Comparison of Telomere Length in Age-Matched Primiparous and Multiparous Brahman Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. White Blood Cell (WBC) Count with Differential
2.3. White Blood Cell (WBC) Isolation and DNA Extraction
2.4. Quantitative PCR
2.5. Statistical Analysis
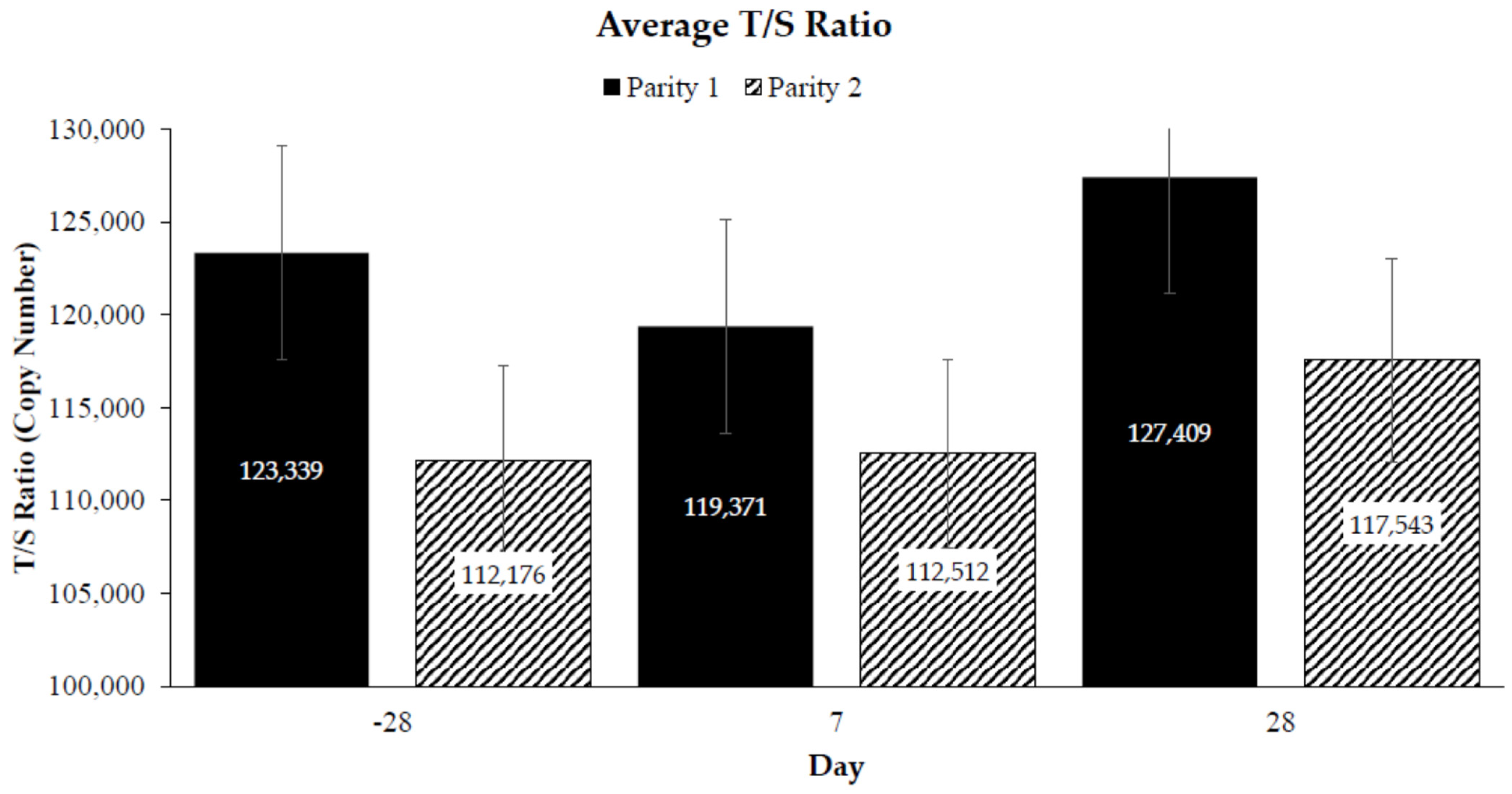
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, E.H.; Gall, J.G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J. Mol. Biol. 1978, 120, 33–53. [Google Scholar] [CrossRef]
- de La Seña, C.; Chowdhary, B.P.; Gustavsson, I. Localization of the Telomeric (TTAGGG)n Sequences in Chromosomes of Some Domestic Animals by Fluorescence in Situ Hybridization. Hereditas 1995, 123, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005, 579, 859–862. [Google Scholar] [CrossRef] [Green Version]
- Greider, C.W.; Blackburn, E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989, 337, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 360–361. [Google Scholar] [CrossRef]
- Lansdorp, P.M. Telomeres and disease. EMBO J. 2009, 28, 2532–2540. [Google Scholar] [CrossRef]
- Li, C.; Stoma, S.; Lotta, L.A.; Warner, S.; Albrecht, E.; Allione, A.; Arp, P.P.; Broer, L.; Buxton, J.L.; Alves, A.D.S.C.; et al. Genome-wide Association Analysis in Humans Links Nucleotide Metabolism to Leukocyte Telomere Length. Am. J. Hum. Genet. 2020, 106, 389–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haussmann, M.F.; Vleck, C.M. Telomere length provides a new technique for aging animals. Oecologia 2002, 130, 325–328. [Google Scholar] [CrossRef]
- Angelier, F.; Costantini, D.; Blevin, P.; Chastel, O. Do glucocorticoids mediate the link between environmental conditions and telomere dynamics in wild vertebrates? A review. Gen. Comp. Endocrinol. 2017, 10, 1016. [Google Scholar] [CrossRef]
- Seeker, L.A.; Ilska, J.J.; Psifidi, A.; Wilbourn, R.V.; Underwood, S.L.; Fairlie, J.; Holland, R.; Froy, H.; Salvo-Chirnside, E.; Bagnall, A.; et al. Bovine telomere dynamics and the association between telomere length and productive lifespan. Sci. Rep. 2018, 8, 12748. [Google Scholar] [CrossRef] [Green Version]
- Ilska-Warner, J.J.; Androniki, P.; Seeker, L.A.; Wilbourn, R.V.; Underwood, S.L.; Fairlie, J.; Whitelaw, B.; Nussey, D.H.; Coffey, M.P.; Banos, G. The genetic architecture of bovine telomere length in early life and association with animal fitness. Front. Genet. 2019, 10, 1048. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.; Dechow, C.; Liu, W.; Harvatine, K.; Ott, T. Hot topic: Association of telomere length with age, herd, and culling in lactating Holsteins. J. Dairy Sci. 2012, 95, 6384–6387. [Google Scholar] [CrossRef]
