The Effects of Onychectomy (Declawing) on Antebrachial Myology across the Full Body Size Range of Exotic Species of Felidae
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. The Motivation behind Onychectomies
1.2. Rates and Legality of Onychectomies
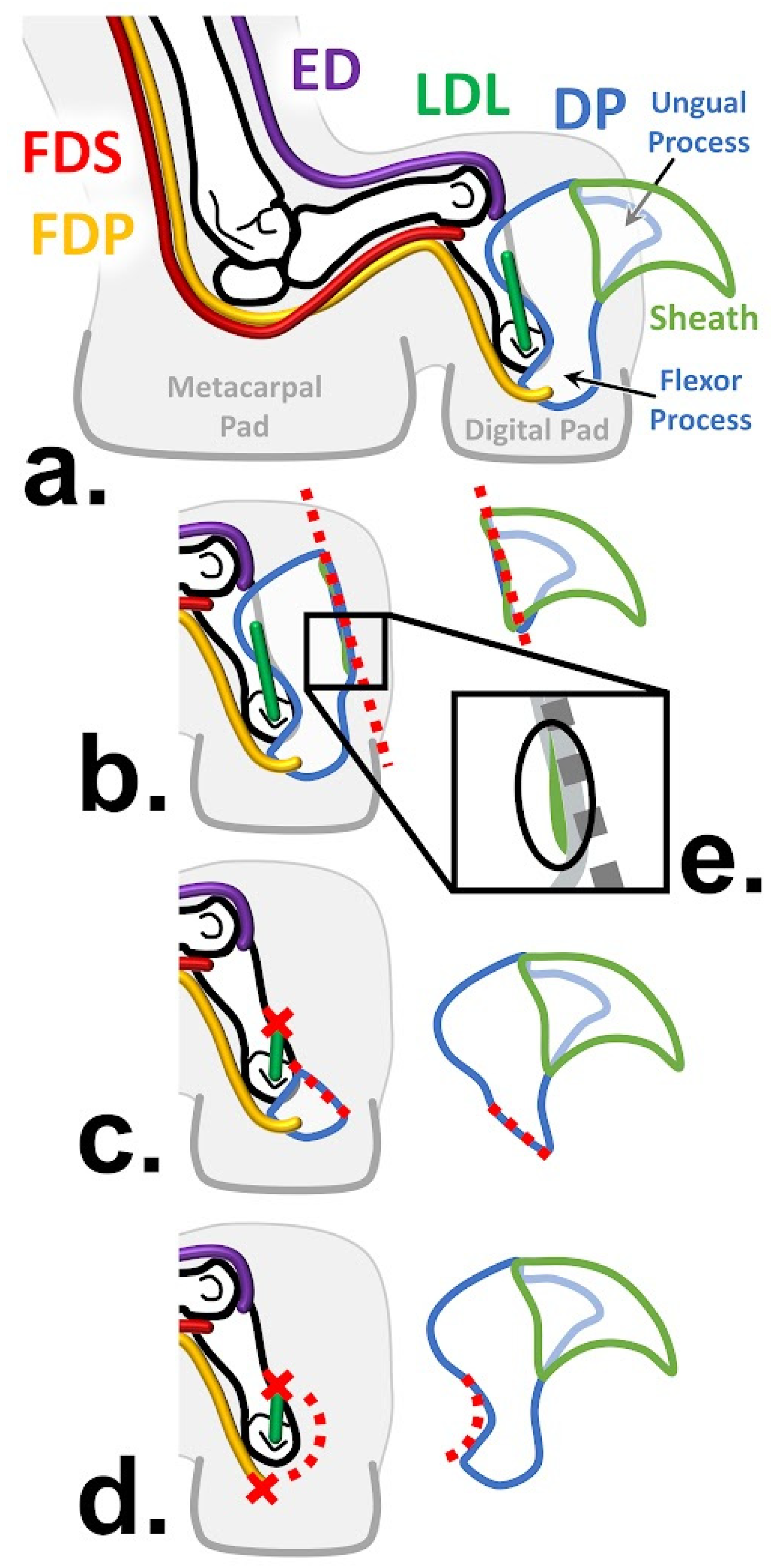
1.3. Antebrachial Anatomy
1.4. Forelimb Function in Felids
1.5. Onychectomies as a Surgery
1.6. The Allometric Problem
2. Hypotheses
3. Materials and Methods
3.1. Sample
3.2. Architectural Variables Studied
3.3. Qualitative Data
4. Results
4.1. Statistical Exclusion
4.2. Allometry across the Sample
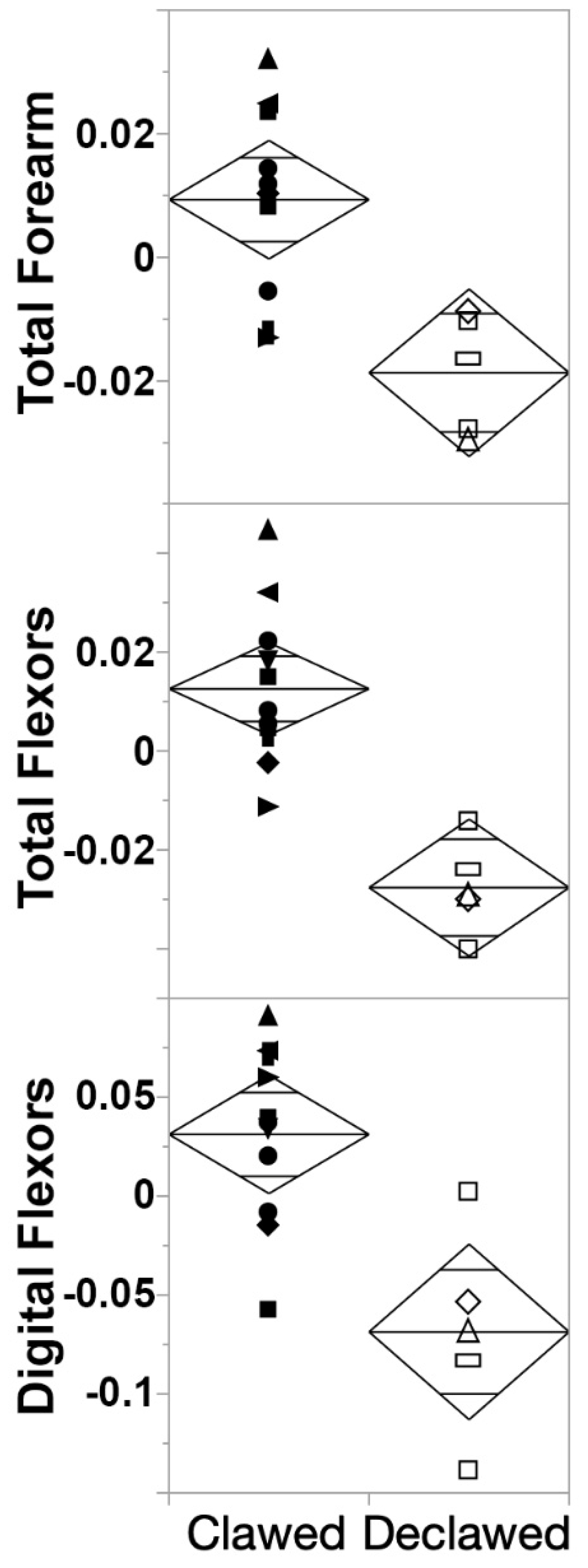
4.3. Differences between Clawed and Declawed Felid Forearms as a Whole
4.4. Differences between Clawed and Declawed Felid Digital Muscles Specifically
4.5. Differences between Clawed and Declawed Felid Wrist and other Non-Digital Forearm Muscles
4.6. Anomalous Myological Qualitative Variation
5. Discussion
5.1. Allometry across the Sample
5.2. Differences between Clawed and Declawed Felid Forearms as a Whole
5.3. Differences between Clawed and Declawed Felid Digital Muscles
