Air Quality Assessment in Pig Farming: The Italian Classyfarm
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Introduction of ClassyFarm, the Italian Risk Assessment in Livestock Farming: The Swine Husbandry
- −
- Level 1 = high risk, unacceptable/negative/dangerous condition, or stress, indicates the possibility that some of the animals are experiencing or may experience a negative situation (“distress”) due to the impossibility of fully enjoying one or more of the 5 freedoms;
- −
- Level 2 = controlled risk or acceptable condition, compatible with the possibility that all herd animals can satisfy their five freedoms and are not subjected to stressful conditions;
- −
- Level 3 = low risk or optimal, positive, and beneficial condition, due not only to full adaptation of the animal to its environment and respect for the five freedoms but also to the possibility of being able to live positive, fulfilling, and satisfying experiences capable of producing “eustress”.
- Area A—Personal and corporate management;
- Area B—Facilities and equipment;
- Area C—Animal-Based Measures (ABMs);
- Area biosafety and biosecurity;
- Great risk and alarm systems.
- Temperature, relative humidity, and dustiness (B 36);
- Harmful gases: ammonia (NH3) and carbon dioxide (CO2) (B 37).
3. Microclimatic Parameters
- −
- The temperature is the average air temperature registered in the room. It is expressed according to the Celsius scale in degrees Celsius (°C);
- −
- Relative humidity is the ratio, expressed as a percentage, between the actual amount of water vapor contained in the air at a given temperature (absolute humidity) and the maximum amount possible (saturated vapor) at the same temperature;
- −
- Air velocity is the ratio of the distance covered by air flow, referred to as time t, and is expressed in meters per second (m/s);
- −
- Although not explicitly discussed in the ClassyFarm system, the heat losses occurring in an animal environment through conduction, convection, radiation, and evaporation may be taken into account during environmental monitoring sessions since the surface thermal insulance of the surface-air boundary layer depends on radiant and convective conditions.
4. Pollutants Generated in Piggeries: Gasses and Particulate Matter
4.1. Carbon Dioxide
4.2. Ammonia
| Year of the Study | Reference | Ammonia Concentration (mg/m3) |
|---|---|---|
| 1974 | [34] | 18 |
| 1980 | [35] | 0.01–1.9 |
| 1981 | [36] | 2.8-15.3 |
| 1982 | [37] | 0.1–18 |
| 1982 | [38] | 1–24 |
| 1991 | [39] | 1.06–9.37 |
| 1996 | [40] | 3.65 |
| 1997 | [41] | 7.08–31.86 |
| 1997 | [42] | 14.66 |
| 1998 | [28] | 5–18 ppm |
| 1999 | [43] | 12–30 |
| 1999 | [44] | 9 ± 1–15 ± 9 ppm |
| 2000 | [45] | 7–15 ppm |
| 2000 | [46] | 2.8–10.6 ppm. |
| 2000 | [47] | 13.88 |
| 2003 | [48] | Less than 30 ppm |
| 2005 | [49] | 1.2–37 ppm. |
| 2017 | [19] | 5.31–7.45 |
| 2022 | [50] | 2.14–5.71 |
4.3. Hydrogen Sulfide
4.4. Particulate Matter
5. Measurement Tips
5.1. The Importance of the Ventilation Rate Measurement
- −
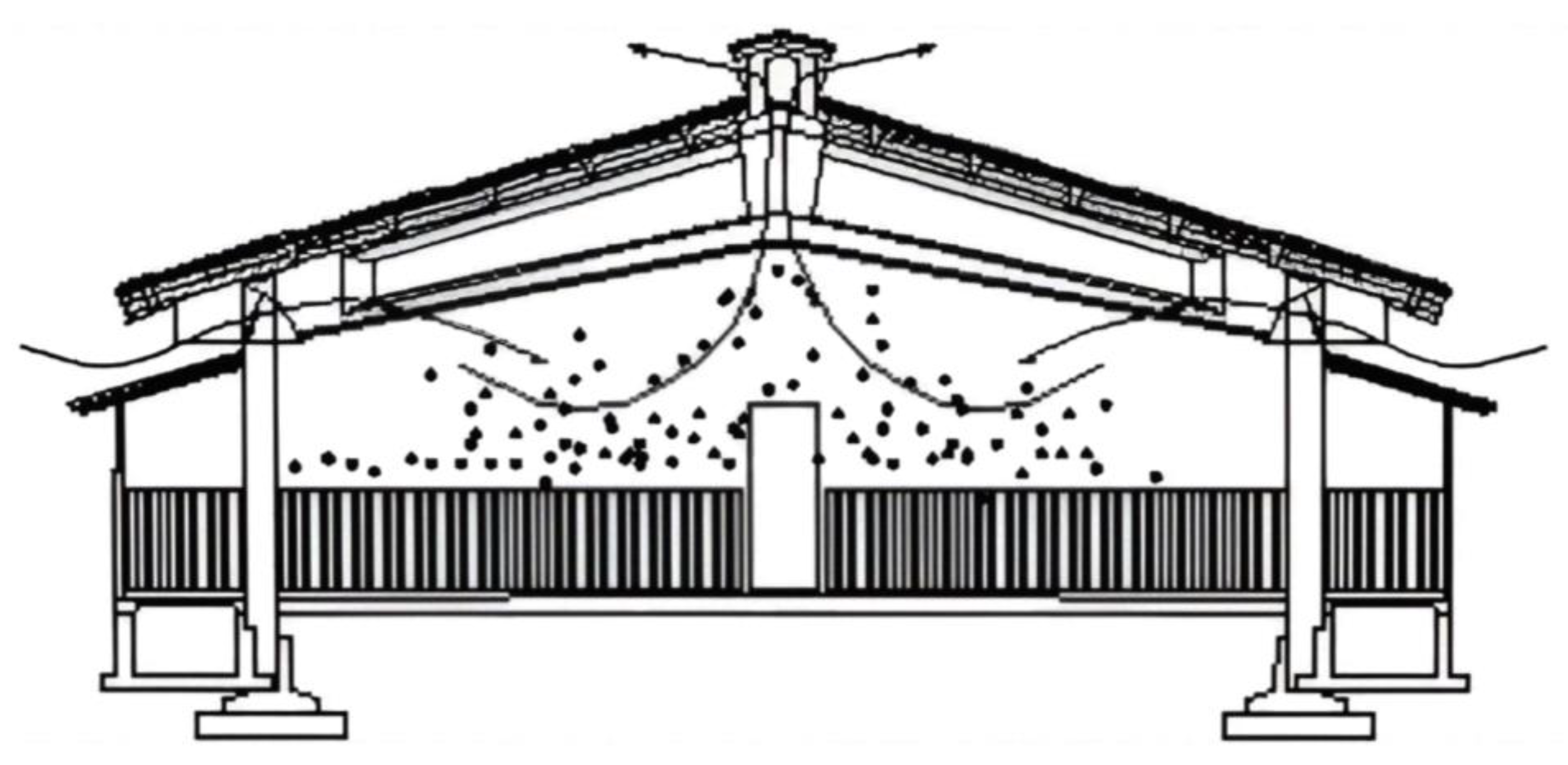
- Natural ventilation (Figure 2) is based on the exchange of air between the outside and the inside of a building without using mechanical fans.
- −
- −
- Mixed systems, characterized by the use of natural ventilation in winter that is aided by mechanical fans in summer (see Figure 4).
- Ei: emission of the pollutant at time i
- Vi: Ventilation rate at time i
- C, exhaust is the gas (i) concentration in the ventilation outlet
- C, inlet is the gas (i) concentration in the ventilation inlet.
5.2. Pollutant Concentration Measurements: Gasses
5.3. Pollutant Concentration Measurements: Particulate Matter
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hughes, B.; Duncan, I. The notion of ethological ‘need’, models of motivation and animal welfare. Anim. Behav. 1988, 36, 1696–1707. [Google Scholar] [CrossRef]
- Main, D.C.J.; Kent, J.P.; Wemelsfelder, F.; Ofner, E.; Tuyttens, F.A.M. Applications for Methods of On-Farm Welfare Assessment. Anim. Welf. 2003, 12, 523–528. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Erasmus, M.A.; Croney, C.C.; Li, C.; Li, Y. A critical review of advancement in scientific research on food animal welfare-related air pollution. J. Hazard. Mater. 2020, 408, 124468. [Google Scholar] [CrossRef] [PubMed]
