Transcriptome-Based Identification of the Muscle Tissue-Specific Expression Gene CKM and Its Regulation of Proliferation, Apoptosis and Differentiation in Chicken Primary Myoblasts
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Sample Collection
2.3. Data Collection
2.4. Rapid Amplification of cDNA Ends (RACE)
2.5. Plasmid Construction and Small Interfering RNA (siRNA) Synthesis
2.6. Isolation, Culture and Transfection of Chicken Primary Myoblasts (CPMs)
2.7. RNA Exaction, cDNA Synthesis, and Quantitative Real-Time PCR (qPCR)
2.8. Cell Counting Kit-8 (CCK-8) Assay
2.9. 5-Ethynyl-2′-Deoxyuridine (EdU) Incorporation Assay
2.10. Flow Cytometry Analyses
2.11. Immunofluorescence Analysis
2.12. Western Blotting (WB)
2.13. RNA-seq and Differential Expression Genes (DEGs) Analysis
2.14. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Analyses
2.15. Statistical Analysis
3. Results
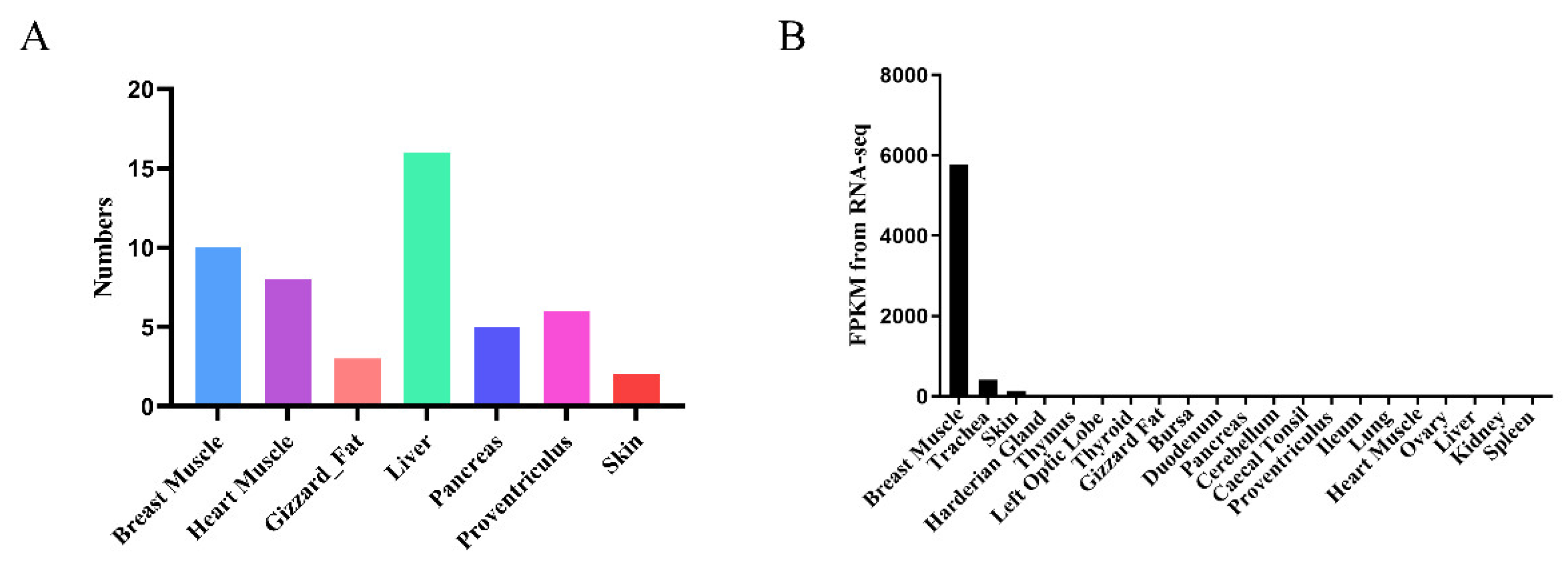
3.1. Identification and Analysis of Chicken Tissue-Specific Genes
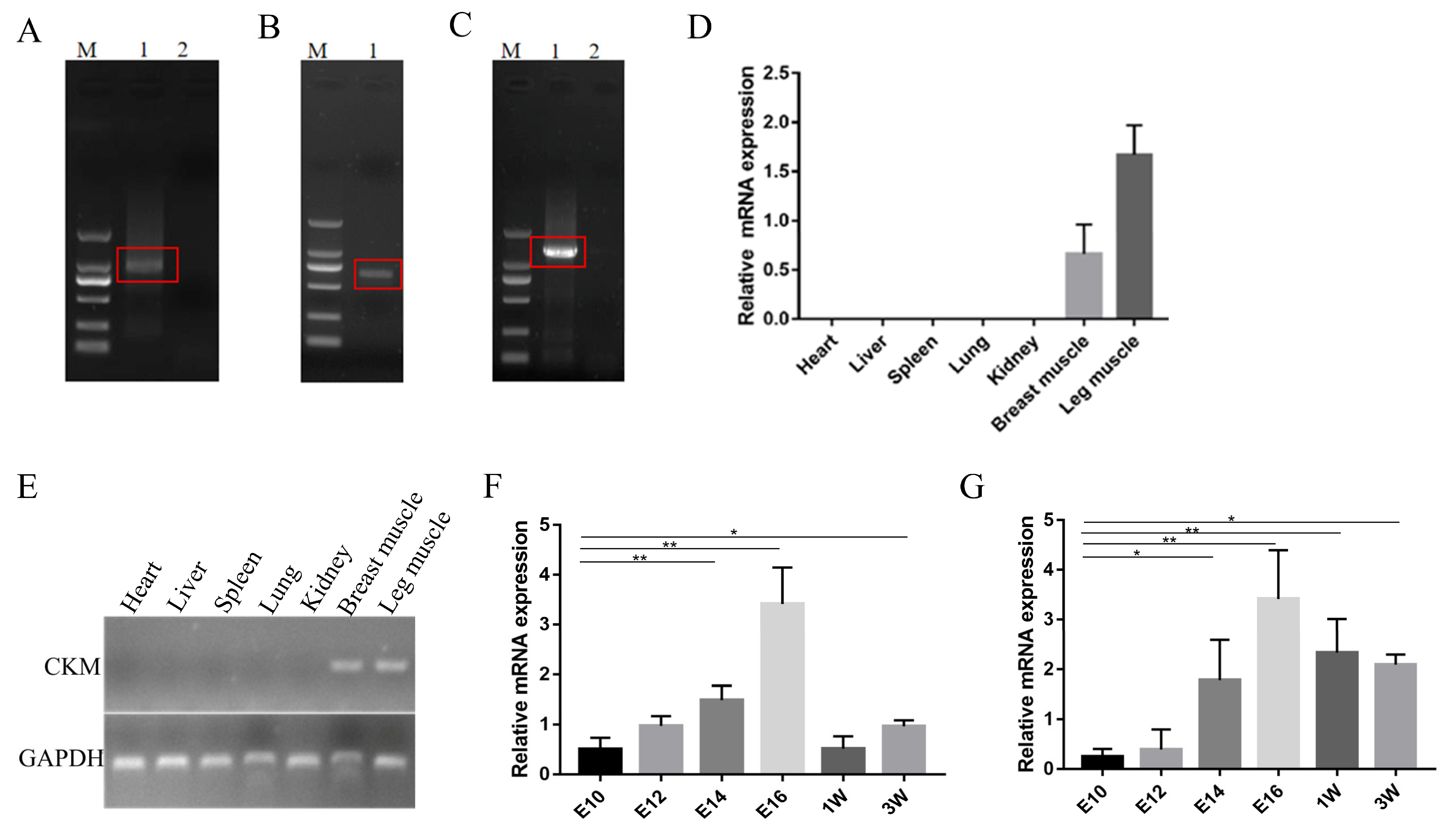
3.2. Expression Pattern of CKM in Chickens
3.3. CKM Inhibits the Proliferation of CPMs
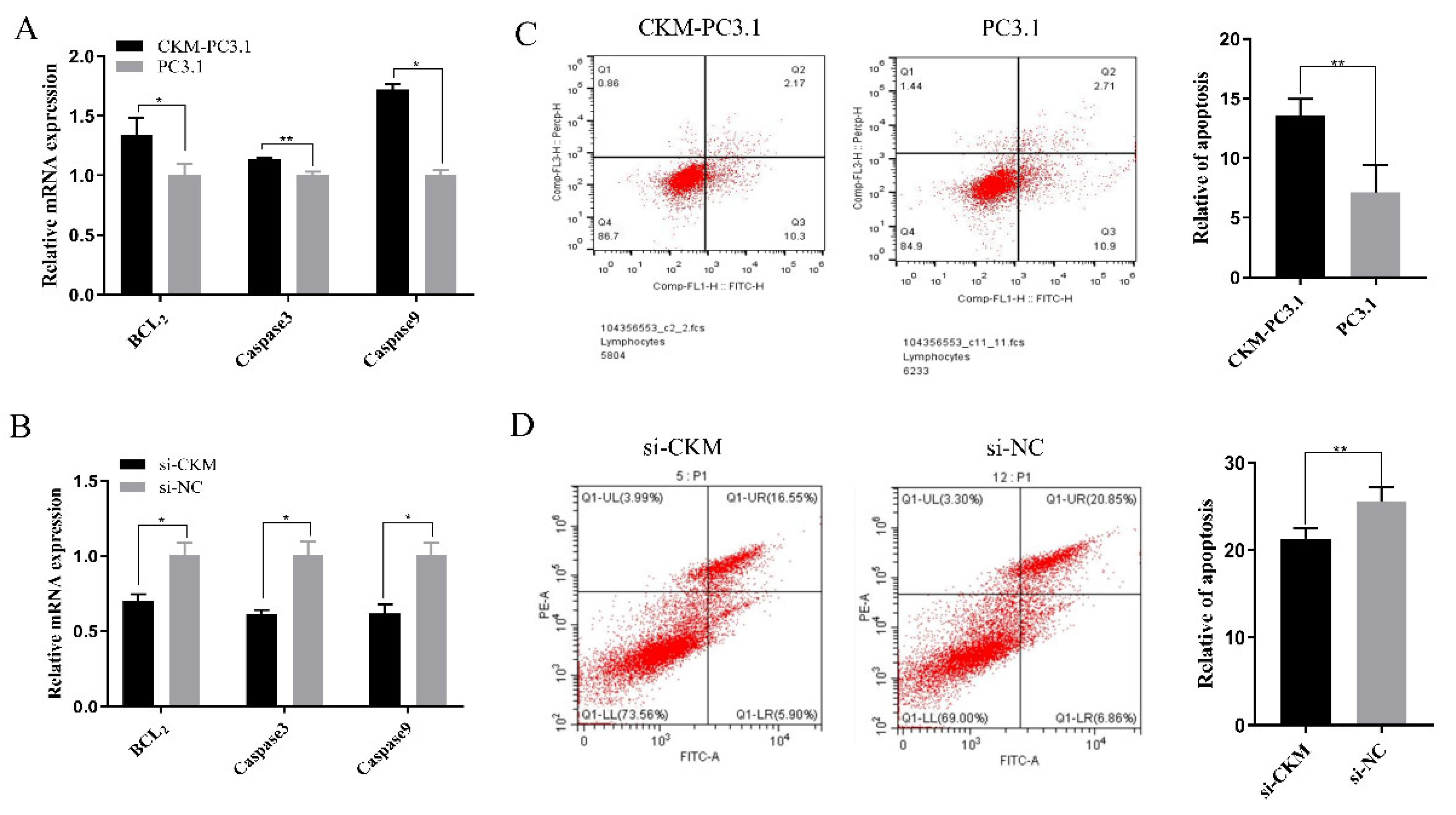
3.4. CKM Promotes Apoptosis in CPMs
3.5. CKM Promotes the Differentiation of CPMs
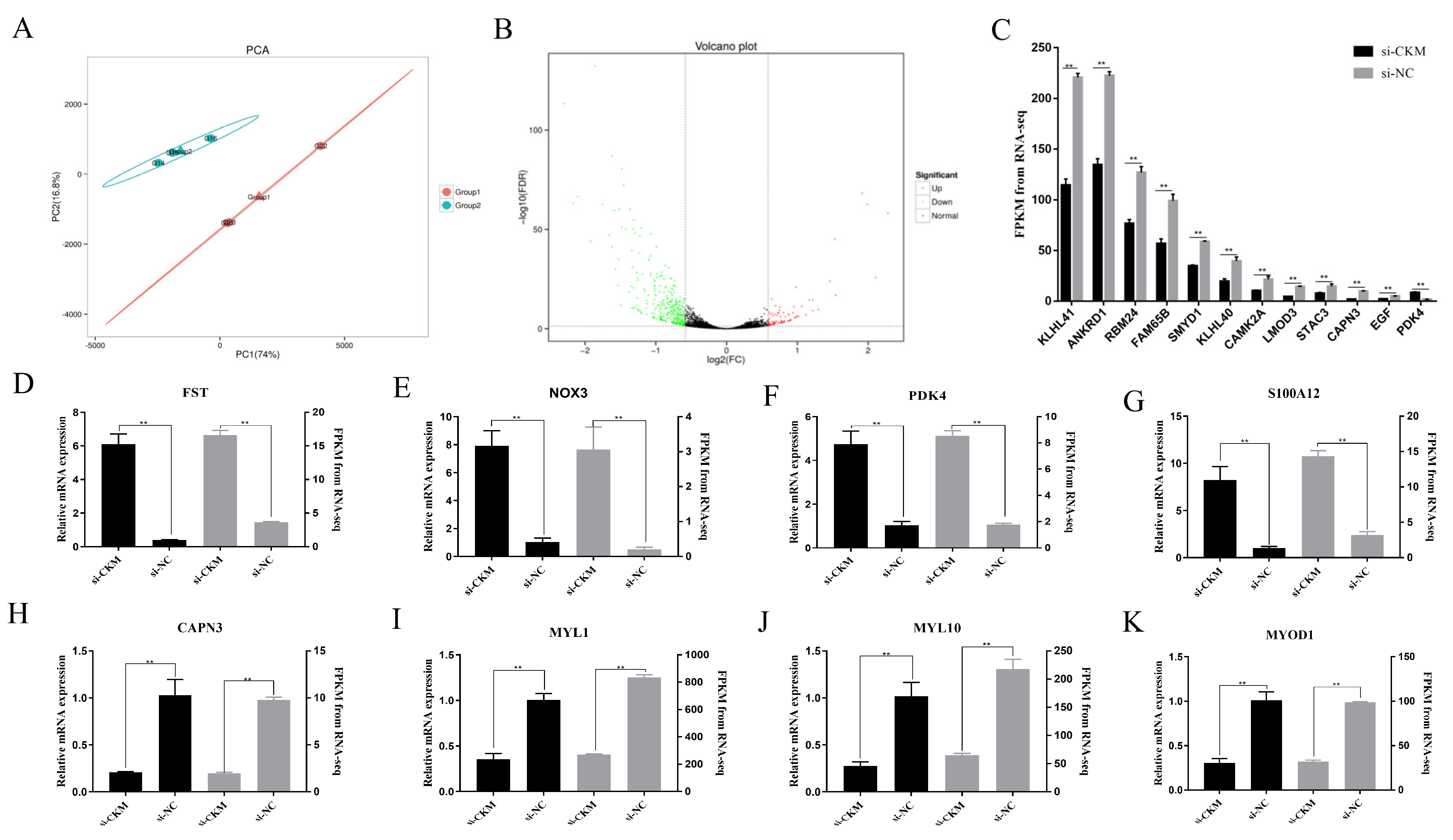
3.6. RNA-seq Analysis of Chicken Primary Myoblasts after CKM Interference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ge, X.; Zhang, Y.; Park, S.; Cong, X.; Gerrard, D.E.; Jiang, H. Stac3 inhibits myoblast differentiation into myotubes. PLoS ONE 2014, 9, e95926. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Lin, Z.; Chen, J.; Chen, G.; Zhang, S.; Liu, M.; Li, H.; He, D.; Liang, S.; Luo, Q.; et al. TMEM182 interacts with integrin beta 1 and regulates myoblast differentiation and muscle regeneration. J. Cachexia Sarcopenia Muscle 2021, 12, 1704–1723. [Google Scholar] [CrossRef] [PubMed]
