Comparison of Nonlinear Growth Models to Estimate Growth Curves in Kivircik Sheep under a Semi-Intensive Production System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Data Collection
2.2. Statistical Analysis
2.2.1. Exploratory Analysis: Principal Component Analysis
2.2.2. Nonlinear Growth Models
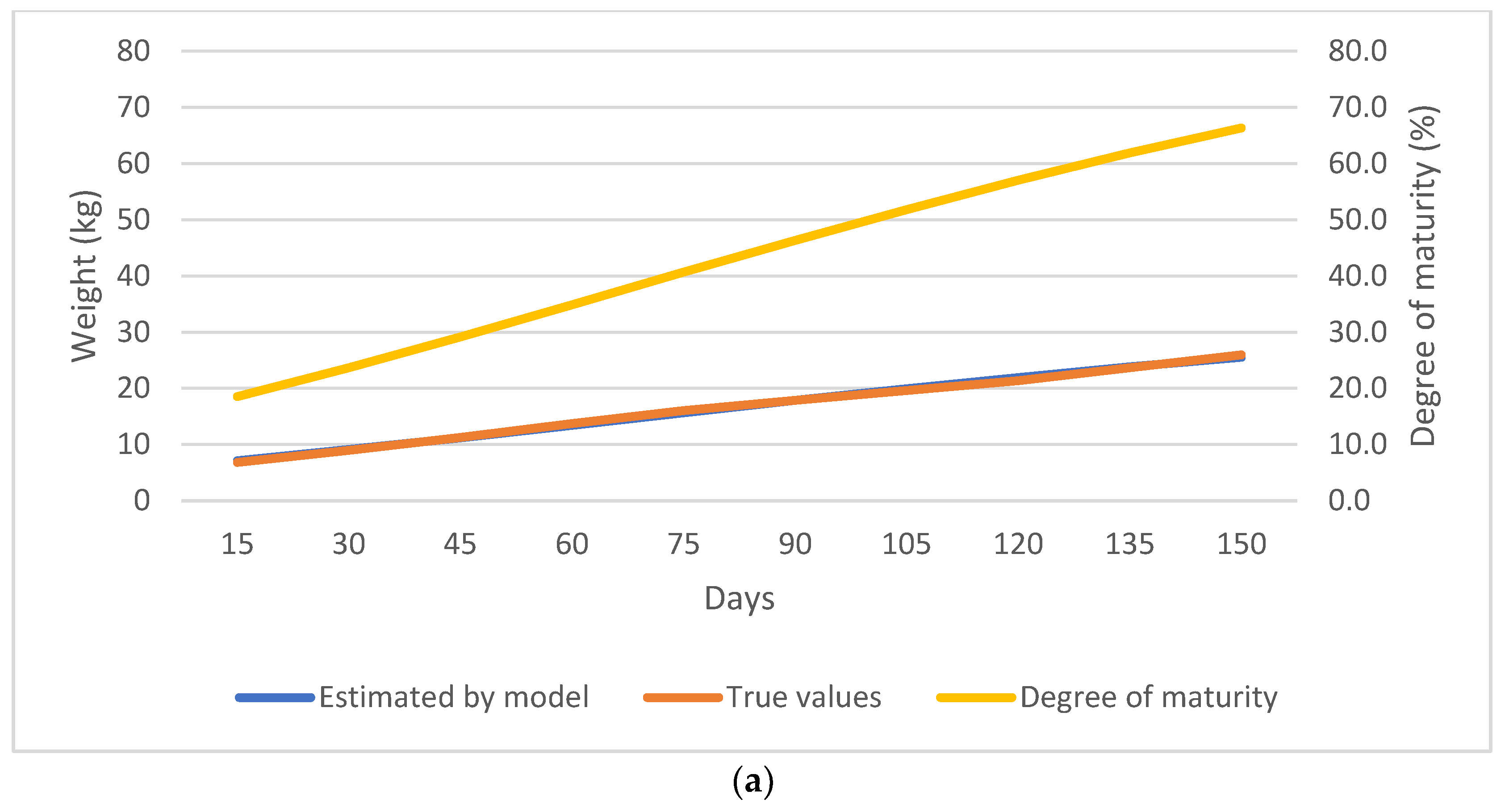
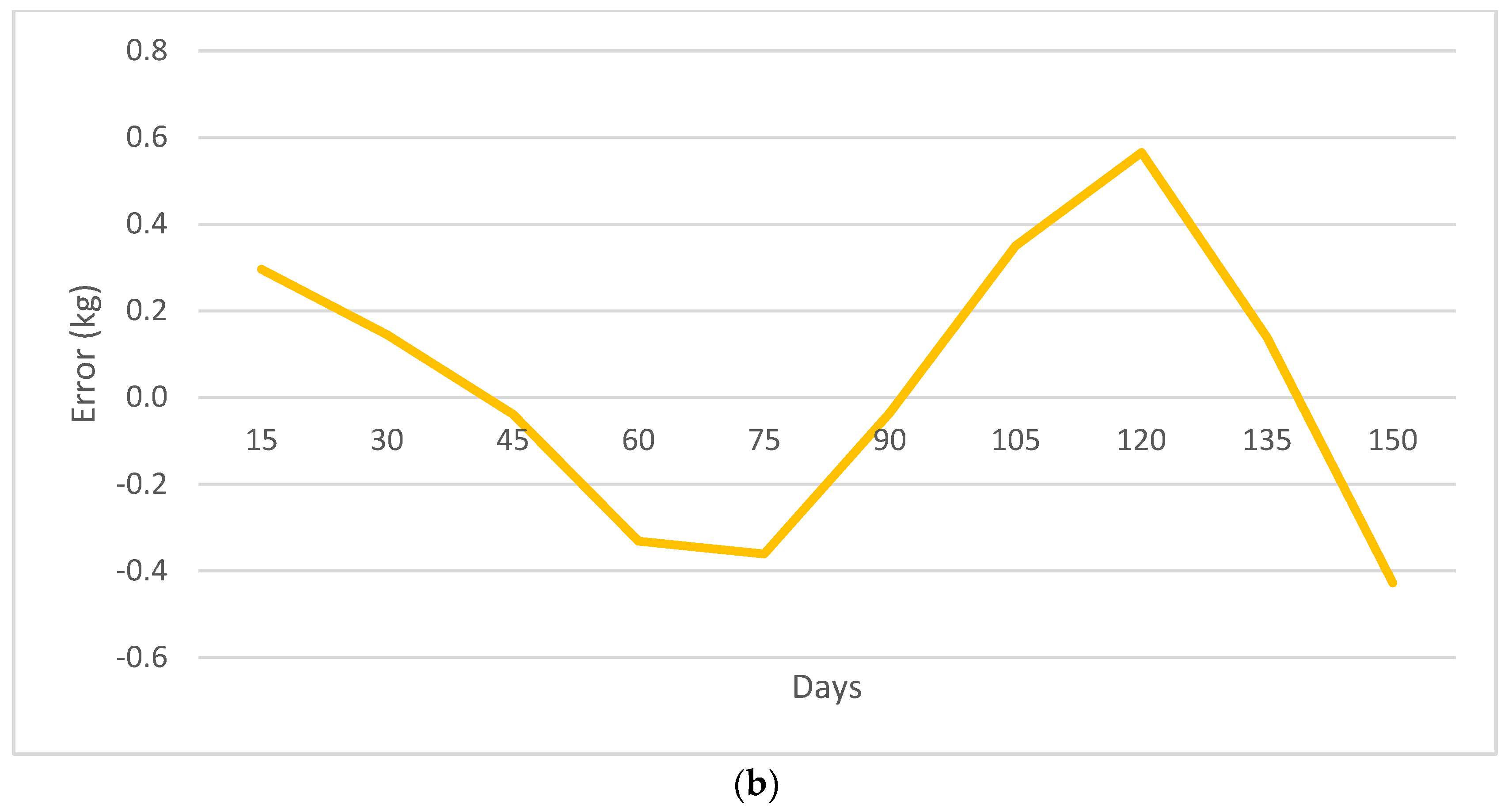
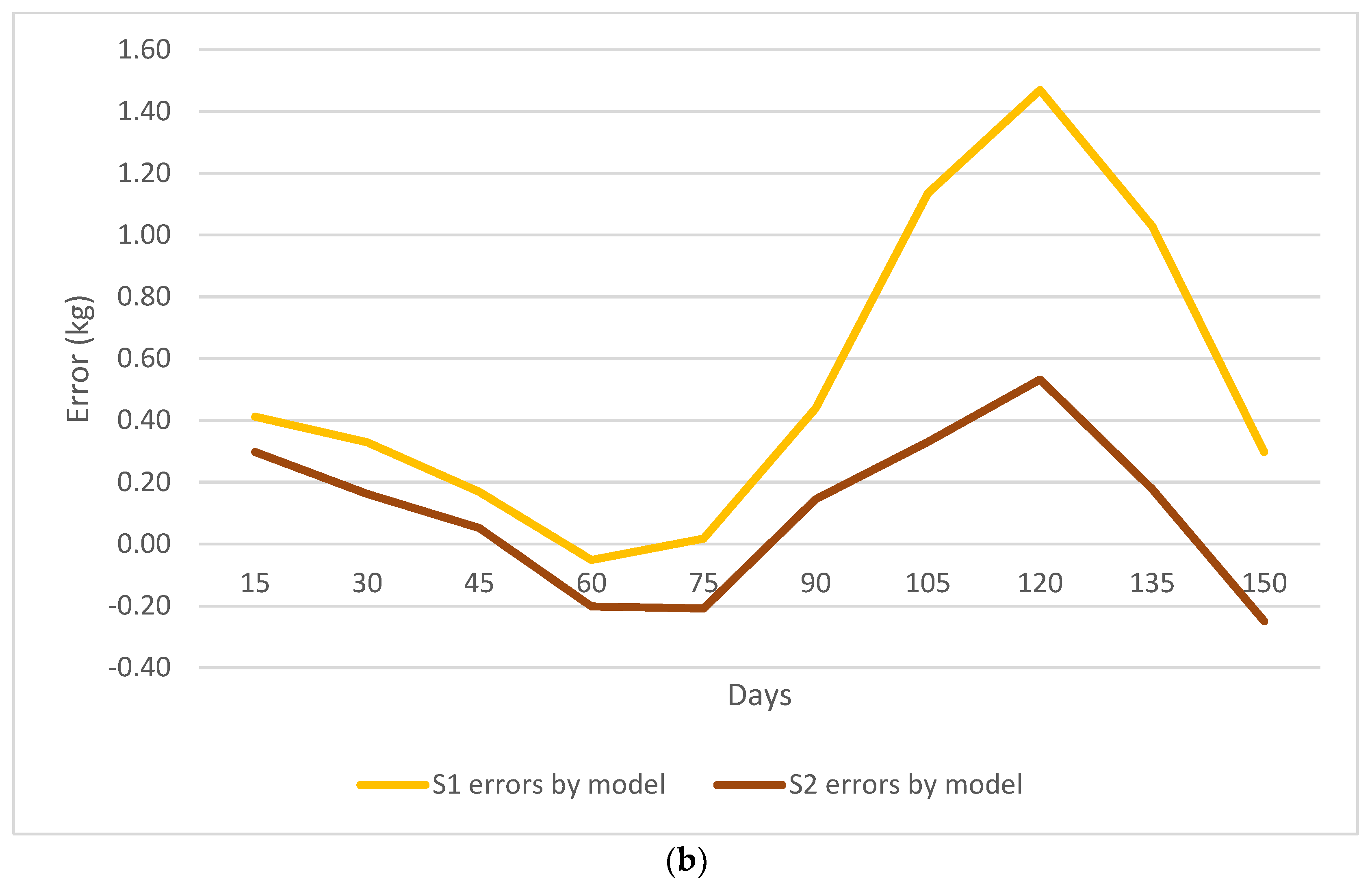
3. Results
3.1. Exploratory Results
3.2. Nonlinear Growth Model Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawrence, T.L.J.; Fowler, V.R. General Aspects of Growth. In Growth of Farm Animals, 2nd ed.; CABI Publishing: Wallingford, UK, 2002; pp. 1–6. [Google Scholar]
- Held, J. Lamb Feeding and Marketing Decisions Can Influence Flock Profitability. In South Dakota Sheep Field Day Proceedings and Research Reports, (8 June 1995). Paper 8. Available online: http://openprairie.sdstate.edu/sd_sheepday_1995/8 (accessed on 10 May 2023).