- Donham, K.J. The Concentration of Swine Production: Effects on Swine Health, Productivity, Human Health, and the Environment. Vet.-Clin. N. Am. Food Anim. Pract. 2000, 16, 559–597. [Google Scholar] [CrossRef]
- Banhazi, T.M.; Seedorf, J.; Rutley, D.L.; Pitchford, W.S. Identification of risk factors for sub-optimal housing conditions in Australian piggeries: Part 3. Environmental parameters. J. Agric. Saf. Health 2008, 14, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Gade, P.B. Welfare of animal production in intensive and organic systems with special reference to Danish organic pig production. Meat Sci. 2002, 62, 353–358. [Google Scholar] [CrossRef]
- Banhazi, T. Modelling and influencing hygiene conditions in Australian livestock buildings. In Livestock Housing. Modern Management to Ensure Optimal Health and Welfare of Farm Animals; Aland, A., Banhazi, T., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 377–390. [Google Scholar] [CrossRef]
- Costa, A.; Domeneghini, C. Pollutants in livestock buildings: Ammonia and dust interplay with the respiratory tract. In Air Quality and Livestock Farming; Banhazi, T., Aland, A., Hartung, J., Eds.; CRC Press: London, UK, 2018; pp. 49–58. ISBN 9781138027039. [Google Scholar]
- Renggaman, A.; Choi, H.L.; Sudiarto, S.I.; Alasaarela, L.; Nam, O.S. Development of pig welfare assessment protocol integrating animal-, environment-, and management-based measures. J. Anim. Sci. Technol. 2015, 57, 1. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.; Wathes, C.; Baldwin, B. The preference of pigs for fresh air over ammoniated air. Appl. Anim. Behav. Sci. 1996, 49, 417–424. [Google Scholar] [CrossRef]
- Almquist, H.J.; Stokstad, E.L.R.; Halbrook, E.R. Supplementary Values of Animal Protein Concentrates in Chick Rations. J. Nutr. 1935, 10, 193–211. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; Karriker, L.A.; Ramirez, A.; Schwartz, K.J.; Stevenson, G.W.; Zhang, J.Q. Diseases of Swine; Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Mount, L. The assessment of thermal environment in relation to pig production. Livest. Prod. Sci. 1975, 2, 381–392. [Google Scholar] [CrossRef]
- Silanikove, N.; Darcan, N.K. Impact of climate change on the dairy industry in temperate zones: Predications on the overall negative impact and on the positive role of dairy goats in adaptation to earth warming. Small Rumin. Res. 2015, 123, 27–34. [Google Scholar] [CrossRef]
- Ferrari, S.; Costa, A.; Guarino, M. Heat stress assessment by swine related vocalizations. Livest. Sci. 2013, 151, 29–34. [Google Scholar] [CrossRef]
- Muirhead, M.R.; Alexander, T.J. Managing Pig Health and Treatment of Disease; 5 M Enterprise: Uckfield, UK, 1997. [Google Scholar]
- Hoff, S.J. Airborne dust in livestock buildings. In Air Quality and Livestock Farming Book Chapter in Banhazi, T.; Aland, A., Hartung, J., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; pp. 3–11. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, Y.S.; Roh, Y.M.; Lee, C.M.; Kim, C.N. Influence of Extreme Seasons on Airborne Pollutant Levels in a Pig-Confinement Building. Arch. Environ. Occup. Health 2007, 62, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Costa, A. Ammonia Concentrations and Emissions from Finishing Pigs Reared in Different Growing Rooms. J. Environ. Qual. 2017, 46, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Giles, L.R.; Bryden, W.L.; Downing, J.L.; Owens, P.C.; Kirby, A.C.; Wynn, P.C. Performance and endo-crine responses of group housed weaner pigs exposed to the air quality of a commercial environment. Livest. Prod. Sci. 2005, 93, 255–262. [Google Scholar] [CrossRef]
- O’connor, E.A.; Parker, M.O.; McLeman, M.A.; Demmers, T.G.; Lowe, J.C.; Cui, L.; Davey, E.L.; Owen, R.C.; Wathes, C.M.; Abeyesinghe, S.M. The impact of chronic environmental stressors on growing pigs, Sus scrofa (Part 1): Stress physiology, production and play behaviour. Animal 2010, 4, 1899–1909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emeash, H.H.; Ali, M.M.; El-Bably, M.A. Effect of some pollutants as stressors on some behavioural patterns and performance of broiler chickens. Vet. Med. J. (Giza) 1997, 45, 307–314. [Google Scholar]