- Esteves de Lima, J.; Relaix, F. Epigenetic Regulation of Myogenesis: Focus on the Histone Variants. Int. J. Mol. Sci. 2021, 22, 12727. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Li, X.; Liu, Z.; Ni, W.; Zhang, X.; Hazi, W.; Ma, Q.; Zhang, Y.; Cao, Y.; Qi, J.; et al. Identification and characterization of long non-coding RNA in prenatal and postnatal skeletal muscle of sheep. Genomics 2019, 111, 133–141. [Google Scholar] [CrossRef]
- Buckingham, M. Gene regulatory networks and cell lineages that underlie the formation of skeletal muscle. Proc. Natl. Acad. Sci. USA 2017, 114, 5830–5837. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Liu, J.; Wang, J.; Zheng, M.; Tan, X.; Xing, S.; Cui, H.; Li, Q.; Zhao, G.; et al. Uncovering the embryonic development-related proteome and metabolome signatures in breast muscle and intramuscular fat of fast-and slow-growing chickens. BMC Genom. 2017, 18, 816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.L.; Ye, J.L.; Yang, J.; Gao, C.Q.; Yan, H.C.; Li, H.C.; Wang, X.Q. mTORC1 Mediates Lysine-Induced Satellite Cell Activation to Promote Skeletal Muscle Growth. Cells 2019, 8, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef]
- Chen, G.; Yin, Y.; Lin, Z.; Wen, H.; Chen, J.; Luo, W. Transcriptome profile analysis reveals KLHL30 as an essential regulator for myoblast differentiation. Biochem. Biophys. Res. Commun. 2021, 559, 84–91. [Google Scholar] [CrossRef]
- Ibrahim, D.; Al-Khalaifah, H.S.; Abdelfattah-Hassan, A.; Eldoumani, H.; Khater, S.I.; Arisha, A.H.; Mohamed, S.A.M.; Ismail, T.A.; Tolba, S.A. Promising Role of Growth Hormone-Boosting Peptide in Regulating the Expression of Muscle-Specific Genes and Related MicroRNAs in Broiler Chickens. Animals 2021, 11, 1906. [Google Scholar] [CrossRef]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992, 281, 21–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenyon, G.L.; Reed, G.H. Creatine kinase: Structure-activity relationships. Adv. Enzymol. Relat. Areas Mol. Biol. 1983, 54, 367–426. [Google Scholar] [CrossRef] [PubMed]
