Genomic Medicine in Canine Periodontal Disease: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
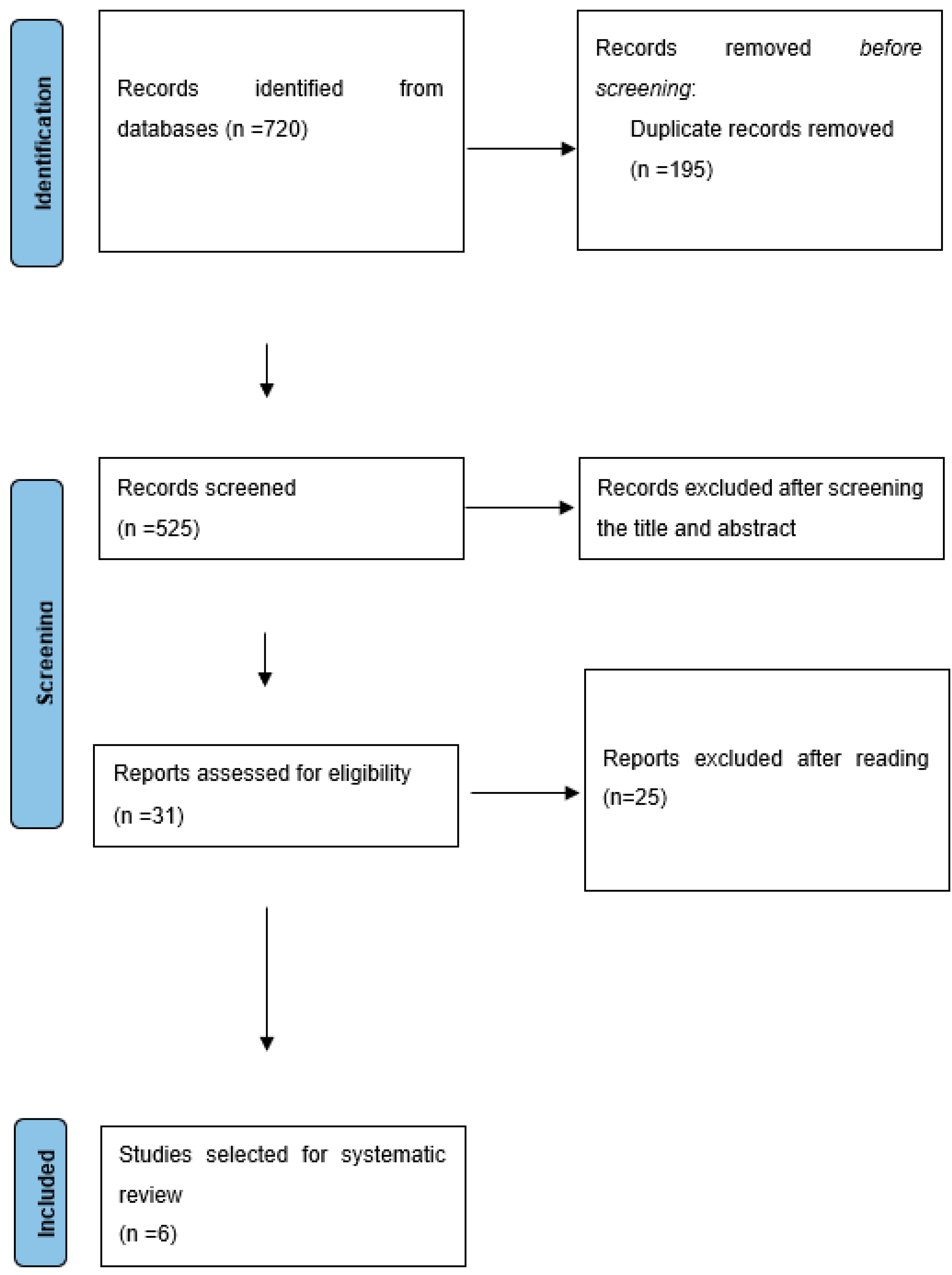
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Sources of Information and Search Strategy
2.3. Risk of Bias in Individual Studies
2.4. Data Analysis
3. Results
3.1. IL6 Gene Variants
3.2. LTF Gene Variants
3.3. IL10 Gene Variants
3.4. IL1A and IL1B Genes Variants
3.5. TLR9 Gene Variants
3.6. RANK Gene Variants
3.7. Risk of Bias Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallis, C.; Marshall, M.; Colyer, A.; O’Flynn, C.; Deusch, O.; Harris, S. A Longitudinal Assessment of Changes in Bacterial Community Composition Associated with the Development of Periodontal Disease in Dogs. Vet. Microbiol. 2015, 181, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, C.; Morinha, F.; Requicha, J.; Martins, T.; Dias, I.; Guedes-Pinto, H.; Bastos, E.; Viegas, C. Canine Periodontitis: The Dog as an Important Model for Periodontal Studies. Vet. J. 2012, 191, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.E. Management of Periodontal Disease: Understanding the Options. Vet. Clin. North. Am. Small Anim. Pr. 2005, 35, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.; Saito, E.K.; Salt, C.; Holcombe, L.J.; Desforges, N.G. Association of Periodontal Disease with Breed Size, Breed, Weight, and Age in Pure-Bred Client-Owned Dogs in the United States. Vet. J. 2021, 275, 105717. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.; Holcombe, L.J. A Review of the Frequency and Impact of Periodontal Disease in Dogs. J. Small Anim. Pr. 2020, 61, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B.A. Periodontal Disease. Top. Companion Anim. Med. 2008, 23, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, C.E. Periodontal Disease in Dogs. Etiopathogenesis, Prevalence, and Significance. Vet. Clin. North. Am. Small Anim. Pr. 1998, 28, 1111–1128. [Google Scholar] [CrossRef]
- Harvey, C.E.; Shofer, F.S.; Laster, L. Association of Age and Body Weight with Periodontal Disease in North American Dogs. J. Vet. Dent. 1994, 11, 94–105. [Google Scholar] [CrossRef]
- Kortegaard, H.E.; Eriksen, T.; Baelum, V. Periodontal Disease in Research Beagle Dogs—An Epidemiological Study: PAPER. J. Small Anim. Pr. 2008, 49, 610–616. [Google Scholar] [CrossRef]
- Kyllar, M.; Witter, K. Prevalence of Dental Disorders in Pet Dogs. Vet. Med. Czech. 2005, 50, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Kwon, D.; Bae, K.; Kim, H.J.; Kim, S.H.; Lee, D.; Lee, J.H. Treponema Denticola as a Prognostic Biomarker for Periodontitis in Dogs. PLoS ONE 2022, 17, e0262859. [Google Scholar] [CrossRef] [PubMed]