- Van Der Merwe, D.A.; Brand, T.S.; Hoffman, L. Precision finishing of South African lambs in feedlots: A review. Trop. Anim. Health Prod. 2020, 52, 2769–2786. [Google Scholar] [CrossRef] [PubMed]
- Wolfová, M.; Wolf, J.; Milerski, M. Calculating economic values for growth and functional traits in non-dairy sheep. J. Anim. Breed. Genet. 2009, 126, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Wolfová, M.; Wolf, J.; Milerski, M. Economic weights of production and functional traits for Merinolandschaf, Romney, Romanov and Sumavska sheep in the Czech Republic. Small Rumin. Res. 2011, 99, 25–33. [Google Scholar] [CrossRef]
- Fitzhugh, H.A.F., Jr. Analysis of Growth Curves and Strategies for Altering Their Shape. J. Anim. Sci. 1976, 42, 1036–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupi, T.; Nogales, S.; León, J.; Barba, C.; Delgado, J. Characterization of commercial and biological growth curves in the Segureña sheep breed. Animal 2015, 9, 1341–1348. [Google Scholar] [CrossRef] [Green Version]
- Brunner, N.; Kühleitner, M. The growth of domestic goats and sheep: A meta study with Bertalanffy-Pütter models. Vet. Anim. Sci. 2020, 10, 100135. [Google Scholar] [CrossRef]
- Mandal, A.; Baneh, H.; Notter, D.R. Modeling the growth curve of Muzaffarnagari lambs from India. Livest. Sci. 2021, 251, 104621. [Google Scholar] [CrossRef]
- Lewis, R.; Emmans, G.; Dingwall, W.; Simm, G. A description of the growth of sheep and its genetic analysis. Anim. Sci. 2002, 74, 51–62. [Google Scholar] [CrossRef] [Green Version]
- López, B.; Lupi, T.; León, J.; López, F.; Agudo, B.; Delgado, J. Characterization of the commercial growth curves of Spanish Merino, Fleischschaf, and crossbred lambs in an associative economy context. Small Rumin. Res. 2018, 164, 8–14. [Google Scholar] [CrossRef]
- Lupi, T.M.; León, J.M.; Nogales, S.; Barba, C.; Delgado, J.V. Genetic parameters of traits associated with the growth curve in Segureña sheep. Animal 2016, 10, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Sharif, N.; Ali, A.; Mohsin, I.; Ahmad, N. Evaluation of nonlinear models to define growth curve in Lohi sheep. Small Rumin. Res. 2021, 205, 106564. [Google Scholar] [CrossRef]
- Teleken, J.T.; Galvão, A.; Robazza, W.D.S. Comparing non-linear mathematical models to describe growth of different animals. Acta Sci. 2017, 39, 73–81. [Google Scholar] [CrossRef]
- Van der Merwe, D.; Brand, T.; Hoffman, L. Application of growth models to different sheep breed types in South Africa. Small Rumin. Res. 2019, 178, 70–78. [Google Scholar] [CrossRef]
- Malhado, C.; Carneiro, P.; Affonso, P.; Souza, A.; Sarmento, J. Growth curves in Dorper sheep crossed with the local Brazilian breeds, Morada Nova, Rabo Largo, and Santa Inês. Small Rumin. Res. 2009, 84, 16–21. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Mokhtari, M.; Saghi, D.; Shahdadi, A. Modeling the growth curve in Kordi sheep: The comparison of non-linear models and estimation of genetic parameters for the growth curve traits. Small Rumin. Res. 2019, 177, 117–123. [Google Scholar] [CrossRef]
- Keçici, P.D.; Yalçintan, H.; Öztürk, N.; Coşkun, R.; Koçak, Ö.; Yilmaz, A.; Ekiz, E. Structural and technical characteristics of purebred Kıvırcık sheep enterprises in Kırklareli province. Kocatepe Vet. J. 2022, 15, 47–62. [Google Scholar]
- Ekiz, B.; Yilmaz, A.; Yakan, A.; Hanoglu, H.; Kaptan, C. Breed influence on finishing performance and meat fatty acid composition in lambs raised under an intensive production system. Large Anim. Rev. 2018, 24, 121–128. [Google Scholar]
- Türk Patent. Available online: https://ci.turkpatent.gov.tr/Files/GeographicalSigns/ca097684-9080-4f85-87d0-8aa512c0382e.pdf (accessed on 5 April 2023).