- Pedersen, S.; Blanes-Vidal, V.; Joergensen, H.; Chwalibog, A.; Haeussermann, A.; Heetkamp, M.J.W.; Aarnink, A.J.A. Carbon Dioxide Production in Animal Houses: A literature review. Agric. Eng. Int. 2008, 10, 1–19. [Google Scholar]
- Gustafsson, G.; Nimmermark, S.; Jeppsson, K.H. Control of emission from livestock buildings and the impact on health, welfare and performance of animals—A review. In Livestock Housing: Modern Management to Ensure Optimal Health and Welfare of Farm Animals; Aland, A., Banhazi, T., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 261–280. [Google Scholar]
- Guizzardi, F.; Saccani, A.; Sanfelice, M.; Guizzardi, S. Gas ambientali tossici e ventilazione. Monitoraggio negli allevamenti suini di un distretto veterinario. Large Anim. Rev. 2006, 12, 25–29. [Google Scholar]
- Jeppsson, K.H. Carbon dioxide emission and water evaporation from deep-litter systems. J. Agric. Eng. Res. 2000, 77, 429–440. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Liu, C.; Huang, L.; Gao, Y.; Yu, M.; Zhao, S.; Li, X. Ammonia Exposure Induced Cilia Dysfunction of Nasal Mucosa in the Piglets. BioMed Res. Int. 2020, 2020, 1705387. [Google Scholar] [CrossRef]
- Koerkamp, P.W.G.; Metz, J.H.M.; Uenk, G.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; White, R.P.; Hartung, J.; Seedorf, J.; et al. Concentration and emission of ammonia in livestock buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 79–95. [Google Scholar] [CrossRef]
- Drummond, J.G.; Curtis, S.E.; Simon, J.; Norton, H.W. Effects of Aerial Ammonia on Growth and Health of Young Pigs1. J. Anim. Sci. 1980, 50, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- De Boer, S.; Morrison, W.D. The Effects of the Quality of the Environment in Livestock Buildings on the Productivity of Swine and the Safety of Humans; Monograph; Department of Agriculture, University of GuelphBroom and Johnson: Guelph, ON, Canada, 1988. [Google Scholar]
- Jones, J.B.; Burgess, L.R.; Webster, A.J.F.; Wathes, C.M. Behavioural responses of pigs to atmospheric ammonia in a chronic choice test. Anim. Sci. 1996, 63, 437–445. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, A.; Chen, Y.; Bao, J.; Xing, H. Intestinal barrier dysfunction induced by ammonia exposure in pigs in vivo and in vitro: The protective role of L-selenomethionine. Ecotoxicol. Environ. Saf. 2022, 248, 114325. [Google Scholar] [CrossRef]
- Broom, D.M.; Jonhson, K.G. Stress and Animal Welfare; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Schaefer, J. Sampling, characterization and analysis of malodous. Agric. Environ. 1977, 3, 121–128. [Google Scholar] [CrossRef]
- Kowalewshy, H.H.; Scheu, R.; Vetter, H. Measurement of odour emissions and immissions. In Effluents from Livestock; Gasser, J.K.R., Ed.; Applied Science Publishers: London, UK, 1980; pp. 609–626. [Google Scholar]
- Hartung, J.; Hilliger, H.J. A method for sampling air in animal houses to analyse trace gases including odorants with the gas chromatograph. Agric. Environ. 1977, 3, 139–145. [Google Scholar] [CrossRef]
- Klarenbeek, J.V.; Jongebreur, A.A.; Beumer, S.C.C. Odour Emission in Pig Fattening Sheds; Report No. 48; IMAG: Wageningen, The Netherlands, 1982. [Google Scholar]
- Van Geelen, M.; Van der Hoek, K.W. Stankbestrijdingstechnieken voor Stallen in de Intensieve Veehouderij; IMAG Publikatie 167: Wageningen, The Netherlands, 1982. [Google Scholar]
- Crook, B.; Robertson, J.F.; Glass, S.A.T.; Botheroyd, E.M.; Lacey, J.; Topping, M.D. Airborne Dust, Ammonia, Microorganisms, and Antigens in Pig Confinement Houses and the Respiratory Health of Exposed Farm Workers; American Industrial Hygiene Association: Falls Church, VA, USA, 1991. [Google Scholar]
- Reynolds, S.J.; Donham, K.J.; Whitten, P.; Merchant, J.A.; Burmeister, L.F.; Popendof, W.J. Longitudinal Evaluation of Dose-Response Relationships for Environmental Exposures and Pulmonary Function in Swine Production Workers. Am. J. Ind. Med. 1996, 29, 33–40. [Google Scholar] [CrossRef]
