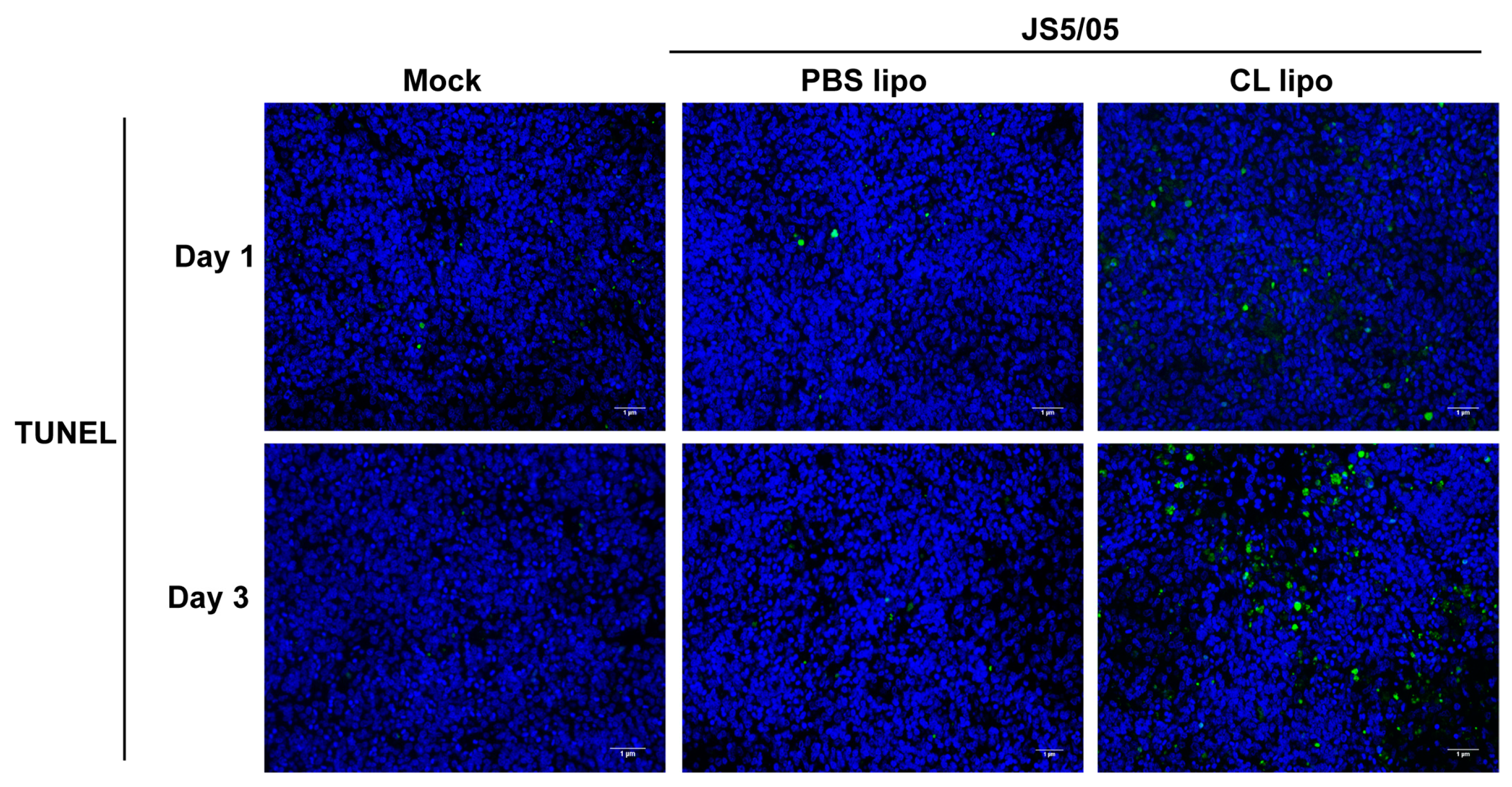
4.6. Macrophage Depletion Enhances Apoptosis Caused by NDV
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, P.J.; Koch, G. Newcastle Disease. In Diseases of Poultry, 14th ed.; Swayne, D.E., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 112–134. [Google Scholar]
- Zhang, D.; Ding, Z.; Xu, X. Pathologic mechanisms of the Newcastle disease virus. Viruses 2023, 15, 864. [Google Scholar] [CrossRef]
- Kaiser, A.; Willer, T.; Sid, H.; Petersen, H.; Baumgartner, W.; Steinberg, P.; Rautenschlein, S. Susceptibility of primary chicken intestinal epithelial cells for low pathogenic avian influenza virus and velogenic viscerotropic Newcastle disease virus. Virus Res. 2016, 225, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.M. Growth of Newcastle disease virus in chicken macrophages. J. Comp. Pathol. 1996, 115, 253–263. [Google Scholar] [CrossRef]
- Butt, S.L.; Moura, V.; Susta, L.; Miller, P.J.; Hutcheson, J.M.; Cardenas-Garcia, S.; Brown, C.C.; West, F.D.; Afonso, C.L.; Stanton, J.B. Tropism of Newcastle disease virus strains for chicken neurons, astrocytes, oligodendrocytes, and microglia. BMC Vet. Res. 2019, 15, 317. [Google Scholar] [CrossRef] [Green Version]
- Xiang, B.; Zhu, W.; Li, Y.; Gao, P.; Liang, J.; Liu, D.; Ding, C.; Liao, M.; Kang, Y.; Ren, T. Immune responses of mature chicken bone-marrow-derived dendritic cells infected with Newcastle disease virus strains with differing pathogenicity. Arch. Virol. 2018, 163, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shi, Q.K.; Han, Z.X.; Fan, Z.; Ai, H.; Chen, L.N.; Li, L.; Liu, T.Y.; Sun, J.F.; Liu, S.W. Newcastle disease virus entry into chicken macrophages via a pH-dependent, dynamin and caveola-mediated endocytic pathway that requires Rab5. J. Virol. 2021, 95, e02288-20. [Google Scholar] [CrossRef]
- Susta, L.; Miller, P.J.; Afonso, C.L.; Brown, C.C. Clinicopathological characterization in poultry of three strains of Newcastle disease virus isolated from recent outbreaks. Vet. Pathol. 2011, 48, 349–360. [Google Scholar] [CrossRef]
- Brown, C.; King, D.J.; Seal, B.S. Pathogenesis of Newcastle disease in chickens experimentally infected with viruses of different virulence. Vet. Pathol. 1999, 36, 125–132. [Google Scholar] [CrossRef]
- Zhang, P.Z.; Ding, Z.; Liu, X.X.; Chen, Y.Y.; Li, J.J.; Tao, Z.; Fei, Y.D.; Xue, C.; Qian, J.; Wang, X.L.; et al. Enhanced replication of virulent Newcastle disease virus in chicken macrophages is due to polarized activation of cells by inhibition of TLR7. Front. Immunol. 2018, 9, 366. [Google Scholar] [CrossRef]
- Cornax, I.; Diel, D.G.; Rue, C.A.; Estevez, C.; Yu, Q.; Miller, P.J.; Afonso, C.L. Newcastle disease virus fusion and haemagglutinin-neuraminidase proteins contribute to its macrophage host range. J. Gen. Virol. 2013, 94, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.J.; Decanini, E.L.; Afonso, C.L. Newcastle disease: Evolution of genotypes and the related diagnostic challenges. Infect. Genet. Evol. 2010, 10, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Wan, H.Q.; Ni, X.X.; Wu, Y.T.; Liu, W.B. Pathotypical and genotypical characterization of strains of Newcastle disease virus isolated from outbreaks in chicken and goose flocks in some regions of China during 1985–2001. Arch. Virol. 2003, 148, 1387–1403. [Google Scholar] [CrossRef]