- T.C. Tarım ve Orman Bakanlığı Koyunculuk Araştırma Enstitüsü Müdürlüğü. Available online: https://arastirma.tarimorman.gov.tr/koyunculuk/Menu/33/Tagem-Projeleri (accessed on 14 April 2023).
- Dudinskaya, E.C.; Naspetti, S.; Arsenos, G.; Caramelle-Holtz, E.; Latvala, T.; Martin-Collado, D.; Orsini, S.; Ozturk, E.; Zanoli, R. European Consumers’ Willingness to Pay for Red Meat Labelling Attributes. Animals 2021, 11, 556. [Google Scholar] [CrossRef]
- Yilmaz, O.; Cengiz, F.; Ertugrul, M.; Wilson, R.T. The domestic livestock resources of Turkey: Sheep breeds and cross-breeds and their conservation status. AGR 2013, 52, 147–163. [Google Scholar] [CrossRef]
- Bangar, Y.C.; Lawar, V.S.; Nimase, R.G.; Nimbalkar, C.A. Comparison of Non-linear Growth Models to Describe the Growth Behaviour of Deccani Sheep. Agric. Res. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Yıldız, G.; Soysal, M.İ.; Gürcan, E.K. Tekirdağ ilinde yetiştirilen Karacabey Merinosu x Kıvırcık melezi kuzularda büyüme eğrisinin farklı modellerle belirlenmesi. JOTAF 2009, 6, 11–19. [Google Scholar]
- Akbaş, Y.; Taşkın, T.; Demirören, E. Farkli modellerin Kivircik ve Dağlik erkek kuzularinin büyüme eğrilerine uyumunun karşilaştirilmasi. Turk. J. Vet. Anim. Sci. 1999, 23, 537–544. [Google Scholar]
- Ekiz, B.; Demirel, G.; Yilmaz, A.; Ozcan, M.; Yalcintan, H.; Kocak, O.; Altinel, A. Slaughter characteristics, carcass quality and fatty acid composition of lambs under four different production systems. Small Rumin. Res. 2013, 114, 26–34. [Google Scholar] [CrossRef]
- Soglia, D.; Sartore, S.; Maione, S.; Schiavone, A.; Dabbou, S.; Nery, J.; Zaniboni, L.; Marelli, S.; Sacchi, P.; Rasero, R. Growth Performance Analysis of Two Italian Slow-Growing Chicken Breeds: Bianca di Saluzzo and Bionda Piemontese. Animals 2020, 10, 969. [Google Scholar] [CrossRef]
- Sariyel, V.; Aygun, A.; Keskin, I. Comparison of growth curve models in partridge. Poult. Sci. 2017, 96, 1635–1640. [Google Scholar] [CrossRef]
- Arnhold, E. Package ‘easyreg’. Pesqui. Agropecu. Bras. 2022, 53, 870–873. [Google Scholar] [CrossRef] [Green Version]
- Narinc, D.; Aksoy, T.; Karaman, E.; Curek, D.I. Analysis of fitting growth models in medium growing chicken raised indoor system. Trends Anim. Vet. Sci. J. 2010, 1, 12–18. [Google Scholar]