- Gustafsson, G. Investigations of Factors Affecting Air Pollutants in Animal Houses. Ann. Agric. Environ. Med. 1997, 4, 203–215. [Google Scholar]
- Cormier, Y.; Duchaine, C.; Israel-Assayag, E.; Bedard, G.; Laviolette, M.; Dosman, J. Effects of repeated swine building exposures on normal naive subjects. Eur. Respir. J. 1997, 10, 1516–1522. [Google Scholar] [CrossRef] [Green Version]
- Demmers, T.; Burgess, L.; Short, J.; Phillips, V.; Clark, J.; Wathes, C. Ammonia emissions from two mechanically ventilated UK livestock buildings. Atmos. Environ. 1999, 33, 217–227. [Google Scholar] [CrossRef]
- Seedorf, J.; Hartung, J. Survey of ammonia concentrations in livestock buildings. J. Agric. Sci. 1999, 133, 433–437. [Google Scholar] [CrossRef]
- Zhu, T.; Pattey, E.; Desjardins, R.L. Relaxed Eddy-Accumulation Technique for Measuring Ammonia Volatilization. Environ. Sci. Technol. 1999, 34, 199–203. [Google Scholar] [CrossRef]
- Ni, J.Q.; Heber, A.J.; Lim, T.T.; Diehl, C.; Duggirala, R.; Haymore, B.; Sutton, A. NH3 emission from a large mechanically ventilated swine building during warm weather. J. Environ. Qual. 2000, 29, 751–758. [Google Scholar] [CrossRef]
- Duchaine, C.; Grimard, Y.; Cormier, Y. Influence of building maintenance, environmental factors, and seasons on airborne contaminants of swine confinement buildings. AIHAJ 2000, 61, 56–63. [Google Scholar] [CrossRef]
- Jacobson, L.D.; Heber, A.J.; Zhang, Y.; Sweeten, J.; Koziel, J.; Hoff, S.J.; Bundy, D.S.; Beasley, D.B.; Baughman, G.R. Air Pollutant Emissions from Confined Animal Buildings in the U.S. In Proceedings of the International Symposium on Gaseous and Odour Emissions from Animal Production Facilities, EurAgEng Horsens, Jutland, Denmark, 1–4 June 2003; pp. 194–202. [Google Scholar]
- Heber, A.J.; Tao, P.C.; Ni, J.Q.; Lim, T.T.; Schmidt, A.M. Two swine finishing building with flushing: Ammonia characteristics. In Proceedings of the Seventh International Symposium, Beijing, China, 10–13 December 2005; pp. 436–443. [Google Scholar]
- Costa, A.; Salvagnini, C.; Buoio, E.; Palmeri, F.; Salvagnini, A.; Tangorra, F.M. Ammonia Emission from a BAT Farrowing Room with Fully Slatted Flooring and Individual Manure Pan. Results of a Yearly Monitoring Survey. In Proceedings of the International Conference on Safety, Health and Welfare in Agriculture and Agro-Food Systems, Sicily, Italy, 3–6 September 2022; pp. 305–313. [Google Scholar] [CrossRef]
- Li, Y.; Pan, L.; Zeng, X.; Zhang, R.; Li, X.; Li, J.; Xing, H.; Bao, J. Ammonia expo-sure causes the imbalance of the gut-brain axis by altering gene networks associated with oxidative metabolism, inflammation and apoptosis. Ecotoxicol. Environ. Saf. 2021, 224, 112668. [Google Scholar] [CrossRef]
- Tang, S.; Xie, J.; Wu, W.; Yi, B.; Liu, L.; Zhang, H. High ammonia exposure regulates lipid metabolism in the pig skeletal muscle via mTOR pathway. Sci. Total. Environ. 2020, 740, 139917. [Google Scholar] [CrossRef]
- Jones, J.B.; Wathes, C.M.; Persaud, K.C.; White, R.P.; Jones, R. Acute and chronic exposure to ammonia and olfactory acuity for n-butanol in the pig. Appl. Anim. Behav. Sci. 2001, 71, 13–28. [Google Scholar] [CrossRef]
- Malayer, J.R.; Brandt, K.E.; Green, M.L.; Kelly, D.T.; Sutton, A.L.; Diekman, M.A. Influence of manure gases on the onset of puberty of replacement gilts. Anim. Sci. 1988, 46, 277–282. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, R.; Li, Y.; Li, J.; Zhao, Q.; Li, X.; Bao, J. Excessive ammonia inhalation causes liver damage and dysfunction by altering gene networks associated with oxidative stress and immune function. Ecotoxicol. Environ. Saf. 2021, 217, 112203. [Google Scholar] [CrossRef]
- Yang, L.-L.; Zhao, Y.; Luo, S.-M.; Ma, J.-Y.; Ge, Z.-J.; Shen, W.; Yin, S. Toxic effects and possible mechanisms of hydrogen sulfide and/or ammonia on porcine oocyte maturation in vitro. Toxicol. Lett. 2018, 285, 20–26. [Google Scholar] [CrossRef]