5.4. Differences between Clawed and Declawed Felid Wrist and Other Non-Digital Forearm Muscles
5.5. Anomalous Myological Variation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, K.; Bailey, T.; Rist, P.; Matthews, A. Comparison of 3 methods of onychectomy. Can. Vet. J. 2014, 55, 255–262. [Google Scholar] [PubMed]
- Swinderski, J.D. Onychectomy and its Alternatives in the Feline Patient. Clin. Tech. Small Anim. Pract. 2002, 17, 158–161. [Google Scholar] [CrossRef]
- Fowler, M.E.; McDonald, S.E. Untoward effects of onychectomy in wild felids and ursids. J. Am. Vet. Med. Assoc. 1982, 181, 1242–1245. [Google Scholar] [PubMed]
- PETA. Cat-Friendly Cities, States, and Countries Where Declawing Is Illegal. Available online: https://www.peta.org/blog/where-declawing-is-illegal/#:~:text=U.S.%20States%20With%20Declawing%20Bans,and%20New%20York%20(2019) (accessed on 2 February 2023).
- Waite, C.J. Starting from scratch: A study in claws and clauses in cat declaw legislation. Drake L. Rev. 2021, 69, 675. [Google Scholar]
- Conrad, J. Milestones—The Paw Project’s Role Advocating Anti-Declawing Legislation. 2014. Available online: https://pawproject.org/legislation/ (accessed on 15 October 2022).