- Narinc, D.; Karaman, E.; Aksoy, T.; Firat, M.Z. Investigation of nonlinear models to describe long-term egg production in Japanese quail. Poult. Sci. 2013, 92, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Yakan, A.; Dalci, M.T. Ankara şartlarinda Akkaraman, Ivesi ve Kivircik irklarinda döl verimi, büyüme ve yaşama gücü. Lalahan Hay. Araşt. Enst. Derg. 2012, 52, 1–10. [Google Scholar]
- Alarslan, E.; Aygün, T. Determination of growth and some morphological traits of Kivircik lambs in Yalova. J. Anim. Prod. 2019, 60, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Selvi, T.N.; Üstüner, H. Some Fertility Traits and Growing Characteristics of Kivircik Sheep Breed in the Extensive Farm Conditions. J. Vet. Med. 2021, 40, 116–119. [Google Scholar] [CrossRef]
- Koops, W.J. Multiphasic Analysis of Growth. Ph.D. Thesis, Department of Animal Breeding, Wageningen Agricultural University, Wageningen, The Netherlands, 16 June 1989. [Google Scholar]
- Robertson, T.B. The Chemical Basis of Growth and Senescence. Monographs of Experimental Biology; J.B. Lippincott Gie.: Philadelphia, PA, USA, 1921. [Google Scholar]
- Zucker, L.; Hall, L.; Young, M.; Zucker, T.F. Quantitative formulation of rat growth. Growth 1941, 5, 415–436. [Google Scholar]
- Boujenane, I. Comparison of nonlinear models for describing the pre-weaning growth of Timahdite lambs and genetic and non-genetic effects for curve parameters. Small Rumin. Res. 2022, 216, 106800. [Google Scholar] [CrossRef]
- Bahreini Behzadi, M.R.; Aslaminejad, A.A.; Sharifi, A.R.; Simianer, H. Comparison of mathematical models for describing the growth of Baluchi sheep. J. Agr. Sci. Tech. 2014, 14, 57–68. [Google Scholar]
- Hossein-Zadeh, N.G. Modeling the growth curve of Iranian Shall sheep using non-linear growth models. Small Rumin. Res. 2015, 130, 60–66. [Google Scholar] [CrossRef]
- Weber, S.H.; dos Santos, S.K.; Heinzen, B.C.; Viana, N.P.; Sotomaior, C.S. Comparison of nonlinear mathematical models for lamb growth analysis. Trop. Anim. Health Prod. 2021, 53, 151. [Google Scholar] [CrossRef]
- Aguilar, C.; Friedli, C.; Cañas, R. The growth curve of animals. Agric. Syst. 1983, 10, 133–147. [Google Scholar] [CrossRef]
- De Paz, C.C.P.; Costa, R.; Enio, C.; Da, C.R.L.D.; Paula, L.L.; Raquel, Q.C. Nonlinear models of Brazilian sheep in adjustment of growth curves. Czech J. Anim. Sci. 2018, 63, 331–338. [Google Scholar] [CrossRef]
- Waheed, A.; Eyduran, E.; Tariq, M.M.; Ahmad, S.; Hameed, T.; Bukhari, F.A. Comparison of the non-linear models defining the growth of Thalli sheep under desert conditions. Pak. J. Zool. 2016, 48, 423–426. [Google Scholar]