- Hoff, S.J.; Bundy, D.S.; Nelson, M.A.; Zelle, B.C.; Jacobson, L.D.; Heber, A.J.; Ni, J.-Q.; Zhang, Y.H.; Koziel, J.A.; Beasley, D.B. Emissions of ammonia, hydrogen sulfide, and odor before, during and after slurry removal from a deep-pit swine finisher. J. Air Waste Manag. Assoc. 2006, 56, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.; Guarino, M.; Navarotto, P.; Savoini, G.; Berckmans, D. Effects of corn milling type on physical charac-teristics and on dustiness of swine diets. Trans. ASABE 2007, 50, 1759–1764. [Google Scholar] [CrossRef]
- Demmers, T.G.M.; Wathes, C.M.; Richards, P.A.; Teer, N.; Taylor, L.L.; Bland, V.; Goodman, J.; Armstrong, D.; Chennells, D.; Done, S.H. Production and disease effects of dust and ammonia on the weaner pig. Landbauforsch Völkenrode SH 2002, 235, 129–138. [Google Scholar]
- Hamon, L.; Andrès, Y.; Dumont, E. Aerial Pollutants in Swine Buildings: A Review of Their Characterization and Methods to Reduce Them. Environ. Sci. Technol. 2012, 46, 12287–12301. [Google Scholar] [CrossRef]
- Cambra-López, M.; Hermosilla, T.; Lai, H.T.L.; Aarnink, A.J.A.; Ogink, N.W.M. Particulate Matter Emitted from Poultry and Pig Houses: Source Identification and Quantification. Trans. ASABE 2011, 54, 629–642. [Google Scholar] [CrossRef] [Green Version]
- Wathes, C.M. Environmental control in pig housing. In Proceedings of the 15th international Pig Veterinary Society Congress, Birmingham, UK, 5–9 July 1998; Volume I, pp. 257–265. [Google Scholar]
- Xie, J.W.; Jin, L.; Luo, X.S.; Zhao, Z.; Li, X.D. Seasonal disparities in airborne bacteria and associated antibiotic re-sistance genes in PM2.5 between urban and rural sites. Environ. Sci. Technol. Lett. 2018, 5, 74–79. [Google Scholar] [CrossRef]
- Song, L.; Wang, C.; Jiang, G.; Ma, J.; Li, Y.; Chen, H.; Guo, J. Bioaerosol is an important transmission route of antibiotic resistance genes in pig farms. Environ. Int. 2021, 154, 106559. [Google Scholar] [CrossRef]
- Pu, S.; Peng, S.; Zhu, J.; Liu, Z.; Long, D.; Lim, T. Characteristics of PM2.5 and Its Correlation with Feed, Manure and NH3 in a Pig-Fattening House. Toxics 2022, 10, 145. [Google Scholar] [CrossRef]
- Done, S.H.; Chennells, D.J.; Gresham, A.C.J.; Williamson, S.; Hunt, B.; Taylor, L.L.; Bland, V.; Jones, P.; Armstrong, D.; White, R.P.; et al. Clinical and pathological responses of weaned pigs to atmospheric ammonia and dust. Vet. Rec. 2005, 157, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Barbari, M.; Gastaldo, A. Le polveri negli edifici zootecnici. L’informatore Agrario 1993, 23, 39–50. [Google Scholar]
- Thorne, P.S.; Kiekhaefer, M.S.; Whitten, P.; Donham, K.J. Comparison of bioaerosol sampling methods in barns housing swine. Appl. Environ. Microbiol. 1992, 58, 2543–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donham, K.J. Hazardous agents in agricultural dusts and methods of evaluation. Am. J. Ind. Med. 1986, 10, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Wathes, C.M.; Demmers, T.G.M.; Teer, N.; White, R.P.; Taylor, L.L.; Bland, V.; Jones, P.; Armstrong, D.; Gresham, A.C.J.; Hartung, J.; et al. Production responses of weaned pigs after chronic exposure to airborne dust and ammonia. Anim. Sci. 2004, 78, 87–97. [Google Scholar] [CrossRef]
- Pikridas, M.; Riipinen, I.; Hildebrandt, L.; Kostenidou, E.; Manninen, H.; Mihalopoulos, N.; Kalivitis, N.; Burkhart, J.F.; Stohl, A.; Kulmala, M.; et al. New particle formation at a remote site in the eastern Mediterranean. J. Geophys. Res. Atmos. 2012, 117, D12205. [Google Scholar] [CrossRef] [Green Version]
- Takai, H.; Pedersen, S. Livestock related fine dust–composition, structure and flows. Landbauforsch Völken-Rode SH 2002, 235, 139–144. [Google Scholar]
- McClendon, C.J.; Gerald, C.L.; Waterman, J.T. Farm animal models of organic dust exposure and toxicity. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Donham, K.J.; Cumro, D.; Reynolds, S. Synergistic Effects of Dust and Ammonia on the Occupational Health Effects of Poultry Production Workers. J. Agromed. 2002, 8, 57–76. [Google Scholar] [CrossRef]
- Baekbo, P. Effects of noxious gases, dust and microorganisms on the incidence and severity of respiratory diseases in pigs. In Proceedings of the 15th International Pig Veterinary Society Congress, Birmingham, UK, 5–9 July 1998; Done, S., Thomson, J., Varley, M., Eds.; Nottingham University Press: Nottingham, UK, 1998; Volume 1, pp. 135–142. [Google Scholar]