- Mase, M.; Imai, K.; Sanada, Y.; Sanada, N.; Yuasa, N.; Imada, T.; Tsukamoto, K.; Yamaguchi, S. Phylogenetic analysis of Newcastle disease virus genotypes isolated in Japan. J. Clin. Microbiol. 2002, 40, 3826–3830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Hu, J.; Hu, S.; Song, Q.; Ding, P.; Zhu, J.; Liu, X.; Wang, X.; Liu, X. High levels of virus replication and an intense inflammatory response contribute to the severe pathology in lymphoid tissues caused by Newcastle disease virus genotype VIId. Arch. Virol. 2015, 160, 639–648. [Google Scholar] [CrossRef]
- Ecco, R.; Brown, C.; Susta, L.; Cagle, C.; Cornax, I.; Pantin-Jackwood, M.; Miller, P.J.; Afonso, C.L. In vivo transcriptional cytokine responses and association with clinical and pathological outcomes in chickens infected with different Newcastle disease virus isolates using formalin-fixed paraffin-embedded samples. Vet. Immunol. Immunopathol. 2011, 141, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Rasoli, M.; Yeap, S.K.; Tan, S.W.; Moeini, H.; Ideris, A.; Bejo, M.H.; Alitheen, N.B.; Kaiser, P.; Omar, A.R. Alteration in lymphocyte responses, cytokine and chemokine profiles in chickens infected with genotype VII and VIII velogenic Newcastle disease virus. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 11–21. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, S.; Meng, C.; Wang, X.; Zhu, J.; Liu, X. Generation of a genotype VII Newcastle disease virus vaccine candidate with high yield in embryonated chicken eggs. Avian Dis. 2011, 55, 391–397. [Google Scholar] [CrossRef]
- Reddy, V.R.; Trus, I.; Desmarets, L.M.; Li, Y.; Theuns, S.; Nauwynck, H.J. Productive replication of nephropathogenic infectious bronchitis virus in peripheral blood monocytic cells, a strategy for viral dissemination and kidney infection in chickens. Vet. Res. 2016, 47, 70. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Liang, Y.; Wang, N.; Cui, L.; Chen, Z.; Wu, H.; Zhu, C.; Wang, Z.; Liu, S.; Li, H. Avian flavivirus infection of monocytes/macrophages by extensive subversion of host antiviral innate immune responses. J. Virol. 2019, 93, e00978-19. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H.A. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Anis, Z.; Morita, T.; Azuma, K.; Ito, H.; Ito, T.; Shimada, A. Histopathological alterations in immune organs of chickens and ducks after experimental infection with virulent 9a5b newcastle disease virus. J. Comp. Pathol. 2013, 149, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Campbell, R.S.; Parede, L. Studies of the pathology of velogenic Newcastle disease: Virus infection in non-immune and immune birds. Avian Pathol. 1991, 20, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Kameka, A.M.; Haddadi, S.; Jamaldeen, F.J.; Moinul, P.; He, X.T.; Nawazdeen, F.H.P.; Bonfield, S.; Sharif, S.; van Rooijen, N.; Abdul-Careem, M.F. Clodronate treatment significantly depletes macrophages in chickens. Can. J. Vet. Res. 2014, 78, 274–282. [Google Scholar] [PubMed]