- Keskin, I.; Dag, B.; Sariyel, V.; Gokmen, M. Estimation of growth curve parameters in Konya Merino sheep. S. Afr. J. Anim. Sci. 2009, 39, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Topal, M.; Ozdemir, M.; Aksakal, V.; Yildiz, N.; Dogru, U. Determination of the best nonlinear function in order to estimate growth in Morkaraman and Awassi lambs. Small Rumin. Res. 2004, 55, 229–232. [Google Scholar] [CrossRef]
- Castillo-Zamora, P.; Macedo-Barragán, R.; Arredondo-Ruiz, V.; Haubi-Segura, C.; Valencia-Posadas, M. Characterization Of Growth Of Socorro Island Merino Lambs. Chil. J. Agric. Anim. Sci. 2022, 38, 363–373. [Google Scholar] [CrossRef]
- Ali, A.; Javed, K.; Zahoor, I.; Anjum, K. Determination of the best non-linear function to describe the growth of Kajli sheep. S. Afr. J. Anim. Sci. 2020, 50, 452–459. [Google Scholar] [CrossRef]
- Dıaz, M.; Velasco, S.; Cañeque, V.; Lauzurica, S.; de Huidobro, F.R.; Pérez, C.; González, J.; Manzanares, C. Use of concentrate or pasture for fattening lambs and its effect on carcass and meat quality. Small Rumin. Res. 2002, 43, 257–268. [Google Scholar] [CrossRef]
- Yalcintan, H.; Ekiz, B.; Kocak, O.; Dogan, N.; Akin, P.D.; Yilmaz, A. Carcass and meat quality characteristics of lambs reared in different seasons. Arch. Anim. Breed. 2017, 60, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, M.S.; Borzi, N.K.; Fozi, M.A.; Behzadi, M.R.B. Evaluation of non-linear models for genetic parameters estimation of growth curve traits in Kermani sheep. Trop. Anim. Health Prod. 2019, 51, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, F.; Eyduran, E.; Raziq, A.; Ali, M.; Huma, Z.E.; Tirink, C.; Sevgenler, H. Modeling and predicting the growth of indigenous Harnai sheep in Pakistan: Non-linear functions and MARS algorithm. Trop. Anim. Health Prod. 2021, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hızlı, H.; Yazgan, E. Comparison of the growth curve models on live weights in terms of different environmental factors in Awassi lambs. Iran. J. Appl. Anim. Sci. 2021, 11, 577–586. [Google Scholar]


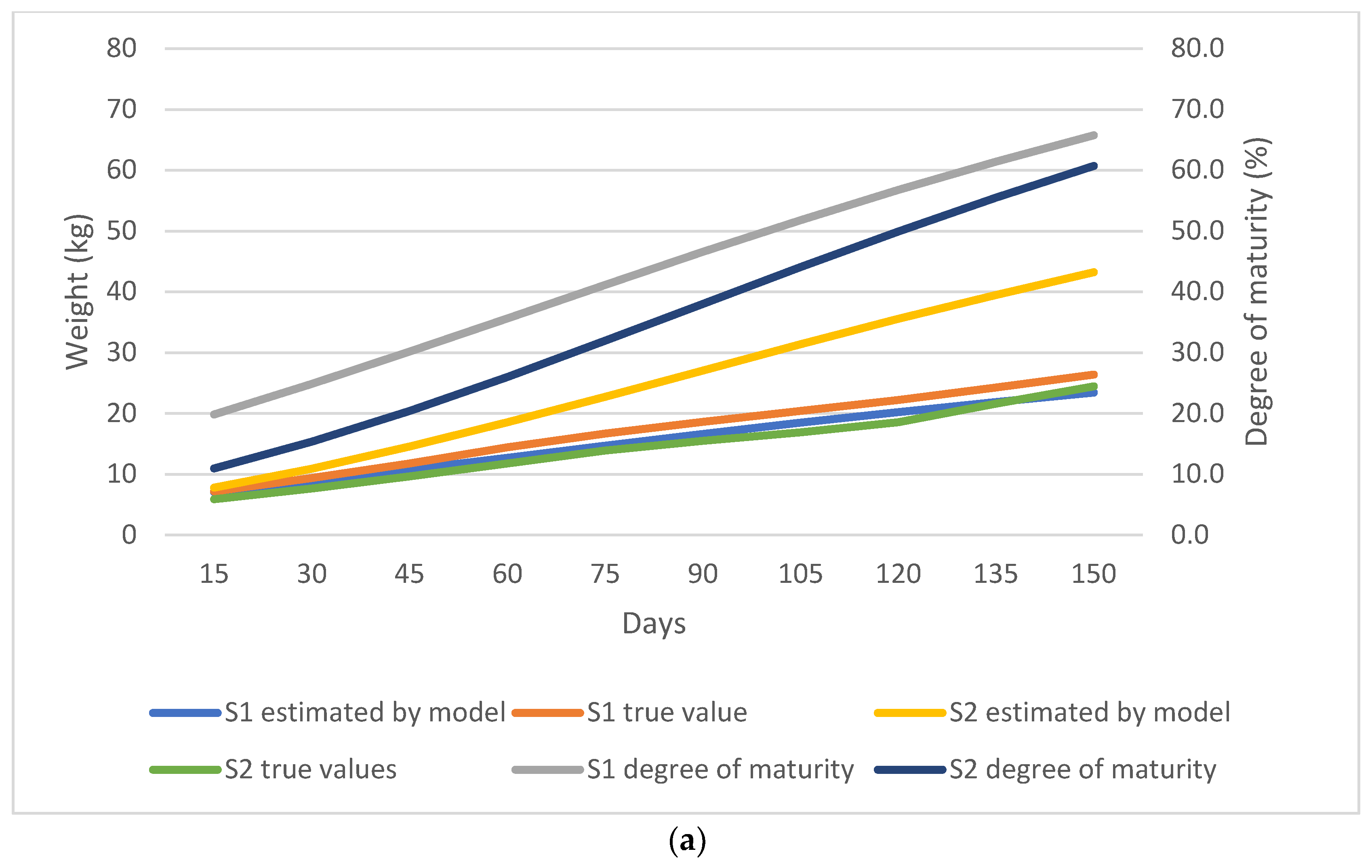
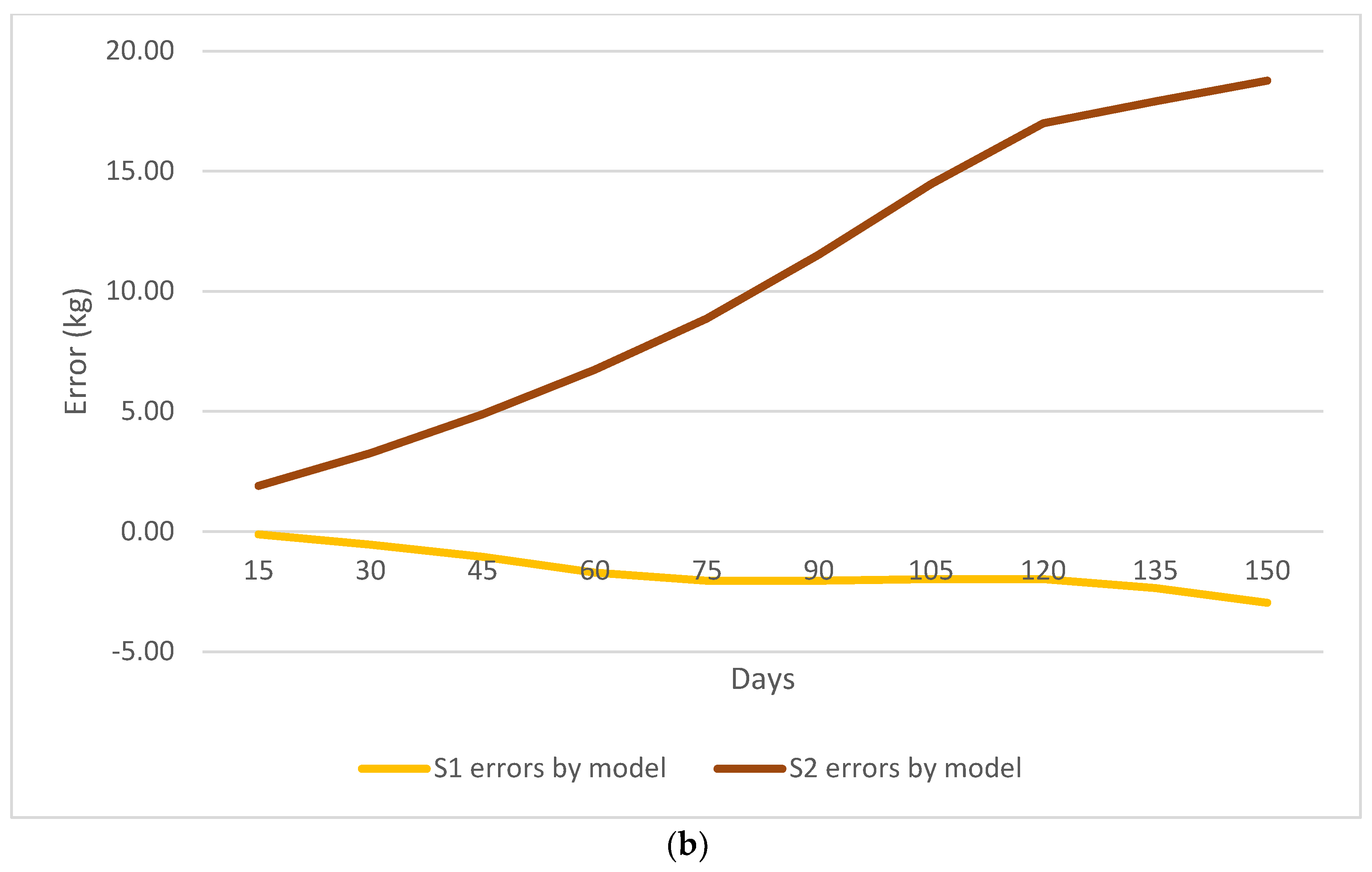
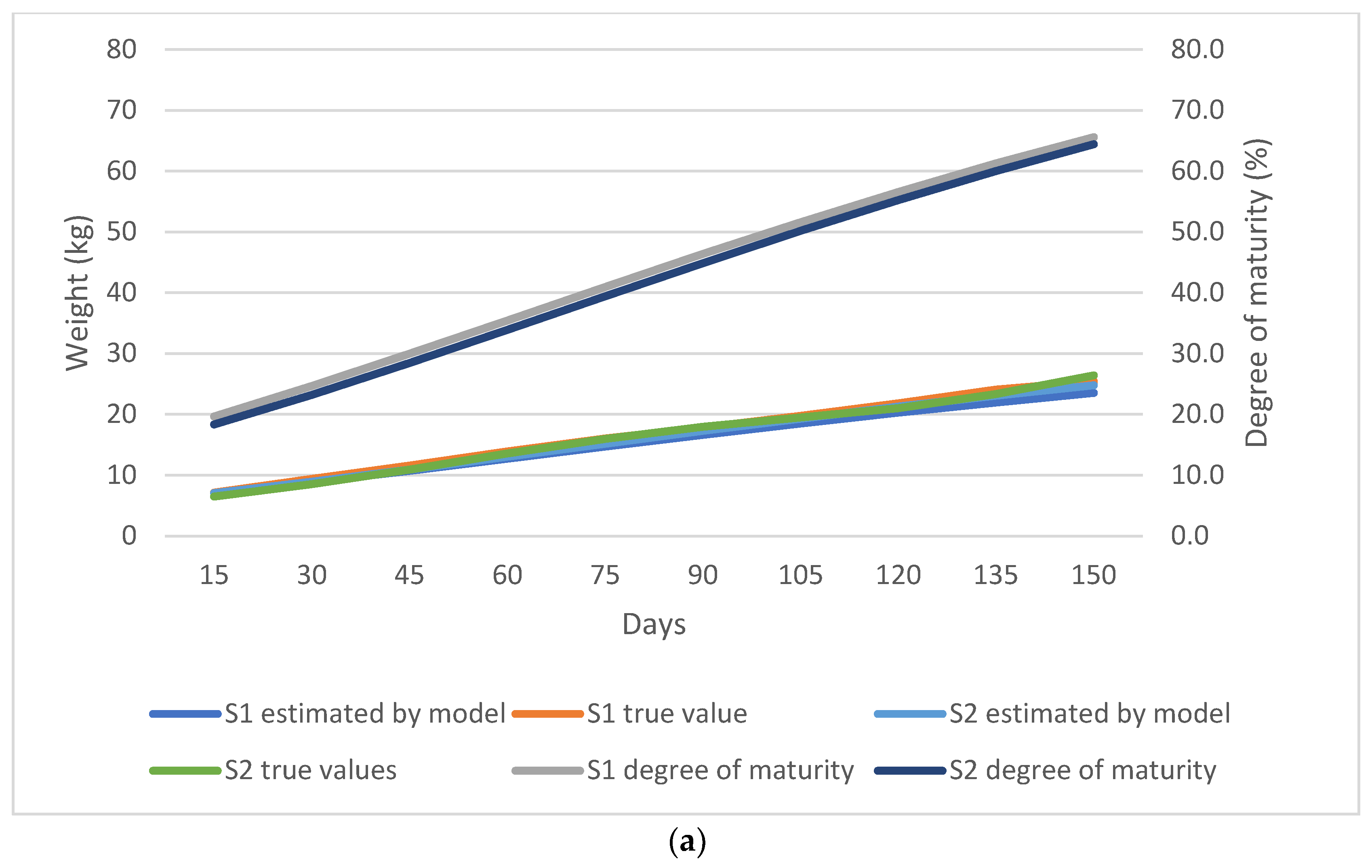

| Item | n | Mean ± Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Birth Weight, kg | 536 | 4.36 ± 0.86 | 2.08 | 7.81 |
| Weight at 15 d, kg | 478 | 6.85 ± 1.34 | 3.31 | 11.34 |
| Weight at 30 d, kg | 608 | 8.98 ± 1.98 | 3.71 | 14.77 |
| Weight at 45 d, kg | 608 | 11.3 ± 2.50 | 4.50 | 18.36 |