- Eppenberger, H.M.; Dawson, D.M.; Kaplan, N.O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J. Biol. Chem. 1967, 242, 204–209. [Google Scholar] [CrossRef]
- Schlegel, J.; Wyss, M.; Eppenberger, H.M.; Wallimann, T. Functional studies with the octameric and dimeric form of mitochondrial creatine kinase. Differential pH-dependent association of the two oligomeric forms with the inner mitochondrial membrane. J. Biol. Chem. 1990, 265, 9221–9227. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, E.; Grove, B.K.; Wallimann, T.; Eppenberger, H.M.; Thornell, L.E. Myofibrillar M-band proteins in rat skeletal muscles during development. Histochemistry 1990, 95, 27–35. [Google Scholar] [CrossRef]
- Fernández-Torres, J.; Martínez-Nava, G.A.; Zamudio-Cuevas, Y.; Barbier, O.C.; Narváez-Morales, J.; Martínez-Flores, K. Ancestral contribution of the muscle-specific creatine kinase (CKM) polymorphism rs4884 in the knee osteoarthritis risk: A preliminary study. Clin. Rheumatol. 2021, 40, 279–285. [Google Scholar] [CrossRef]
- Moat, S.J.; Korpimäki, T.; Furu, P.; Hakala, H.; Polari, H.; Meriö, L.; Mäkinen, P.; Weeks, I. Characterization of a Blood Spot Creatine Kinase Skeletal Muscle Isoform Immunoassay for High-Throughput Newborn Screening of Duchenne Muscular Dystrophy. Clin. Chem. 2017, 63, 908–914. [Google Scholar] [CrossRef] [Green Version]
- Crum-Cianflone, N.F. Bacterial, fungal, parasitic, and viral myositis. Clin. Microbiol. Rev. 2008, 21, 473–494. [Google Scholar] [CrossRef] [Green Version]
- Saenger, A.K. A tale of two biomarkers: The use of troponin and CK-MB in contemporary practice. Clin. Lab. Sci. J. Am. Soc. Med. Technol. 2010, 23, 134–140. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Serum creatine kinase in patients with rheumatic diseases. Clin. Rheumatol. 2000, 19, 296–300. [Google Scholar] [CrossRef]