- Seeker, L.A.; Ilska, J.J.; Psifidi, A.; Wilbourn, R.V.; Underwood, S.L.; Fairlie, J.; Holland, R.; Froy, H.; Bagnall, A.; Whitelaw, B.; et al. Longitudinal changes in telomere length and associated genetic parameters in dairy cattle analysed using random regression models. PLoS ONE 2018, 13, e0192864. [Google Scholar] [CrossRef] [Green Version]
- Asghar, M.; Bensch, S.; Tarka, M.; Hansson, B.; Hasselquist, D. Maternal and genetic factors determine early life telomere length. Proc. R. Soc. B Boil. Sci. 2015, 282, 20142263. [Google Scholar] [CrossRef]
- Fairlie, J.; Holland, R.; Pilkington, J.G.; Pemberton, J.M.; Harrington, L.; Nussey, D.H. Lifelong leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell 2016, 15, 140–148. [Google Scholar] [CrossRef]
- Haussmann, M.F.; Marchetto, N.M. Telomeres: Linking stress and survival, ecology and evolution. Curr. Zool. 2010, 56, 714–727. [Google Scholar] [CrossRef]
- Haussmann, M.F.; Heidinger, B.J. Telomere dynamics may link stress exposure and ageing across generations. Biol. Lett. 2015, 11, 20150396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bateson, M. Cumulative stress in research animals: Telomere attrition as a biomarker in a welfare context? Bioessays 2015, 38, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Iannuzzi, A.; Albarella, S.; Parma, P.; Galdiero, G.; D’Anza, E.; Pistucci, R.; Peretti, V.; Ciotola, F. Characterization of telomere length in Agerolese cattle breed, correlating blood and milk samples. Anim. Genet. 2022, 53, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, T.; Coffey, M.; Mrode, R.; Wall, E. Understanding the genetics of survival in dairy cows. J. Dairy Sci. 2013, 96, 3296–3309. [Google Scholar] [CrossRef] [Green Version]
- Mayr, B.; Korb, H.; Oppeneiger, T.; Demetz, F.; Egger, J. Highly characteristic and individual specific telomere length patterns in cattle. Veter J. 2007, 174, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Tilesi, F.; Di Domenico, E.G.; Pariset, L.; Bosco, L.; Willems, D.; Valentini, A.; Ascenzioni, F. Telomere Length Diversity in Cattle Breeds. Diversity 2010, 2, 1118–1129. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Mu, T.; Ma, Y.; Wang, X.; Ma, Y. Analysis of Longevity Traits in Holstein Cattle: A Review. Front. Genet. 2022, 12, 695543. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Lin, J.; Dhabhar, F.S.; Wolkowitz, O.M.; Puterman, E.; Karan, L.; Blackburn, E.H. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav. Immun. 2010, 24, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotrschal, A.; Ilmonen, P.; Penn, D.J. Stress impacts telomere dynamics. Biol. Lett. 2007, 3, 128–130. [Google Scholar] [CrossRef]