- Wilson, C.; Bain, M.; DePorter, T.; Beck, A.; Grassi, V.; Landsberg, G. Owner observations regarding cat scratching behavior: An internet-based survey. J. Feline Med. Surg. 2016, 18, 791–797. [Google Scholar] [CrossRef]
- Hernandez-Divers, S.M. Exotic felid medicine. In Proceedings of the American Association of Zoo Veterinarians Conference, Los Angeles, CA, USA, 11–17 October 2008. [Google Scholar]
- Becker, C.; Raadschelders, J. Ohio’s Dangerous Wild Animal Act of 2012: Enactment, Implementation and Evaluation; Columbia University Press: New York, NY, USA, 2014. [Google Scholar]
- Cuff, A.R.; Sparkes, E.L.; Randau, M.; Pierce, S.E.; Kitchener, A.C.; Goswami, A.; Hutchinson, J.R. The scaling of postcranial muscles in cats (Felidae) I: Forelimb, cervical, and thoracic muscles. J. Anat. 2016, 229, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Song, Y.; Baker, J.S.; Fekete, G.; Ugbolue, U.C.; Li, S.; Gu, Y. The biomechanical characteristics of a feline distal forelimb: A finite element analysis study. Comput. Biol. Med. 2021, 129, 104174. [Google Scholar] [CrossRef]
- Meachen-Samuels, J.; Van Valkenburgh, B. Forelimb indicators of prey-size preference in the Felidae. J. Morphol. 2009, 270, 729–744. [Google Scholar] [CrossRef]
- Grigg, E.K.; Kogan, L.R. Owners’ Attitudes, Knowledge, and Care Practices: Exploring the Implications for Domestic Cat Behavior and Welfare in the Home. Animals 2019, 9, 978. [Google Scholar] [CrossRef] [Green Version]
- American Veterinary Medical Association. Literature Review on the Welfare Implications of Declawing of Domestic Cats; American Veterinary Medical Association: Schaumburg, IL, USA, 2019; pp. 1–11. [Google Scholar]
- McKeown, D.; Luescher, A.; Machum, M. The problem of destructive scratching by cats. Can. Vet. J. 1988, 29, 1017–1018. [Google Scholar]
- Atwood-Harvey, D. Death or declaw: Dealing with moral ambiguity in a veterinary hospital. Soc. Anim. 2005, 13, 315–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landsberg, G.M. Cat owners’ attitudes toward declawing. Anthrozoös 1991, 4, 192–197. [Google Scholar] [CrossRef]
- Mison, M.B.; Bohart, G.H.; Walshaw, R.; Winters, C.A.; Hauptman, J.G. Use of carbon dioxide laser for onychectomy in cats. J. Am. Vet. Med. Assoc. 2002, 221, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, H. Letting the (big) cat(s) out of the bag: A case for federal prohibition of exotic big cat ownership in Pakistan. J. Anim. Environ. Law 2023. forthcoming. [Google Scholar] [CrossRef]
- Patronek, G.J. Assessment of claims of short- and long-term complications associated with onychectomy in cats. J. Am. Vet. Med. Assoc. 2001, 219, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Patronek, G.J.; Beck, A.M.; Glickman, L.T. Dynamics of dog and cat populations in a community. J. Am. Vet. Med. Assoc. 1997, 210, 637–642. [Google Scholar]
- Wagner, A.E.; Hellyer, P.W. Survey of anesthesia techniques and concerns in private veterinary practice. J. Am. Vet. Med. Assoc. 2000, 217, 1652–1657. [Google Scholar] [CrossRef]
- Lockhart, L.E.; Motsinger-Reif, A.A.; Simpson, W.M.; Posner, L.P. Prevalence of onychectomy in cats presented for veterinary care near Raleigh, NC and educational attitudes toward the procedure. Vet. Anaesth. Analg. 2014, 41, 48–53. [Google Scholar] [CrossRef]
- Cohick, C. The Forgotten Cool Cats and Kittens: How a Lack of Federal Oversight in the USDA Led to Inhumane Loopholes in the Exploitation of Big Cats in America. Admin. L. Rev. Accord 2020, 6, 125. [Google Scholar]