- Morandi, L.; Angelini, C.; Prelle, A.; Pini, A.; Grassi, B.; Bernardi, G.; Politano, L.; Bruno, C.; De Grandis, D.; Cudia, P.; et al. High plasma creatine kinase: Review of the literature and proposal for a diagnostic algorithm. Neurol. Sci. 2006, 27, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, J.; Heerschap, A.; Oerlemans, F.; Ruitenbeek, W.; Jap, P.; ter Laak, H.; Wieringa, B. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell 1993, 74, 621–631. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhuang, R.; Zhao, G.; Liu, Y.; Su, Y.; Wang, W.; Xi, X.; Yang, Y.; Han, X.; Xie, S.; et al. Identification of the CKM Gene as a Potential Muscle-Specific Safe Harbor Locus in Pig Genome. Genes 2022, 13, 921. [Google Scholar] [CrossRef]
- Wei, C.; Niu, Y.; Chen, B.; Qin, P.; Wang, Y.; Hou, D.; Li, T.; Li, R.; Wang, C.; Yin, H.; et al. Genetic effect of an InDel in the promoter region of the NUDT15 and its effect on myoblast proliferation in chickens. BMC Genom. 2022, 23, 138. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. MCP 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaiz Noya, M.; Berti, F.; Dietrich, S. Comprehensive expression analysis for the core cell cycle regulators in the chicken embryo reveals novel tissue-specific synexpression groups and similarities and differences with expression in mouse, frog and zebrafish. J. Anat. 2022, 241, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, F.; Gao, H.; Zhang, X.; Li, X.; Xiao, D. CAPN3: A muscle-specific calpain with an important role in the pathogenesis of diseases (Review). Int. J. Mol. Med. 2021, 48, 203. [Google Scholar] [CrossRef] [PubMed]
- Grifone, R.; Xie, X.; Bourgeois, A.; Saquet, A.; Duprez, D.; Shi, D.L. The RNA-binding protein Rbm24 is transiently expressed in myoblasts and is required for myogenic differentiation during vertebrate development. Mech. Dev. 2014, 134, 1–15. [Google Scholar] [CrossRef]
- Tan, X.; Rotllant, J.; Li, H.; De Deyne, P.; Du, S.J. SmyD1, a histone methyltransferase, is required for myofibril organization and muscle contraction in zebrafish embryos. Proc. Natl. Acad. Sci. USA 2006, 103, 2713–2718. [Google Scholar] [CrossRef]