- Reichert, S.; Stier, A.; Zahn, S.; Arrive, M.; Bize, P.; Massemin, S.; Criscuolo, F. Increased brood size leads to persistent eroded telomeres. Front. Ecol. Evol. 2014, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Sudyka, J.; Arct, A.; Drobniak, S.; Dubiec, A.; Gustafsson, L.; Cichoń, M. Experimentally increased reproductive effort alters telomere length in the blue tit (Cyanistes caeruleus). J. Evol. Biol. 2014, 27, 2258–2264. [Google Scholar] [CrossRef] [Green Version]
- FASS. Guide for Care and Use of Agricultural Animals in Research and Teaching, 3rd ed.; Federation of Animal Science Societies: Champaign, IL, USA, 2010. [Google Scholar]
- Herd, D.B.; Sprott, L.R. Body Condition, Nutrition, and Reproduction of Beef Cows; Texas Agricultural Extension Service Bull; B-1526; Texas Agricultural Extension Service: College Station, TX, USA, 1986. [Google Scholar]
- Triplett, B.L.; Neuendorff, D.A.; Randel, R.D. Influence of undegraded intake protein supplementation on milk production, weight gain, and reproductive performance in postpartum Brahman cows. J. Anim. Sci. 1995, 73, 3223–3229. [Google Scholar] [CrossRef] [Green Version]
- Texas A&M Veterinary Medical Diagnostic Laboratory. How to Properly Prepare a Blood Smear. 2022. Available online: https://tvmdl.tamu.edu/2022/02/21/how-to-properly-prepare-a-blood-smear/ (accessed on 7 July 2023).
- Woronzoff-Dashkoff, K.K. The Wright-Giemsa stain: Secrets revealed. Clin. Lab. Med. 2002, 22, 15–23. [Google Scholar] [CrossRef]
- Lynch, E.C. Peripheral blood smear. Chapter 155. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworth: Boston, MA, USA, 1990. Available online: https://www.ncbi.nlm.nih.gov/books/NBK263/ (accessed on 7 July 2023).
- Wood, R.D. Hematology of bovids. In Schalm’s Veterinary Hematology, 7th ed.; Brooks, M.B., Harr, K.E., Seelig, D.M., Wardrop, K.J., Weiss, D.J., Eds.; Chapter 111; John Wiley & Sons, Inc.: New York, NY, USA, 2022. [Google Scholar]
- Seeker, L.A.; Holland, R.; Underwood, S.; Fairlie, J.; Psifidi, A.; Ilska, J.J.; Bagnall, A.; Whitelaw, B.; Coffey, M.; Banos, G.; et al. Method Specific Calibration Corrects for DNA Extraction Method Effects on Relative Telomere Length Measurements by Quantitative PCR. PLoS ONE 2016, 11, e0164046. [Google Scholar] [CrossRef] [Green Version]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [Green Version]
- Merck Veterinary Manual. Available online: https://www.merckvetmanual.com/multimedia/table/hematology-complete-blood-count-reference-ranges (accessed on 7 July 2023).