- Dunn, R.H.; Beresheim, A.; Gubatina, A.; Bitterman, K.; Butaric, L.; Bejes, K.; Kennedy, S.; Markham, S.; Miller, D.; Mrvoljak, M.; et al. Muscular anatomy of the forelimb of tiger (Panthera tigris). J. Anat. 2022, 241, 119–144. [Google Scholar] [CrossRef]
- Dickinson, E.; Boettcher, M.L.; Smith, M.R.; Worden, N.A.; Swindell, S.R.; Seelye, J.S.; Pastor, F.; Hartstone-Rose, A. Myological variation in the forearm anatomy of Callitrichidae and Lemuridae. J. Anat. 2021, 239, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, C.; Naples, V.; Ross, E.; Carlon, B. Comparative analysis of paw pad structure in the clouded leopard (Neofelis nebulosa) and domestic cat (Felis catus). Anat. Rec. 2009, 292, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, C.F.; Nichols, T.R. The mechanical actions of muscles predict the direction of muscle activation during postural perturbations in the cat hindlimb. J. Neurophysiol. 2014, 111, 900–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, H.N.; Russell, A.P.; Laroiya, R.; Powell, G.L. Claw retraction and protraction in the carnivora: Skeletal microvariation in the phalanges of the Felidae. J. Morphol. 1996, 229, 289–308. [Google Scholar] [CrossRef]
- Gonyea, W.; Ashworth, R. The form and function of retractile claws in the Felidae and other representative carnivorans. J. Morphol. 1975, 145, 229–238. [Google Scholar] [CrossRef]
- Böhmer, C.; Fabre, A.-C.; Taverne, M.; Herbin, M.; Peigné, S.; Herrel, A. Functional relationship between myology and ecology in carnivores: Do forelimb muscles reflect adaptations to prehension? Biol. J. Linn. Soc. 2019, 127, 661–680. [Google Scholar] [CrossRef]
- Patel, S.; Potty, A.; Taylor, E.J.; Sorene, E.D. Collateral ligament injuries of the metacarpophalangeal joint of the thumb: A treatment algorithm. Strateg. Trauma Limb Reconstr. 2010, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- DePorter, T.L.; Elzerman, A.L. Common feline problem behaviors: Destructive scratching. J. Feline Med. Surg. 2019, 21, 235–243. [Google Scholar] [CrossRef]
- Cartmill, M. Climbing. In Functional Vertebrate Morphology; Harvard University Press: Cambridge, MA, USA; London, UK, 1985; pp. 73–88. [Google Scholar]
- Julik, E.; Zack, S.; Adrian, B.; Maredia, S.; Parsa, A.; Poole, M.; Starbuck, A.; Fisher, R.E. Functional Anatomy of the Forelimb Muscles of the Ocelot (Leopardus pardalis). J. Mammal Evol. 2012, 19, 277–304. [Google Scholar] [CrossRef]
- Morales, M.M.; Moyano, S.R.; Ortiz, A.M.; Ercoli, M.D.; Aguado, L.I.; Cardozo, S.A.; Giannini, N.P. Comparative myology of the ankle of Leopardus wiedii and L. geoffroyi (Carnivora: Felidae): Functional consistency with osteology, locomotor habits and hunting in captivity. Zoology 2018, 126, 46–57. [Google Scholar] [CrossRef]
- Carlon, B.; Hubbard, C. Hip and thigh anatomy of the clouded leopard (Neofelis nebulosa) with comparisons to the domestic cat (Felis catus). Anat. Rec. 2012, 295, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Mellen, J.D. A Comparative Analysis of Scent-Marking, Social and Reproductive Behavior in 20 Species of Small Cats (Felis). Am. Zool. 1993, 33, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.F.; Townsend, K.E.B.; Adrian, B.; Levy, S.; Marsh, S.; Hassur, R.; Manfredi, K.; Echols, M.S. Functional Adaptations in the Forelimb of the Snow Leopard (Panthera uncia). Integr. Comp. Biol. 2021, 61, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Peddie, J. Onychectomy in the exotic feline. In Current Techniques in Small Animal Surgery, 2nd ed.; Bojrab, M.J., Ed.; Teton NewMedia: Jackson, WY, USA, 1983. [Google Scholar]