- Done, S.H. The Relationship between Climate, Respiratory Disease and Pig Performance, Studies at National Pig Performance Testing Stations. Ph.D. Thesis, Royal College of Veterinary Surgeons, London, UK, 1995. [Google Scholar]
- Hartung, J. Effects of bioaerosol related particulate matter on animal health. In Proceedings PM in and from Agriculture; Landbauforschung Völkenrode Sonderheft 235: Braunschweig, Germany, 2002; pp. 119–123. [Google Scholar]
- Costa, A.; Colosio, C.; Gusmara, C.; Guarino, M.; Sala, V. Effects of disinfectant fogging procedure on dust, ammonia concentration, aerobic bacteria and fungal spores in a farrowing-weaning room. Ann. Agric. Environ. Med. 2014, 21, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Donham, K.J.; Cumro, D. Setting maximum dust exposure levels for people and animals in livestock facilities. In Proceedings of the International Symposium on Dust Control in Animal Production Facilities, Aarhus, Denmark, 30 May–2 June 1999; pp. 93–110. [Google Scholar]
- Luiken, R.E.; Van Gompel, L.; Bossers, A.; Munk, P.; Joosten, P.; Hansen, R.B.; Knudsen, B.E.; García-Cobos, S.; Dewulf, J.; Aarestrup, F.M.; et al. Farm dust resistomes and bacterial microbiomes in European poultry and pig farms. Environ. Int. 2020, 143, 105971. [Google Scholar] [CrossRef]
- Van Gompel, L.; Luiken, R.E.C.; Sarrazin, S.; Munk, P.; Knudsen, B.E.; Hansen, R.B.; Bossers, A.; Aarestrup, F.M.; Dewulf, J.; Wagenaar, J.A.; et al. The antimicrobial resistome in relation to antimicrobial use and biosecurity in pig farming, a metagenome-wide association study in nine European countries. J. Antimicrob. Chemother. 2019, 74, 865–876. [Google Scholar] [CrossRef]
- De Rooij, M.M.T.; Hoek, G.; Schmitt, H.; Janse, I.; Swart, A.; Maassen, C.B.M.; Schalk, M.; Heederik, D.J.J.; Wouters, I.M. Insights into Livestock-Related Microbial Concentrations in Air at Residential Level in a Livestock Dense Area. Environ. Sci. Technol. 2019, 53, 7746–7758. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Liu, J.; Dong, S.; Zhao, K.; Chen, L.; Chen, Z.; Sun, Y.; Guo, Z. The Distribution Characteristics of Aerosol Bacteria in Different Types of Pig Houses. Animals 2022, 12, 1540. [Google Scholar] [CrossRef]
- Kumari, P.; Choi, H.L. Seasonal Variability in Airborne Biotic Contaminants in Swine Confinement Buildings. PLoS ONE 2014, 9, e112897. [Google Scholar] [CrossRef]
- Nichols, G.P.; Fontenot, J.D.; Gibbons, J.P.; Sanders, M. Evaluation of volumetric modulated Arc therapy for postmastectomy treatment. Radiat. Oncol. 2014, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Labuza, T.P. Water activity and food preservation. In Handbook of Food Preservation; Marcel Dekker: New York, NJ, USA, 1999; pp. 339–382. [Google Scholar]
- Pessoa, J.; Montoro, J.C.; Nunes, T.P.; Norton, T.; McAloon, C.; Manzanilla, E.G.; Boyle, L. Environmental Risk Factors Influence the Frequency of Coughing and Sneezing Episodes in Finisher Pigs on a Farm Free of Respiratory Disease. Animals 2022, 12, 982. [Google Scholar] [CrossRef]
- Kumari, P.; Woo, C.; Yamamoto, N.; Choi, H.L. Variations in abundance, diversity and community composition of airborne fungi in swine houses across seasons. Sci. Rep. 2016, 6, 37929. [Google Scholar] [CrossRef] [Green Version]
- Nucci, M.; Anaissie, E. Fusarium Infections in Immunocompromised Patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Heber, A.J.; Ni, J.Q.; Cortus, E.L.; Lim, T.T.; Bogan, B.W. National study of livestock air quality in USA. In Proceedings of the International Symposium on Health Environment and Animal Welfare (ISHEAW-2011), Chongqing, China, 19–22 October 2011; Chinese Society of Agricultural Engineering: Beijing, China, 2011; pp. 42–66. [Google Scholar]
- Dai, X.; Ni, J.Q.; Pan, Q. Monitoring of temperature, Humidity and air quality inside pig weaner house in eastern China. Chin. Soc. Agric. Mach. 2016, 47, 315–322. [Google Scholar]