- Pollack, A.Z.; Rivers, K.; Ahrens, K.A. Parity associated with telomere length among US reproductive age women. Hum. Reprod. 2018, 33, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauch, C.; Becker, P.H.; Verhulst, S. Telomere length reflects phenotypic quality and costs of reproduction in a long-lived seabird. Proc. R. Soc. B Boil. Sci. 2013, 280, 20122540. [Google Scholar] [CrossRef]
- Laubenthal, L.; Hoelker, M.; Frahm, J.; Dänicke, S.; Gerlach, K.; Südekum, K.-H.; Sauerwein, H.; Häussler, S. Short communication: Telomere lengths in different tissues of dairy cows during early and late lactation. J. Dairy Sci. 2016, 99, 4881–4885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beard, J.K.; Musgrave, J.A.; Hanford, K.J.; Funston, R.N.; Mulliniks, J.T. The effect of dam age on heifer progeny performance and longevity. Transl. Anim. Sci. 2019, 3, 1710–1713. [Google Scholar] [CrossRef] [Green Version]
- Meesters, M.; Van Eetvelde, M.; Martens, D.S.; Nawrot, T.S.; Dewulf, M.; Govaere, J.; Opsomer, G. Prenatal environment impacts telomere length in newborn dairy heifers. Sci. Rep. 2023, 13, 4672. [Google Scholar] [CrossRef] [PubMed]
- Froy, H.; Underwood, S.L.; Dorrens, J.; Seeker, L.A.; Watt, K.; Wilbourn, R.V.; Pilkington, J.G.; Harrington, L.; Pemberton, J.M.; Nussey, D.H. Heritable variation in telomere length predicts mortality in Soay sheep. Proc. Natl. Acad. Sci. USA 2021, 118, e2020563118. [Google Scholar] [CrossRef]
- Kyo, S.; Takakura, M.; Kanaya, T.; Zhuo, W.; Fujimoto, K.; Nishio, Y.; Orimo, A.; Inoue, M. Estrogen activates telomerase. Cancer Res 1999, 59, 5917–5921. [Google Scholar]
- Effros, R.B.; Dagarag, M.; Spaulding, C.; Man, J. The role of CD8+ T-cell replicative senescence in human aging. Immunol. Rev. 2005, 205, 147–157. [Google Scholar] [CrossRef]
- Choi, J.; Fauce, S.R.; Effros, R.B. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain, Behav. Immun. 2008, 22, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2018, 177, 37–45. [Google Scholar] [CrossRef]
- Murphy, M.G.; Boland, M.P.; Roche, J.F. Pattern of follicular growth and resumption of ovarian activity in post-partum beef suckler cows. Reproduction 1990, 90, 523–533. [Google Scholar] [CrossRef] [Green Version]
- Wood, C.E. Control of parturition in ruminants. J. Reprod. Fertil. Suppl. 1999, 54, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panelli, D.M.; Diwan, M.; Cruz, G.I.; Leonard, S.A.; Chueh, J.; Gotlib, I.H.; Bianco, K. An exploratory analysis of leukocyte telomere length among pregnant and non-pregnant people. Brain Behav. Immun. 2022, 25, 100506. [Google Scholar] [CrossRef]
- Panelli, D.M.; Leonard, S.A.; Wong, R.J.; Becker, M.; Mayo, J.A.; Wu, E.; Girsen, A.I.; Gotlib, I.H.; Aghaeepour, N.; Druzin, M.L.; et al. Leukocyte telomere dynamics across gestation in uncomplicated pregnancies and associations with stress. BMC Pregnancy Childbirth 2022, 22, 381–392. [Google Scholar] [CrossRef]
- Panelli, D.M.; Mayo, J.A.; Wong, R.J.; Becker, M.; Maric, I.; Wu, E.; Gotlib, I.H.; Aghaeepour, N.; Druzin, M.L.; Stevenson, D.K.; et al. Shorter maternal leukocyte telomere length following cesarean birth: Implications for future research. Amer. J. Obstet. Gynecol 2023, 228, S456–S457, (Abstract# 709). [Google Scholar] [CrossRef]
- Abu-Awwad, S.-A.; Craina, M.; Gluhovschi, A.; Ciordas, P.D.; Marian, C.; Boscu, L.; Bernad, E.; Iurciuc, M.; Abu-Awwad, A.; Iurciuc, S.; et al. Linking Pregnancy and Long-Term Health: The Impact of Cardiovascular Risk on Telomere Shortening in Pregnant Women. Medicina 2023, 59, 1012. [Google Scholar] [CrossRef] [PubMed]