- Morinha, F.; Albuquerque, C.; Requicha, J.; Dias, I.; Leitão, J.; Gut, I.; Guedes-Pinto, H.; Viegas, C.; Bastos, E. Detection and Characterization of Interleukin-6 Gene Variants in Canis Familiaris: Association Studies with Periodontal Disease. Gene 2011, 485, 139–145. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Sheilesh, D. Risk Factors for Periodontitis. J. Int. Acad. Periodontol. 2005, 7, 3–7. [Google Scholar] [CrossRef]
- Jia, X.-W.; Yuan, Y.-D.; Yao, Z.-X.; Wu, C.-J.; Chen, X.; Chen, X.-H.; Lin, Y.-M.; Meng, X.-Y.; Zeng, X.-T.; Shao, J. Association between IL-4 and IL-4R Polymorphisms and Periodontitis: A Meta-Analysis. Dis. Markers 2017, 2017, 8021279. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Preshaw, P.M.; Donaldson, P.T. Cytokine Gene Polymorphism and Immunoregulation in Periodontal Disease. Periodontol. 2000 2004, 35, 158–182. [Google Scholar] [CrossRef]
- Laine, M.L.; Loos, B.G.; Crielaard, W. Gene Polymorphisms in Chronic Periodontitis. Int. J. Dent. 2010, 2010, 324719. [Google Scholar] [CrossRef] [Green Version]
- Wayne, R.K.; Ostrander, E.A. Lessons Learned from the Dog Genome. Trends Genet. 2007, 23, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.G.; Shearin, A.L.; Ostrander, E.A. Man’s Best Friend Becomes Biology’s Best in Show: Genome Analyses in the Domestic Dog. Annu. Rev. Genet. 2010, 44, 309–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nares, S. The Genetic Relationship to Periodontal Disease. Periodontol. 2000 2003, 32, 36–49. [Google Scholar] [CrossRef]
- Kinane, D.F.; Shiba, H.; Hart, T.C. The Genetic Basis of Periodontitis. Periodontol. 2000 2005, 39, 91–117. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-Wide Association Studies. Nat. Rev. Methods Prim. 2021, 1, 59. [Google Scholar] [CrossRef]
- Wilbe, M.; Jokinen, P.; Truvé, K.; Seppala, E.H.; Karlsson, E.K.; Biagi, T.; Hughes, A.; Bannasch, D.; Andersson, G.; Hansson-Hamlin, H.; et al. Genome-Wide Association Mapping Identifies Multiple Loci for a Canine SLE-Related Disease Complex. Nat. Genet. 2010, 42, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.K.; Sigurdsson, S.; Ivansson, E.; Thomas, R.; Elvers, I.; Wright, J.; Howald, C.; Tonomura, N.; Perloski, M.; Swofford, R.; et al. Genome-Wide Analyses Implicate 33 Loci in Heritable Dog Osteosarcoma, Including Regulatory Variants near CDKN2A/B. Genome Biol. 2013, 14, R132. [Google Scholar] [CrossRef]
- Karyadi, D.M.; Karlins, E.; Decker, B.; vonHoldt, B.M.; Carpintero-Ramirez, G.; Parker, H.G.; Wayne, R.K.; Ostrander, E.A. A Copy Number Variant at the KITLG Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit. PLoS Genet. 2013, 9, e1003409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, J.J.; White, M.E.; Boyle, M.; Shannon, L.M.; Casal, M.L.; Castelhano, M.G.; Center, S.A.; Meyers-Wallen, V.N.; Simpson, K.W.; Sutter, N.B.; et al. Imputation of Canine Genotype Array Data Using 365 Whole-Genome Sequences Improves Power of Genome-Wide Association Studies. PLoS Genet. 