- Garg, A.; O’Rourke, J.; Long, C.; Doering, J.; Ravenscroft, G.; Bezprozvannaya, S.; Nelson, B.R.; Beetz, N.; Li, L.; Chen, S.; et al. KLHL40 deficiency destabilizes thin filament proteins and promotes nemaline myopathy. J. Clin. Investig. 2014, 124, 3529–3539. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Martinez, A.; Cenik, B.K.; Bezprozvannaya, S.; Chen, B.; Bassel-Duby, R.; Liu, N.; Olson, E.N. KLHL41 stabilizes skeletal muscle sarcomeres by nonproteolytic ubiquitination. eLife 2017, 6, e26439. [Google Scholar] [CrossRef]
- Rufenach, B.; Van Petegem, F. Structure and function of STAC proteins: Calcium channel modulators and critical components of muscle excitation-contraction coupling. J. Biol. Chem. 2021, 297, 100874. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Kawahara, G.; Gupta, V.A.; Rozkalne, A.; Beauvais, A.; Kunkel, L.M.; Gussoni, E. Fam65b is important for formation of the HDAC6-dysferlin protein complex during myogenic cell differentiation. FASEB J. 2014, 28, 2955–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.H.; Wang, A.; Dai, W.; Chen, S.; Ding, Y.; Sun, L.V. Lmod3 promotes myoblast differentiation and proliferation via the AKT and ERK pathways. Exp. Cell Res. 2020, 396, 112297. [Google Scholar] [CrossRef]
- Samaras, S.E.; Almodóvar-García, K.; Wu, N.; Yu, F.; Davidson, J.M. Global deletion of Ankrd1 results in a wound-healing phenotype associated with dermal fibroblast dysfunction. Am. J. Pathol. 2015, 185, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Ciano, M.; Mantellato, G.; Connolly, M.; Paul-Clark, M.; Willis-Owen, S.; Moffatt, M.F.; Cookson, W.; Mitchell, J.A.; Polkey, M.I.; Hughes, S.M.; et al. EGF receptor (EGFR) inhibition promotes a slow-twitch oxidative, over a fast-twitch, muscle phenotype. Sci. Rep. 2019, 9, 9218. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Du, X.; Wen, L.; Li, Y.; Qin, J.; Chen, Z.; Huang, Y.; Wu, X.; Luo, H.; Lin, Y.; et al. Transcriptome analysis reveals the involvement of ubiquitin-proteasome pathway in the regulation of muscle growth of rice flower carp. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 41, 100948. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Ge, K.; Li, M.; Yang, L.; Jin, S.; Zhang, C.; Chen, X.; Geng, Z. Egg-laying and brooding stage-specific hormonal response and transcriptional regulation in pituitary of Muscovy duck (Cairina moschata). Poult. Sci. 2019, 98, 5287–5296. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, X.; Zhang, G.; Chen, F.; He, M.; Zhang, T.; Wang, J.; Xie, K.; Dai, G. Transcriptome for the breast muscle of Jinghai yellow chicken at early growth stages. PeerJ 2020, 8, e8950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Cao, J.; Zhang, J.; Ge, L.; Zhang, H.; Liu, X. Skeletal Muscle Transcriptome Analysis of Hanzhong Ma Duck at Different Growth Stages Using RNA-Seq. Biomolecules 2021, 11, 315. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Yang, X.; Liang, C.; Raza, S.H.A.; Pan, Y.; Zhang, K.; Zan, L. Integration of RNA-seq and ATAC-seq identifies muscle-regulated hub genes in cattle. Front. Vet. Sci. 2022, 9, 925590. [Google Scholar] [CrossRef]
- Bouwman, F.G.; van Ginneken, M.M.; van der Kolk, J.H.; van Breda, E.; Mariman, E.C. Novel markers for tying-up in horses by proteomics analysis of equine muscle biopsies. Comp. Biochem. Physiol. Part D Genom. Proteom. 2010, 5, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, A.; Wang, Q.; Wang, H.; Dong, D.; Liu, L. Transcriptome analysis of embryonic muscle development in Chengkou Mountain Chicken. BMC Genom. 2021, 22, 431. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, X.; Xue, H.; Zou, X.; Liu, G.; Deng, M.; Sun, B.; Guo, Y.; Liu, D.; Li, Y. RNA-Seq Reveals miRNA and mRNA Co-regulate Muscle Differentiation in Fetal Leizhou Goats. Front. Vet. Sci. 2022, 9, 829769. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Cai, B.; Ma, M.; Zhou, Z.; Kong, S.; Zhang, J.; Zhang, X.; Nie, Q. circPTPN4 regulates myogenesis via the miR-499-3p/NAMPT axis. J. Anim. Sci. Biotechnol. 2022, 13, 2. [Google Scholar] [CrossRef]