- Heber, A.J.; Ni, J.-Q.; Lim, T.T.; Tao, P.-C.; Schmidt, A.M.; Koziel, J.A.; Beasley, D.B.; Hoff, S.J.; Nicolai, R.E.; Jacobson, L.D.; et al. Quality assured measurements of animal building emissions: Gas concentrations. J. Air Waste Manag. Assoc. 2006, 56, 1472–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinsey, J.S.; Swift, J.; Bursey, J. Characterization of fugitive mercury emissions from the cell building at a US chlor-alkali plant. Atmos. Environ. 2004, 38, 623–631. [Google Scholar] [CrossRef]
- Krause, K.; Janssen, J. Measuring and simulation of the distribution of ammonia in animal houses. Room Vent 1990, 90, 1–12. [Google Scholar]
- Scholtens, R.; Van’t Ooster, A. Performance and accuracy of methods for measuring natural ventilation rates and ammonia emission from naturally ventilated livestock houses. In Proceedings of the Report N. 94-C-033—12th CIGR World Congress and AgEng′94 Conference: Agricultural Engineering, Milano, Italy, 29 August–1 September 1994. 13p. [Google Scholar]
- Van’t Klooster, C.E.; Heitlager, B.P. Determination of minimum ventilation rate in pig houses with natural ventilation based on carbon-dioxide balance. J. Agric. Eng. Res. 1994, 57, 279–287. [Google Scholar] [CrossRef]
- Pedersen, S.; Takai, H.; Johnsen, J.; Metz, J.; Koerkamp, P.G.; Uenk, G.; Phillips, V.; Holden, M.; Sneath, R.; Short, J.; et al. A Comparison of Three Balance Methods for Calculating Ventilation Rates in Livestock Buildings. J. Agric. Eng. Res. 1998, 70, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Demmers, T.G.M. Ammonia removal from exhaust air in intensive animal housing. In Proceedings of the Symposium on Biotechniques for Air Pollution abatement and Odour Control Policies, Maastricht, The Netherlands, 27–29 October 1991. [Google Scholar]
- Muller, H.J. Rates of odour and pollutant gas emission from animal housing: Measurement and analysis. Land-Technik 1994, 49, 360–371. [Google Scholar]
- Berckmans, D.; Vandenbroeck, P.; Goedseels, V. Sensor for continuous ventilation rate measurement in livestock buildings. J. Indoor Air 1991, 3, 323–336. [Google Scholar] [CrossRef]
- Costa, A.; Borgonovo, F.; Leroy, T.; Berckmans, D.; Guarino, M. Dust concentration variation in relation to animal activity in a pig barn. Biosyst. Eng. 2009, 104, 118–124. [Google Scholar] [CrossRef]
- Costa, A.; Guarino, M. Definition of yearly emission factor of dust and greenhouse gases through continuous measurements in swine husbandry. Atmos. Environ. 2009, 43, 1548–1556. [Google Scholar] [CrossRef]
- Calvet, S.; Gates, R.S.; Zhang, G.; Estellés, F.; Ogink, N.W.; Pedersen, S.; Berckmans, D. Measuring gas emissions from livestock buildings: A review on uncertainty analysis and error sources. Biosyst. Eng. 2013, 116, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Hassouna, M.; Calvet, S.; Gates, R.; Hayes, E.; Schrade, S. Measurement of Gaseous Emissions from Animal Housing. In Introduction to Biosystems Engineering; Holden, N.M., Wolfe, M.L., Ogejo, J.A., Cummins, E.J., Eds.; American Society of Agricultural and Biological Engineers (ASABE) and Virginia Tech Publishing: St Joseph, MI, USA, 2020; ISBN 978-1-949373-97-4. [Google Scholar] [CrossRef]
- Cambra-López, M.; Aarnink, A.J.A.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef]
- Kulkarni, P.; Baron, P.A.; Willeke, K. Introduction to aerosol characterization. In Aerosol Measurement: Principles, Techniques, and Applications; John Wiley & Sans: Hoboken, NJ, USA, 2011; pp. 3–13. [Google Scholar]




| Weight | Temperature Range |
|---|---|
| 5–14 kg | 24–29 °C |
| 14–23 kg | 21–27 °C |
| 23–34 kg | 16–21 °C |
| 34–82 kg | 13–21 °C |
| >82 kg | 10–21 °C |
| Weight (kg) | |||
|---|---|---|---|
| 20 | 60 | 100 | |
| Temperature (°C) | |||
| SUMMER | |||
| Draught-free insulated shelter | 14 | 12 | 7 |
| Draught-free shelter | 17 | 15 | 11 |
| Insulated shelter with straw bedding | 10 | 8 | 2 |
| WINTER | |||
| Ventilated insulated shelter | 18 | 16 | 13 |
| Ventilated shelter | 23 | 20 | 17 |
| Non-insulated shelter with concrete floor | 29 | 25 | 22 |
| Harmful Gas | Acceptable Concentration |
|---|---|
| NH3 (Ammonia) | 10 ppm |
| CO2 (Carbon Dioxide) | 3000 ppm |
| H2S (Hydrogen Sulfide) | 0.5 ppm (5 ppm during evacuation) |