- Ruch-Gallie, R.; Hellyer, P.W.; Schoenfeld-Tacher, R.; Kogan, L.R. Survey of practices and perceptions regarding feline onychectomy among private practitioners. J. Am. Vet. Med. Assoc. 2016, 249, 291–298. [Google Scholar] [CrossRef]
- Tobias, K.S. Feline onychectomy at a teaching institution: A retrospective study of 163 cases. Vet. Surg. 1994, 23, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Riegel, R.J.; Godbold, J.C., Jr. Laser Therapy in Veterinary Medicine: Photobiomodulation; Wiley-Blackwell: Hoboken, NJ, USA, 2017; p. 512. [Google Scholar]
- Ripley, W.; McCarnan, H. Declawing an African lion: A challenge for choice of instrument. Can. Vet. J. 1974, 15, 353. [Google Scholar]
- Conrad, J.; Wendelburg, K.; Santinelli, S.; Park, A. Deleterious effects of onychectomy (Declawing) in exotic felids and a reparative surgical technique: A preliminary report. In Proceedings of the 35th Conference of the American Association of Zoo Veterinarians, Milwaukee, WI, USA, 5–10 October 2002; pp. 5–10. [Google Scholar]
- Wilson, D.E.; Mittermeier, R.A. Handbook of the Mammals of the World, 1st ed.; Lynx Edicions: Barcelona, Spain, 2009; Volume 1, p. 727. [Google Scholar]
- Chi, K.-J.; Louise Roth, V. Scaling and mechanics of carnivoran footpads reveal the principles of footpad design. J. R. Soc. Interface 2010, 7, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Biewener, A.A. Biomechanical consequences of scaling. J. Exp. Biol. 2005, 208, 1665–1676. [Google Scholar] [CrossRef] [Green Version]
- Doube, M.; Wiktorowicz-Conroy, A.; Christiansen, P.; Hutchinson, J.R.; Shefelbine, S. Three-dimensional geometric analysis of felid limb bone allometry. PLoS ONE 2009, 4, e4742. [Google Scholar] [CrossRef]
- Alexander, R.M.; Jayes, A.S.; Maloiy, G.M.O.; Wathuta, E.M. Allometry of the leg muscles of mammals. J. Zool. 1981, 194, 539–552. [Google Scholar] [CrossRef]
- Hartstone-Rose, A.; Hertzig, I.; Dickinson, E. Bite force and masticatory muscle architecture adaptations in the dietarily diverse Musteloidea (Carnivora). Anat. Rec. 2019, 302, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Hartstone-Rose, A.; Dickinson, E.; Deutsch, A.R.; Worden, N.; Hirschkorn, G.A. Masticatory muscle architectural correlates of dietary diversity in Canidae, Ursidae, and across the order Carnivora. Anat. Rec. 2022, 305, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.L.; Crouch, M.; Allen, K.L.; Marchi, D.; Pastor, F.; Hartstone-Rose, A. Scaling of primate forearm muscle architecture as it relates to locomotion and posture. Anat. Rec. 2018, 301, 484–495. [Google Scholar] [CrossRef] [Green Version]
- Ward, S.R.; Sarver, J.J.; Eng, C.M.; Kwan, A.; Würgler-Hauri, C.C.; Perry, S.M.; Williams, G.R.; Soslowsky, L.J.; Lieber, R.L. Plasticity of muscle architecture after supraspinatus tears. J. Orthop. Sports Phys. Ther. 2010, 40, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Boettcher, M.L.; Leonard, K.C.; Dickinson, E.; Aujard, F.; Herrel, A.; Hartstone-Rose, A. The forearm musculature of the gray mouse lemur (Microcebus murinus): An ontogenetic study. Anat. Rec. 2020, 303, 1354–1363. [Google Scholar] [CrossRef]
- Boettcher, M.L.; Leonard, K.C.; Dickinson, E.; Herrel, A.; Hartstone-Rose, A. Extraordinary grip strength and specialized myology in the hyper-derived hand of Perodicticus potto? J. Anat. 2019, 235, 931–939. [Google Scholar] [CrossRef]
- Hartstone-Rose, A.; Perry, J.; Morrow, C.J. Bite force estimation and the fiber architecture of felid masticatory muscles. Anat. Rec. 2012, 295, 1336–1351. [Google Scholar] [CrossRef]