- Wu, S.; Su, R.; Jia, H. Cyclin B2 (CCNB2) Stimulates the Proliferation of Triple-Negative Breast Cancer (TNBC) Cells In Vitro and In Vivo. Dis. Markers 2021, 2021, 5511041. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Gao, X.; Li, Y.; Lin, J.; Chen, L.; Chang, L.; Chen, G.; Guan, Y.; Pan, L.K.; et al. CCND1 Amplification Contributes to Immunosuppression and Is Associated With a Poor Prognosis to Immune Checkpoint Inhibitors in Solid Tumors. Front. Immunol. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Zhang, D.; Ran, J.; Li, J.; Yu, C.; Cui, Z.; Amevor, F.K.; Wang, Y.; Jiang, X.; Qiu, M.; Du, H.; et al. miR-21-5p Regulates the Proliferation and Differentiation of Skeletal Muscle Satellite Cells by Targeting KLF3 in Chicken. Genes 2021, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L.; Serfas, M.S.; Tyner, A.L. p21--negative regulator of the cell cycle. Proc. Soc. Exp. Biol. Med. 1996, 213, 138–149. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.Y.; Chen, H.; Wu, Y.C.; Zhang, L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020, 53, e12739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Kuida, K. Caspase-9. Int. J. Biochem. Cell Biol. 2000, 32, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.; Yao, Y.; Ma, L.; Wang, X.; Shi, X.; Yang, G.; Li, X. The long noncoding RNA MyHC IIA/X-AS contributes to skeletal muscle myogenesis and maintains the fast fiber phenotype. J. Biol. Chem. 2020, 295, 4937–4949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrovidov, S.; Hosseini, V.; Ahadian, S.; Fujie, T.; Parthiban, S.P.; Ramalingam, M.; Bae, H.; Kaji, H.; Khademhosseini, A. Skeletal muscle tissue engineering: Methods to form skeletal myotubes and their applications. Tissue Eng. Part B Rev. 2014, 20, 403–436. [Google Scholar] [CrossRef]