| Pollutant | Effect |
|---|---|
| Ammonia (NH3) | Eye and/or respiratory tract inflammation, anorexia, irritation of the mucous membranes of the respiratory tract, reduction of immunity, and specific illnesses. |
| Carbon Dioxide (CO2) | Increased respiratory rate, breathing difficulties, possible light-headedness, dizziness, and unconsciousness. |
| Hydrogen Sulphide (H2S) | Eye and/or respiratory system inflammation, smell disturbances, anorexia, nausea, and diarrhoea. |
| Dust | Irritation of the respiratory and ocular system, the respirable fraction (<5 µm) can combine with harmful pollutants and originate the secondary particulate matter. |
| Analysis Classification | Substrate | Techniques | |
|---|---|---|---|
| All | Collect dust for chemical, physical, or biochemical evaluations | Electrostatic precipitation | Gravimetry Tapered element oscillating microbalance (TEOM) Beta-ray attenuation principle Photometry |
| Physical | MASS SAMPLING Concentration of particles (number and mass) | Total dust | |
| Particle separation (Filtering by size) | Cyclone separator Cascade impactor Vertical elutriator | ||
| PARTICLE COUNTING Particle-size distribution (PSD) (number and mass) | Light scattering | ||
| Beta attenuation Laser | |||
| Laser light microscopy | |||
| Scanning electron microscopy | |||
| Chemical | Source apportionment | Scanning electron microscopy with X-ray (SEM-EDX) | |
| Inorganic compound | X-ray fluorescence | ||
| proton induced X-ray emission | |||
| Organic compound | atomic absorption spectrophotometry inductively coupled plasma with atomic emission spectroscopy inductively coupled plasma with mass spectroscopy | ||
| Toxins | Standard analytical methods (i.e., Limulus Amebocyte Lysate assay) | Endotoxin, aflatoxin, | |
| Allergens | Standard analytical methods | Protein analysis Antigenic analysis | |
| Microbiological | Number of viable and non-viable bacteria/viruses/fungi | Andersen microbial sampler (AMS) | |
| Nuclepore filtration and elution method (NFE) | |||
| all-glass impinger method (AGI) | |||
| Ventilation Rate Measuring Technique | Error | Reference |
|---|---|---|
| Impeller anemometer | 2–25% | Novalynx Corporation, Davis Instruments, [93] |
| Hot wire anemometer | 0.5–25% | [94,95] |
| CO2 balance | 15–40% | [96,97] |
| Thermic balance | 30–100% | [28,96] |
| Tracer gas | 10–50% | [41,98,99] |
| Ventilation rate control through a free running impeller | 3% | [100,101,102] |
| Equipment | Utility | Compounds | Accuracy | Detection Limit | |
|---|---|---|---|---|---|
| Chemical Technique | Reactant colorimetric Tubes | approximative measurements | all compounds | 85–98% | depends on analyzed compounds |
| Chemical + Physical Technique | Acid traps + Colorimetry | - | all compounds soluble in aqueous solutions | all compounds soluble in aqueous solutions | depends on analyzed compounds |
| Physical Technique | Gas Chromatography | pseudocontinuous measurements | depends on detectors | depends on detectors | depends on analyzed compounds |
| Optical Technique | Infrared photoacoustic detector | continuous measurements | all compounds | all compounds | ~0.01–0.02 ppmv |
| Optical Technique | Fourier transform infrared (FTIR) Spectroscopy | - | NH3, CH4, CO2, N2O | NH3, CH4, CO2, N2O | ~10 ppbv |
| Chemical Technique | Proton Transfer Reaction-Mass Spectrometry | continuous & real-time measurements | molecules with affinity to protons | molecules with affinity to protons | ~1 ppbv |
| Physical Technique | Chemiluminescence NO Analyzer | - | NOx and NH3 | NOx and NH3 | ~0.2 ppmv |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buoio, E.; Cialini, C.; Costa, A. Air Quality Assessment in Pig Farming: The Italian Classyfarm. Animals 2023, 13, 2297. https://doi.org/10.3390/ani13142297
Buoio E, Cialini C, Costa A. Air Quality Assessment in Pig Farming: The Italian Classyfarm. Animals. 2023; 13(14):2297. https://doi.org/10.3390/ani13142297
Chicago/Turabian StyleBuoio, Eleonora, Chiara Cialini, and Annamaria Costa. 2023. "Air Quality Assessment in Pig Farming: The Italian Classyfarm" Animals 13, no. 14: 2297. https://doi.org/10.3390/ani13142297