- Leonard, K.C.; Worden, N.; Boettcher, M.; Dickinson, E.; Omstead, K.M.; Burrows, A.; Hartstone-Rose, A. Anatomical and ontogenetic influences on muscle density. Sci. Rep. 2021, 11, 2114. [Google Scholar] [CrossRef] [PubMed]
- Leonard, K.; Worden, N.; Boettcher, M.; Dickinson, E.; Hartstone-Rose, A. Effects of freezing and short-term fixation on muscle mass, volume and density. Anat. Rec. 2022, 305, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.; Leonard, K.C.; Herrel, A.; Hartstone-Rose, A. The soft-tissue anatomy of the highly derived hand of Perodicticus relative to the more generalized Nycticebus. In Evolution, Ecology and Conservation of Lorises and Pottos; Nekaris, A., Burrows, A.M., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 76–96. [Google Scholar]
- Herrel, A.; De Smet, A.; Aguirre, L.F.; Aerts, P. Morphological and mechanical determinants of bite force in bats: Do muscles matter? J. Exp. Biol. 2008, 211, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, G.-H. Funktionelle morphologie der kaumuskulatur. VEB G 1961, 262. [Google Scholar]
- Perry, J.M.G.; Hartstone-Rose, A.; Wall, C.E. The jaw adductors of strepsirrhines in relation to body size, diet, and ingested food size. Anat. Rec. 2011, 294, 712–728. [Google Scholar] [CrossRef] [PubMed]


| Species (Common Name) | Specimen a | Sex | Claw Status | Body Mass (kg) |
|---|---|---|---|---|
| Caracal caracal (Caracal) | 202148 | Female | Clawed | 11.79 |
| Felis nigripes (Black-footed cat) | 202143 | Unknown | Clawed | 3.90 b |
| Leopardus pardalis (Ocelot) | 202136 | Female | Clawed | 11.30 |
| Leopardus pardalis (Ocelot) | 202147 | Male | Clawed | 7.48 |
| Leopardus pardalis (Ocelot) | 202137 | Male | Clawed | 14.70 |
| Leptailurus serval (Serval) | 202140 | Male | Clawed | 12.93 |
| Lynx rufus (Bobcat) | 202146 | Male | Clawed | 11.34 |
| Panthera leo (Lion) | 202150 | Male | Clawed | 176.40 |
| Panthera pardus (Leopard) | 202141 | Male | Clawed | 43.09 |
| Panthera tigris (Tiger) | 202134 | Female | Clawed | 93.44 |
| Panthera tigris (Tiger) | 202144 | Female | Clawed | 142.88 |
| Panthera tigris (Tiger) c | 202157 | Female | Clawed | 140.16 |
| Prionailurus viverrinus (Fishing cat) | 202149 | Unknown | Clawed | 11.05 b |
| Leptailurus serval (serval) | 202135 | Male | Declawed | 11.88 |
| Lynx rufus (bobcat) | 202105 | Male | Declawed | 14.24 |
| Puma concolor (cougar) | 202145 | Male | Declawed | 61.69 |
| Panthera tigris (tiger) | 202127 | Female | Declawed | 115.21 |
| Panthera tigris (tiger) | 202152 | Male | Declawed | 147.87 |
| MM | PCSA | FL | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | Sample | Y-Intercept | Slope (β) | Lower β CL | Upper β CL | r2 | Y-Intercept | Slope (β) | Lower β CL | Upper β CL | r2 | Y-Intercept | Slope (β) | Lower β CL | Upper β CL | r2 |
| Total forearm | Clawed | −0.86 | 1.12 | 0.99 | 1.26 | 0.98 | −0.93 | 1.32 a | 1.17 a | 1.50 | 0.98 | −0.90 | 0.93 | 0.53 | 1.60 | 0.73 |
| Declawed | −0.95 | 1.16 | 0.95 | 1.41 | 0.99 | −0.89 | 1.25 a | 1.06 a | 1.49 | 0.99 | −1.01 | 1.00 | 0.45 | 2.24 | 0.88 | |
| Total flexors | Clawed | −0.95 | 1.12 a | 1.02 a | 1.22 | 0.99 | −1.10 | 1.36 a | 1.22 a | 1.51 | 0.98 | −0.80 | 0.77 | 0.41 | 1.47 | 0.64 |
| Declawed | −1.10 | 1.19 a | 1.03 a | 1.38 | 0.99 | −1.18 | 1.35 a | 1.16 a | 1.56 | 0.99 | −0.96 | 0.88 | - | - | 0.24 | |
| Total extensors | Clawed | −1.03 | 1.12 | 0.96 | 1.31 | 0.97 | −1.32 | 1.39 a | 1.05 a | 1.85 | 0.90 | −0.74 | 0.84 | 0.44 | 1.60 | 0.67 |
| Declawed | −0.97 | 1.07 | 0.79 | 1.45 | 0.97 | −0.89 | 1.08 | 0.73 | 1.61 | 0.96 | −1.10 | 1.12 a | 1.09 a | 1.15 | 1.00 | |