- Ren, K.; Crouzier, T.; Roy, C.; Picart, C. Polyelectrolyte multilayer films of controlled stiffness modulate myoblast cells differentiation. Adv. Funct. Mater. 2008, 18, 1378–1389. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Gu, X.; Hu, D.; Hao, Y. Co-culture with synovial tissue in patients with rheumatoid arthritis suppress cell proliferation by regulating MAPK pathway in osteoblasts. Am. J. Transl. Res. 2019, 11, 3317–3327. [Google Scholar]
- Sakelliou, A.; Fatouros, I.G.; Athanailidis, I.; Tsoukas, D.; Chatzinikolaou, A.; Draganidis, D.; Jamurtas, A.Z.; Liacos, C.; Papassotiriou, I.; Mandalidis, D.; et al. Evidence of a Redox-Dependent Regulation of Immune Responses to Exercise-Induced Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 2840643. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Wang, Y.; Hou, D.; Zhang, Y.; Zhang, B.; Niu, Y.; Ji, H.; Tian, Y.; Liu, X.; Kang, X.; et al. Transcriptome-Based Identification of the Muscle Tissue-Specific Expression Gene CKM and Its Regulation of Proliferation, Apoptosis and Differentiation in Chicken Primary Myoblasts. Animals 2023, 13, 2316. https://doi.org/10.3390/ani13142316
Chen B, Wang Y, Hou D, Zhang Y, Zhang B, Niu Y, Ji H, Tian Y, Liu X, Kang X, et al. Transcriptome-Based Identification of the Muscle Tissue-Specific Expression Gene CKM and Its Regulation of Proliferation, Apoptosis and Differentiation in Chicken Primary Myoblasts. Animals. 2023; 13(14):2316. https://doi.org/10.3390/ani13142316
Chicago/Turabian StyleChen, Bingjie, Yanxing Wang, Dan Hou, Yushi Zhang, Bochun Zhang, Yufang Niu, Haigang Ji, Yadong Tian, Xiaojun Liu, Xiangtao Kang, and et al. 2023. "Transcriptome-Based Identification of the Muscle Tissue-Specific Expression Gene CKM and Its Regulation of Proliferation, Apoptosis and Differentiation in Chicken Primary Myoblasts" Animals 13, no. 14: 2316. https://doi.org/10.3390/ani13142316