2019, 15, e1008003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momozawa, Y.; Merveille, A.C.; Battaille, G.; Wiberg, M.; Koch, J.; Willesen, J.L.; Proschowsky, H.F.; Gouni, V.; Chetboul, V.; Tiret, L.; et al. Genome Wide Association Study of 40 Clinical Measurements in Eight Dog Breeds. Sci. Rep. 2020, 10, 6520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Risch, N.; Merikangas, K. The Future of Genetic Studies of Complex Human Diseases. Science/AAAS 1996, 273, 1516–1517. [Google Scholar] [CrossRef] [Green Version]
- Morinha, F.; Albuquerque, C.; Requicha, J.; Dias, I.; Leitao, J.; Gut, I.; Guedes-Pinto, H.; Viegas, C.; Bastos, E. Analysis of New Lactotransferrin Gene Variants in a Case-Control Study Related to Periodontal Disease in Dog. Mol. Biol. Rep. 2012, 39, 4673–4681. [Google Scholar] [CrossRef]
- Albuquerque, C.; Morinha, F.; Requicha, J.; Dias, I.; Guedes-Pinto, H.; Viegas, C.; Bastos, E. A Case-Control Study between Interleukin-10 Gene Variants and Periodontal Disease in Dogs. Gene 2014, 539, 75–81. [Google Scholar] [CrossRef]
- Albuquerque, C.; Morinha, F.; Magalhães, J.; Requicha, J.; Dias, I.; Guedes-Pinto, H.; Bastos, E.; Viegas, C. Variants in the Interleukin-1 Alpha and Beta Genes, and the Risk for Periodontal Disease in Dogs. J. Genet. 2015, 94, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Anjo, N.; Leite-Pinheiro, F.; Ribeiro, R.; Requicha, J.F.; Lourenço, A.L.; Dias, I.; Viegas, C.; Bastos, E. Toll-like Receptor 9 Gene in Periodontal Disease—A Promising Biomarker. Gene 2019, 687, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Anjo, N.; Requicha, J.; Teixeira, A.; Dias, I.; Viegas, C.; Bastos, E. Genomic Medicine in Periodontal Disease: Old Issue, New Insights. J. Vet. Dent. 2022, 39, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L. Suggested Guidelines for Systematic Reviews of Periodontal Genetic Association Studies. J. Clin. Periodontol. 2013, 40, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W.; Rishniw, M. Periodontal Disease Is Associated with Cognitive Dysfunction in Aging Dogs: A Blinded Prospective Comparison of Visual Periodontal and Cognitive Questionnaire Scores. Open Vet. J. 2021, 11, 210–216. [Google Scholar] [CrossRef]
- Pereira dos Santos, J.D.; Cunha, E.; Nunes, T.; Tavares, L.; Oliveira, M. Relation between Periodontal Disease and Systemic Diseases in Dogs. Res. Vet. Sci. 2019, 125, 136–140. [Google Scholar] [CrossRef]
- Glickman, L.T.; Glickman, N.W.; Moore, G.E.; Goldstein, G.S.; Lewis, H.B. Evaluation of the Risk of Endocarditis and Other Cardiovascular Events on the Basis of the Severity of Periodontal Disease in Dogs. J. Am. Vet. Med. Assoc. 2009, 234, 486–494. [Google Scholar] [CrossRef]