| Weight at 60 d, kg | 607 | 13.8 ± 3.01 | 5.52 | 22.86 |
| Weight at 75 d, kg | 610 | 16.0 ± 3.43 | 5.97 | 26.37 |
| Weight at 90 d, kg | 600 | 17.9 ± 3.73 | 5.71 | 29.50 |
| Weight at 105 d, kg | 571 | 19.6 ± 4.04 | 5.34 | 32.90 |
| Weight at 120 d, kg | 550 | 21.4 ± 4.41 | 5.06 | 33.95 |
| Weight at 135 d, kg | 519 | 23.7 ± 4.61 | 10.00 | 38.81 |
| Weight at 150 d, kg | 498 | 26.0 ± 5.07 | 12.21 | 40.65 |
| Variable | Subgroups | n | Relative Frequency (%) |
|---|---|---|---|
| Year | 2014 | 261 | 42.6 |
| 2015 | 104 | 17.0 | |
| 2016 | 247 | 40.4 | |
| Sex | Male | 299 | 48.9 |
| Female | 313 | 51.1 | |
| Birth type | Single | 467 | 76.3 |
| Twin | 145 | 23.7 | |
| Season of birth * | Winter | 280 | 45.8 |
| Spring | 332 | 54.2 |
| Variable | Fit Statistics | Nonlinear Models | |||
|---|---|---|---|---|---|
| Gompertz | Logistic | von Bertalanffy | Brody | ||
| All | R2adj | 0.71 | 0.72 | 0.72 | 0.72 |
| AIC | 30,960 | 30,994 | 30,950 | 30,921 | |
| BIC | 30,986 | 31,021 | 30,977 | 30,947 | |
| RMS | 13.5 | 13.6 | 13.5 | 13.4 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Sex | |||||
| R2adj | 0.73 | 0.72 | 0.73 | 0.73 | |
| Male | AIC | 14,234 | 14,252 | 14,228 | 14,219 |
| BIC | 14,257 | 14,276 | 14,252 | 14,243 | |
| RMS | 14.6 | 14.7 | 14.6 | 14.6 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Female | R2adj | 0.73 | 0.72 | 0.73 | 0.73 |
| AIC | 16,503 | 16,520 | 16,499 | 16,477 | |
| BIC | 16,527 | 16,544 | 16,523 | 16,502 | |
| RMS | 11.7 | 11.8 | 11.7 | 11.6 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Birth type | |||||
| R2adj | 0.75 | 0.75 | 0.75 | 0.73 | |
| AIC | 22,881 | 22,909 | 22,873 | 16,477 | |
| Single | BIC | 22,906 | 22,935 | 22,899 | 16,501 |
| RMS | 11.6 | 11.7 | 11.6 | 11.6 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| R2adj | 0.68 | 0.67 | 0.68 | - | |
| Twin | AIC | 7548 | 7554 | 7546 | - |
| BIC | 7569 | 7575 | 7567 | - | |
| RMS | 14.7 | 14.8 | 14.7 | - | |
| p-value | <0.001 | <0.001 | <0.001 | - | |
| Season of birth | |||||
| R2adj | 0.72 | 0.72 | 0.72 | 0.72 | |
| Spring | AIC | 13,296 | 13,306 | 13,294 | 13,292 |
| BIC | 13,320 | 13,329 | 13,318 | 13,315 | |
| RMS | 12.0 | 12.1 | 12.0 | 12.0 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Winter | R2adj | 0.72 | 0.72 | 0.72 | 0.72 |
| AIC | 30,945 | 30,980 | 30,936 | 30,921 | |
| BIC | 30,972 | 31,007 | 30,962 | 30,947 | |
| RMS | 13.5 | 13.5 | 13.4 | 13.4 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Variable | Nonlinear Models | ||||