- Welsh, T.H.; Kochran, K.; Earnhardt, A.L.; Cardoso, R.C.; Hairgrove, T.B.; Long, C.R.; Riley, D.; Randel, R.D. PSVIII-B-3 Comparison of Telomere Length in Leukocytes of Control and Prenatally Stressed Brahman Bull and Heifer Calves. J. Anim. Sci. 2022, 100, 314. [Google Scholar] [CrossRef]
- Whiteman, V.E.; Goswami, A.; Salihu, H.M. Telomere length and fetal programming: A review of recent scientific advances. Am. J. Reprod. Immunol. 2017, 77, e12661. [Google Scholar] [CrossRef] [Green Version]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of telomere length across human tissues. Science 2020, 369, eaaz6876. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nawrot, T.S.; Van Der Stukken, C.; Tylus, D.; Sleurs, H.; Peusens, M.; Alfano, R.; Langie, S.A.; Plusquin, M.; Martens, D.S. Different epigenetic signatures of newborn telomere length and telomere attrition rate in early life. Aging 2021, 13, 14630–14650. [Google Scholar] [CrossRef] [PubMed]
- Vinayagamurthy, S.; Bagri, S.; Mergny, J.-L.; Chowdhury, S. Telomeres expand sphere of influence: Emerging molecular impact of telomeres in non-telomeric functions. Trends Genet. 2023, 39, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Xu, Y.; Wan, H.; Yan, R.; Guo, J.; Skory, R.; Yan, L.; Wu, X.; Sun, F.; Chen, G.; et al. Neurulation of the cynomolgus monkey embryo achieved from 3D blastocyst culture. Cell 2023, 186, 2078–2091.e18. [Google Scholar] [CrossRef] [PubMed]

| Sample Day | ||||
|---|---|---|---|---|
| Parity | Day − 28 | Day + 7 | Day + 28 | |
| BW, kg | Parity 1 | 499 ± 15 | 507 ± 17 | 513 ± 9 |
| Parity 2 | 526 ± 14 | 521 ± 15 | 547 ± 10 | |
| BCS | Parity 1 | 7.00 ± 0.17 | 6.75 ± 0.21 | 6.63 ± 0.16 |
| Parity 2 | 6.90 ± 0.22 | 6.73 ± 0.22 | 6.68 ± 0.23 | |
| Target Gene | Gene Name | IDT Ref# 1 | Primer | Sequence |
|---|---|---|---|---|
| Telomere | Telomere | 207839206 207839207 | Forward Reverse | 5′-ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT-3′ 5′-TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA-3′ |
| β2G | Beta-2-Globulin | 207839208 207839209 | Forward Reverse | 5′-CGGCGGCGGGCGGCGCGGGCTGGGCGGGAAGGCCCATGGCAAGAAGG-3′ 5′-GCCGGCCCGCCGCGCCCGTCCCGCCGCTCACTCAGCCAC-AAAGG-3′ |
| Standard Name | IDT Ref # 1 | Strand | Sequence |
|---|---|---|---|
| Bovine Telomere | 206903826 | Sense | 5′-TTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTA GGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGG-3′ |
| Antisense | 5′-CCCTAACCCTAACCCTAACCCTAACCCTAACC CTAACCCTAACCCTAACCCTAACCCTAACCCTAACCCTAACCCTAA-3′ | ||
| Bovine Beta-2-Globulin | 206903823 | Sense | 5′-GGTGAAGGCCCATGGCAGAAGGTGCTAGATT CCTTTAGTAATGGCATGAAGCATCTCGATGACCTCAAGGGCACCTTTGCGCTGAGTGAGCTG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Daniel, S.E.; Kochan, K.J.; Long, C.R.; Riley, D.G.; Randel, R.D.; Welsh, T.H., Jr. Comparison of Telomere Length in Age-Matched Primiparous and Multiparous Brahman Cows. Animals 2023, 13, 2325. https://doi.org/10.3390/ani13142325
O’Daniel SE, Kochan KJ, Long CR, Riley DG, Randel RD, Welsh TH Jr. Comparison of Telomere Length in Age-Matched Primiparous and Multiparous Brahman Cows. Animals. 2023; 13(14):2325. https://doi.org/10.3390/ani13142325
Chicago/Turabian StyleO’Daniel, Sydney E., Kelli J. Kochan, Charles R. Long, David G. Riley, Ronald D. Randel, and Thomas H. Welsh, Jr. 2023. "Comparison of Telomere Length in Age-Matched Primiparous and Multiparous Brahman Cows" Animals 13, no. 14: 2325. https://doi.org/10.3390/ani13142325
APA StyleO’Daniel, S. E., Kochan, K. J., Long, C. R., Riley, D. G., Randel, R. D., & Welsh, T. H., Jr. (2023). Comparison of Telomere Length in Age-Matched Primiparous and Multiparous Brahman Cows. Animals, 13(14), 2325. https://doi.org/10.3390/ani13142325