- Glickman, L.T.; Glickman, N.W.; Moore, G.E.; Lund, E.M.; Lantz, G.C.; Pressler, B.M. Association between Chronic Azotemic Kidney Disease and the Severity of Periodontal Disease in Dogs. Prev. Vet. Med. 2011, 99, 193–200. [Google Scholar] [CrossRef]
- Rawlinson, J.E.; Goldstein, R.E.; Reiter, A.M.; Attwater, D.Z.; Harvey, C.E. Association of Periodontal Disease with Systemic Health Indices in Dogs and the Systemic Response to Treatment of Periodontal Disease. J. Am. Vet. Med. Assoc. 2011, 238, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Baciu, S.F.; Mesaroș, A.; Kacso, I.M. Chronic Kidney Disease and Periodontitis Interplay—A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 1298. [Google Scholar] [CrossRef]
- Rahimi, A.; Afshari, Z. Periodontitis and Cardiovascular Disease: A Literature Review. ARYA Atheroscler. 2021, 17, 1. [Google Scholar] [CrossRef]
- Padilla, C.; Lobos, O.; Hubert, E.; González, C.; Matus, S.; Pereira, M.; Hasbun, S.; Descouvieres, C. Periodontal Pathogens in Atheromatous Plaques Isolated from Patients with Chronic Periodontitis. J. Periodontal. Res. 2006, 41, 350–353. [Google Scholar] [CrossRef]
- Paraskevas, S.; Huizinga, J.D.; Loos, B.G. A Systematic Review and Meta-Analyses on C-Reactive Protein in Relation to Periodontitis. J. Clin. Periodontol. 2008, 35, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Lee, H.; Kim, J.-W.; Song, T.-J. Association between Periodontal Disease Status and Risk of Atrial Fibrillation: A Nationwide Population-Based Cohort Study. BMC Oral Health 2023, 23, 461. [Google Scholar] [CrossRef] [PubMed]
- Kouanda, B.; Sattar, Z.; Geraghty, P. Periodontal Diseases: Major Exacerbators of Pulmonary Diseases? Pulm. Med. 2021, 2021, 4712406. [Google Scholar] [CrossRef]
- Albuquerque-Souza, E.; Sahingur, S.E. Periodontitis, Chronic Liver Diseases, and the Emerging Oral-Gut-Liver Axis. Periodontol. 2000 2022, 89, 125–141. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Matin, P.; White, M.B.; Fagan, A.; Golob Deeb, J.; Acharya, C.; Dalmet, S.S.; Gillevet, P.M.; Sahingur, S.E. Periodontal Therapy Favorably Modulatesthe Oral-Gut-Hepatic Axis in Cirrhosis. Hepatol. Nutr. 2018, 315, 824–837. [Google Scholar]
- Acharya, C.; Sahingur, S.E.; Bajaj, J.S. Microbiota, Cirrhosis, and the Emerging Oral-Gut-Liver Axis. JCI Insight 2017, 2, e94416. [Google Scholar] [CrossRef]
- Alakhali, M.S.; Al-Maweri, S.A.; Al-Shamiri, H.M.; Al-haddad, K.; Halboub, E. The Potential Association between Periodontitis and Non-Alcoholic Fatty Liver Disease: A Systematic Review. Clin. Oral Investig. 2018, 22, 2965–2974. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Betrapally, N.S.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; White, M.B.; Unser, A.; Thacker, L.R.; Sanyal, A.J.; Kang, D.J.; et al. Salivary Microbiota Reflects Changes in Gut Microbiota in Cirrhosis with Hepatic Encephalopathy. Hepatology 2015, 62, 1260–1271. [Google Scholar] [CrossRef]