|---|---|---|---|---|---|
| Gompertz | Logistic | von Bertalanffy | Brody | ||
| All | A | 38.52 ± 1.41 | 31.71 ± 0.67 | 44.09 ± 2.21 | 105.27 ± 24.58 |
| B | 1.97 ± 0.02 | 4.36 ± 0.08 | 0.51 ± 0.001 | 0.96 ± 0.01 | |
| K | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.0004 | 0.002 ± 0.0004 | |
| Sex | |||||
| A | 44.19 ± 2.88 | 35.04 ± 1.27 | 52.43 ± 4.86 | 270.90 ± 273.66 | |
| Male | B | 2.07 ± 0.04 | 4.69 ± 4.69 | 0.53 ± 0.01 | 0.98 ± 0.02 |
| K | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.001 ± 0.001 | |
| A | 35.24 ± 1.51 | 29.62 ± 0.74 | 39.59 ± 2.28 | 75.35 ± 15.34 | |
| Female | B | 1.91 ± 0.03 | 4.15 ± 0.10 | 0.50 ± 0.01 | 0.94 ± 0.01 |
| K | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.002 ± 0.001 | |
| Birth type | |||||
| A | 35.63 ± 1.05 | 30.59 ± 0.54 | 39.37 ± 1.53 | 75.35 ± 15.34 | |
| Single | B | 1.88 ± 0.02 | 4.08 ± 0.08 | 0.49 ± 0.004 | 0.94 ± 0.01 |
| K | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.0005 | 0.002 ± 0.001 | |
| A | 71.20 ± 19.37 | 41.96 ± 5.25 | 124.37 ± 67.76 | - | |
| Twin | B | 2.61 ± 0.23 | 6.50 ± 0.72 | 0.66 ± 0.05 | - |
| K | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.003 ± 0.001 | - | |
| Season of birth | |||||
| A | 35.89 ± 1.73 | 30.09 ± 0.85 | 40.38 ± 2.63 | 78.53 ± 18.73 | |
| Spring | B | 1.89 ± 0.03 | 4.05 ± 0.11 | 0.49 ± 0.01 | 0.94 ± 0.01 |
| K | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.002 ± 0.001 | |
| A | 38.48 ± 1.40 | 31.70 ± 0.67 | 44.02 ± 2.20 | 105.27 ± 24.58 | |
| Winter | B | 1.97 ± 0.02 | 4.35 ± 0.08 | 0.51 ± 0.01 | 0.96 ± 0.01 |
| K | 0.01 ± 0.0005 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.002 ± 0.0004 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturk, N.; Kecici, P.D.; Serva, L.; Ekiz, B.; Magrin, L. Comparison of Nonlinear Growth Models to Estimate Growth Curves in Kivircik Sheep under a Semi-Intensive Production System. Animals 2023, 13, 2379. https://doi.org/10.3390/ani13142379
Ozturk N, Kecici PD, Serva L, Ekiz B, Magrin L. Comparison of Nonlinear Growth Models to Estimate Growth Curves in Kivircik Sheep under a Semi-Intensive Production System. Animals. 2023; 13(14):2379. https://doi.org/10.3390/ani13142379
Chicago/Turabian StyleOzturk, Nursen, Pembe Dilara Kecici, Lorenzo Serva, Bulent Ekiz, and Luisa Magrin. 2023. "Comparison of Nonlinear Growth Models to Estimate Growth Curves in Kivircik Sheep under a Semi-Intensive Production System" Animals 13, no. 14: 2379. https://doi.org/10.3390/ani13142379