| Wrist flexors | Clawed | −1.17 | 1.15 | 0.87 | 1.52 | 0.88 | −1.33 | 1.38 | 0.96 | 1.99 | 0.82 | −1.45 | 1.10 | 0.44 | 2.73 | 0.52 |
| Declawed | −1.12 | 1.13 | 0.70 | 1.81 | 0.95 | −1.05 | 1.20 | 0.79 | 1.83 | 0.96 | −1.71 | 1.30 | 0.69 | 2.42 | 0.92 | |
| Digital flexors | Clawed | −1.05 | 1.14 a | 1.02 a | 1.28 | 0.98 | −1.45 | 1.49 a | 1.12 a | 1.99 | 0.88 | −0.84 | 0.85 | 0.25 | 2.92 | 0.44 |
| Declawed | −1.27 | 1.23 | 0.84 | 1.80 | 0.96 | −1.81 | 1.60 | 0.61 | 4.17 | 0.86 | −1.05 | 1.03 | - | - | 0.18 | |
| Wrist extensors | Clawed | −1.11 | 1.13 | 0.93 | 1.38 | 0.95 | −1.54 | 1.47 a | 1.02 a | 2.12 | 0.85 | −0.68 | 0.83 | 0.36 | 1.93 | 0.59 |
| Declawed | −0.99 | 1.05 | 0.81 | 1.38 | 0.98 | −1.00 | 1.11 | 0.80 | 1.54 | 0.97 | −0.97 | 1.05 | 0.92 | 1.19 | 1.00 | |
| Digital extensors | Clawed | −1.11 | 1.06 | 0.96 | 1.17 | 0.98 | −1.24 | 1.16 | 0.94 | 1.44 | 0.93 | −1.12 | 1.05 | 0.70 | 1.57 | 0.79 |
| Declawed | −1.21 | 1.11 | 0.74 | 1.69 | 0.96 | −1.07 | 1.01 | 0.53 | 1.90 | 0.91 | −1.55 | 1.37 a | 1.03 a | 1.82 | 0.98 | |
| BR | Clawed | −1.34 | 1.14 | 0.89 | 1.46 | 0.91 | −2.03 | 1.38 | 0.84 | 2.27 | 0.73 | −0.91 | 1.31 | 0.46 | 3.71 | 0.48 |
| Declawed | −1.77 | 1.39 a | 1.08 a | 1.79 | 0.98 | −1.80 | 1.17 | 0.78 | 1.75 | 0.96 | −1.93 | 1.96 | 0.92 | 4.19 | 0.89 | |
| Sup. | Clawed | −1.53 | 1.21 | 0.99 | 1.48 | 0.94 | −1.48 | 1.25 a | 1.13 a | 1.37 | 0.98 | −1.82 | 1.25 | 0.72 | 2.16 | 0.70 |
| Declawed | −1.84 | 1.37 a | 1.10 a | 1.70 | 0.99 | −2.10 | 1.57 a | 1.20 a | 2.07 | 0.98 | −1.36 | 0.97 | 0.70 | 1.35 | 0.97 | |
| PT | Clawed | −1.02 | 0.96 | 0.75 | 1.22 | 0.91 | −1.22 | 1.12 | 0.97 | 1.29 | 0.97 | −0.96 | 0.84 | 0.16 | 4.27 | 0.40 |
| Declawed | −1.31 | 1.15 | 0.96 | 1.36 | 0.99 | −1.49 | 1.27 a | 1.05 a | 1.54 | 0.99 | −1.05 | 0.93 | 0.48 | 1.81 | 0.91 | |
| PQ | Clawed | −1.32 | 1.07 | 0.95 | 1.21 | 0.98 | −1.46 | 1.26 a | 1.07 a | 1.49 | 0.95 | −1.47 | 0.98 | 0.42 | 2.29 | 0.54 |
| Declawed | −1.60 | 1.25 | 0.88 | 1.78 | 0.97 | −1.47 | 1.27 | 0.93 | 1.73 | 0.97 | −1.88 | 1.23 | 0.72 | 2.09 | 0.93 | |
| FCR | Clawed | −1.16 | 1.01 | 0.74 | 1.39 | 0.86 | −1.21 | 1.04 | 0.62 | 1.77 | 0.71 | −1.79 | 1.42 | 0.63 | 3.21 | 0.56 |
| Declawed | −1.29 | 1.09 | 0.86 | 1.39 | 0.98 | −1.27 | 1.07 | 0.79 | 1.43 | 0.98 | −1.41 | 1.18 | 0.78 | 1.78 | 0.96 | |
| FCU | Clawed | −1.38 | 1.24 a | 1.06 a | 1.46 | 0.96 | −1.64 | 1.52 a | 1.21 a | 1.91 | 0.93 | −1.32 | 0.99 | 0.36 | 2.69 | 0.53 |
| Declawed | −1.36 | 1.22 | 0.82 | 1.81 | 0.96 | −1.29 | 1.29 a | 1.01 a | 1.65 | 0.98 | −1.99 | 1.41 | - | - | 0.77 | |
| PL | Clawed | −1.65 | 1.40 | 0.41 | 4.74 | 0.83 | −2.05 | 1.77 | - | - | 0.67 | −1.76 | 1.29 | - | - | 0.50 |
| Declawed | −1.56 | 1.32 a | 1.06 a | 1.63 | 1.00 | −1.89 | 1.59 | 0.50 | 5.09 | 0.93 | −1.34 | 1.05 | - | - | 0.72 | |
| FDS | Clawed | −2.13 | 1.66 | 0.69 | 3.95 | 0.57 | −2.94 | 2.25 | 0.39 | 13.09 | 0.43 | −1.19 | 0.92 | 0.59 | 1.43 | 0.79 |
| Declawed | −2.91 | 2.00 | 0.16 | 25.61 | 0.78 | −4.12 | 2.73 | - | - | 0.71 | −0.83 | 0.73 | 0.29 | 1.85 | 0.86 | |
| FDP | Clawed | −1.01 | 1.08 a | 1.00 a | 1.17 | 0.99 | −1.34 | 1.34 a | 1.10 a | 1.62 | 0.94 | −0.82 | 0.87 | 0.35 | 2.17 | 0.52 |
| Declawed | −1.06 | 1.08 | 0.90 | 1.30 | 0.99 | −1.16 | 1.14 | 0.81 | 1.59 | 0.97 | −1.10 | 1.12 | - | - | 0.86 | |
| ECU | Clawed | −1.29 | 1.14 a | 1.01 a | 1.28 | 0.98 | −1.63 | 1.45 a | 1.16 a | 1.82 | 0.93 | −1.40 | 1.03 | - | - | 0.32 |
| Declawed | −1.43 | 1.21 | 0.89 | 1.66 | 0.97 | −1.62 | 1.41 | 0.84 | 2.35 | 0.94 | −1.24 | 0.93 | 0.38 | 2.31 | 0.87 | |