- Abdul-Cader, M.S.; Ehiremen, G.; Nagy, E.; Abdul-Careem, M.F. Low pathogenic avian influenza virus infection increases the staining intensity of KUL01+cells including macrophages yet decrease of the staining intensity of KUL01+cells using clodronate liposomes did not affect the viral genome loads in chickens. Vet. Immunol. Immunopathol. 2018, 198, 37–43. [Google Scholar] [CrossRef]
- Coleman, C.M.; Sisk, J.M.; Halasz, G.; Zhong, J.; Beck, S.E.; Matthews, K.L.; Venkataraman, T.; Rajagopalan, S.; Kyratsous, C.A.; Frieman, M.B. CD8+ T cells and macrophages regulate pathogenesis in a mouse model of Middle East respiratory syndrome. J. Virol. 2017, 91, e01825-16. [Google Scholar] [CrossRef] [Green Version]
- Rivas, C.; Djeraba, A.; Musset, E.; van Rooijen, N.; Baaten, B.; Quéré, P. Intravenous treatment with liposome-encapsulated dichloromethylene bisphosphonate (Cl2MBP) suppresses nitric oxide production and reduces genetic resistance to Marek’s disease. Avian Pathol. 2003, 32, 139–149. [Google Scholar]
- Tate, M.D.; Pickett, D.L.; van Rooijen, N.; Brooks, A.G.; Reading, P.C. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J. Virol. 2010, 84, 7569–7580. [Google Scholar] [CrossRef] [Green Version]
- Roscic-Mrkic, B.; Schwendener, R.A.; Odermatt, B.; Zuniga, A.; Pavlovic, J.; Billeter, M.A.; Cattaneo, R. Roles of macrophages in measles virus infection of genetically modified mice. J. Virol. 2001, 75, 3343–3351. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Duan, Z.; Hu, S.; Kai, Y.; Wang, X.; Song, Q.; Zhong, L.; Sun, Q.; Wu, Y.; Liu, X. Lack of detection of host associated differences in Newcastle disease viruses of genotype VIId isolated from chickens and geese. Virol. J. 2012, 9, 197. [Google Scholar] [CrossRef] [Green Version]
| Group | dpi a | Lesion Severity b |
|---|---|---|
| PBS liposomes | 1 | + |
| 3 | +++ | |
| Clodronate liposomes | 1 | ++ |
| 3 | ++++ | |
| Control | 1 | − |
| 3 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, J.; Deng, J.; Chen, Q.; Liao, T.; Hu, J.; Chen, Y.; Hu, S.; Hu, Z.; Liu, X. Role of Macrophages in the Pathogenesis of Genotype VII Newcastle Disease Virus in Chickens. Animals 2023, 13, 2239. https://doi.org/10.3390/ani13132239
Ni J, Deng J, Chen Q, Liao T, Hu J, Chen Y, Hu S, Hu Z, Liu X. Role of Macrophages in the Pathogenesis of Genotype VII Newcastle Disease Virus in Chickens. Animals. 2023; 13(13):2239. https://doi.org/10.3390/ani13132239
Chicago/Turabian StyleNi, Jie, Jing Deng, Qing Chen, Tianxing Liao, Jiao Hu, Yu Chen, Shunlin Hu, Zenglei Hu, and Xiufan Liu. 2023. "Role of Macrophages in the Pathogenesis of Genotype VII Newcastle Disease Virus in Chickens" Animals 13, no. 13: 2239. https://doi.org/10.3390/ani13132239