| ECRL | Clawed | −1.45 | 1.27 a | 1.08 a | 1.48 | 0.98 | −2.07 | 1.54 | 0.85 | 2.79 | 0.82 | −0.70 | 1.03 | - | - | 0.35 |
| Declawed | −1.35 | 1.16 | 0.89 | 1.51 | 0.99 | −1.85 | 1.31 a | 1.10 a | 1.56 | 1.00 | −0.39 | 0.87 | 0.44 | 1.72 | 0.96 | |
| ECRB | Clawed | −0.75 | 0.75 b | 0.60 | 0.93 b | 0.97 | −1.12 | 0.99 | 0.53 | 1.85 | 0.81 | −0.83 | 0.87 | - | - | 0.26 |
| Declawed | −0.99 | 0.95 | 0.37 | 2.42 | 0.94 | −1.04 | 0.90 | - | - | 0.79 | −1.19 | 1.19 | 0.56 | 2.53 | 0.96 | |
| Muscle | p-Value (MM) | p-Value (PCSA) | F-Ratio (MM) | F-Ratio (PCSA) | DF |
|---|---|---|---|---|---|
| Total forearm | 0.054 | 0.003 * | 4.48 | 13.31 | 1, 13 |
| Total flexors | 0.005 * | <0.001 * | 11.03 | 26.67 | 1, 14 |
| Total extensors | 0.585 | 0.372 | 0.31 | 0.85 | 1, 13 |
| Wrist flexors | 0.629 | 0.870 | 0.24 | 0.03 | 1, 14 |
| Digital flexors | <0.001 * | 0.001* | 17.96 | 16.03 | 1, 14 |
| Wrist extensors | 0.890 | 0.744 | 0.02 | 0.11 | 1, 13 |
| Digital Extensors | 0.288 | 0.052 | 1.22 | 4.52 | 1, 14 |
| BR | 0.135 | 0.164 | 2.52 | 2.15 | 1, 14 |
| Sup. | 0.030 * | <0.001 * | 5.84 | 31.17 | 1, 14 |
| PT | 0.934 | 0.148 | 0.01 | 2.35 | 1, 14 |
| PQ | 0.794 | 0.884 | 0.07 | 0.02 | 1, 14 |
| FCR | 0.884 | 0.537 | 0.02 | 0.40 | 1, 14 |
| FCU | 0.706 | 0.970 | 0.15 | <0.01 | 1, 13 |
| PL | 0.564 | 0.290 | 0.37 | 1.31 | 1, 7 |
| FDS | 0.017 * | 0.012 * | 7.53 | 8.58 | 1, 13 |
| FDP | <0.001 * | 0.001 * | 19.52 | 20.12 | 1, 14 |
| ECU | 0.235 | 0.182 | 1.55 | 1.99 | 1, 13 |
| ECRL | 0.012 * | 0.011 * | 9.33 | 10.09 | 1, 10 |
| ECRB | 0.320 | 0.510 | 1.11 | 0.47 | 1, 9 |
| Category | Body Mass (kg) | Proportion MM | Proportion PCSA |
|---|---|---|---|
| Total forearm | 10 | 79.32 | |
| 40 | 75.15 | ||
| 140 | 71.57 | ||
| Total flexors | 10 | 68.62 | 68.35 |
| 40 | 76.58 | 68.38 | |
| 140 | 84.57 | 68.41 | |
| Digital extensors | 10 | 87.11 | |
| 40 | 75.37 | ||
| 140 | 66.13 | ||
| Digital flexors | 10 | 52.54 | 38.25 |
| 40 | 58.79 | 41.10 | |
| 140 | 65.07 | 43.85 | |
| SUP | 10 | 50.65 | 43.37 |
| 40 | 65.51 | 58.33 | |
| 140 | 82.65 | 76.23 | |
| ECRL | 10 | 76.04 | |
| 40 | 66.74 | ||
| 140 | 59.32 | ||
| FDP | 10 | 72.59 | 65.53 |
| 40 | 72.91 | 55.69 | |
| 140 | 73.21 | 48.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, L.L.; Piersanti, S.J.; Berger, A.; Kida, N.A.; Deutsch, A.R.; Bertok, K.; Humphries, L.; Lassiter, A.; Hartstone-Rose, A. The Effects of Onychectomy (Declawing) on Antebrachial Myology across the Full Body Size Range of Exotic Species of Felidae. Animals 2023, 13, 2462. https://doi.org/10.3390/ani13152462
Martens LL, Piersanti SJ, Berger A, Kida NA, Deutsch AR, Bertok K, Humphries L, Lassiter A, Hartstone-Rose A. The Effects of Onychectomy (Declawing) on Antebrachial Myology across the Full Body Size Range of Exotic Species of Felidae. Animals. 2023; 13(15):2462. https://doi.org/10.3390/ani13152462
Chicago/Turabian StyleMartens, Lara L., Sarah Jessica Piersanti, Arin Berger, Nicole A. Kida, Ashley R. Deutsch, Kathryn Bertok, Lauren Humphries, Angela Lassiter, and Adam Hartstone-Rose. 2023. "The Effects of Onychectomy (Declawing) on Antebrachial Myology across the Full Body Size Range of Exotic Species of Felidae" Animals 13, no. 15: 2462. https://doi.org/10.3390/ani13152462
APA StyleMartens, L. L., Piersanti, S. J., Berger, A., Kida, N. A., Deutsch, A. R., Bertok, K., Humphries, L., Lassiter, A., & Hartstone-Rose, A. (2023). The Effects of Onychectomy (Declawing) on Antebrachial Myology across the Full Body Size Range of Exotic Species of Felidae. Animals, 13(15), 2462. https://doi.org/10.3390/ani13152462