- Buduneli, N.; Baylas, H.; Buduneli, E.; Türkoǧlu, O.; Köse, T.; Dahlen, G. Periodontal Infections and Pre-Term Low Birth Weight: A Case-Control Study. J. Clin. Periodontol. 2005, 32, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, P.; Mahendra, J. Toll-like Receptors: A Key Marker for Periodontal Disease and Preterm Birth—A Contemporary Review. J. Clin. Diagn. Res. 2015, 9, ZE14–ZE17. [Google Scholar] [CrossRef] [PubMed]
- DeBowes, L.J. The Effects of Dental Disease on Systemic Disease. Vet. Clin. North. Am. Small Anim. Pr. 1998, 28, 1057–1062. [Google Scholar] [CrossRef]
- Kuo, L.C.; Polson, A.M.; Kang, T. Associations between Periodontal Diseases and Systemic Diseases: A Review of the Inter-Relationships and Interactions with Diabetes, Respiratory Diseases, Cardiovascular Diseases and Osteoporosis. Public Health 2008, 122, 417–433. [Google Scholar] [CrossRef]
- Peng, J.; Chen, J.; Liu, Y.; Lyu, J.; Zhang, B. Association between Periodontitis and Osteoporosis in United States Adults from the National Health and Nutrition Examination Survey: A Cross-Sectional Analysis. BMC Oral Health 2023, 23, 254. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.Y. Osteoporosis and Periodontal Diseases—An Update on Their Association and Mechanistic Links. Periodontol. 2000 2022, 89, 99–113. [Google Scholar] [CrossRef]
- Pizzo, G.; Guiglia, R.; Russo, L.L.; Campisi, G. Dentistry and Internal Medicine: From the Focal Infection Theory to the Periodontal Medicine Concept. Eur. J. Intern. Med. 2010, 21, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shen, L. Advances and Trends in Omics Technology Development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef]
- Wörheide, M.A.; Krumsiek, J.; Kastenmüller, G.; Arnold, M. Multi-Omics Integration in Biomedical Research—A Metabolomics-Centric Review. Anal. Chim. Acta 2021, 1141, 144–162. [Google Scholar] [CrossRef]
- Veenstra, T.D. Omics in Systems Biology: Current Progress and Future Outlook. Proteomics 2021, 21, e2000235. [Google Scholar] [CrossRef]
- Carlos, G.; dos Santos, F.P.; Fröehlich, P.E. Canine Metabolomics Advances. Metabolomics 2020, 16, 16. [Google Scholar] [CrossRef]
- Overmyer, K.A.; Rhoads, T.W.; Merrill, A.E.; Ye, Z.; Westphall, M.S.; Acharya, A.; Shukla, S.K.; Coon, J.J. Proteomics, Lipidomics, Metabolomics, and 16S DNA Sequencing of Dental Plaque from Patients with Diabetes and Periodontal Disease. Mol. Cell. Proteom. 2021, 20, 100126. [Google Scholar] [CrossRef] [PubMed]
- Rezende, T.M.B.; Lima, S.M.F.; Petriz, B.A.; Silva, O.N.; Freire, M.S.; Franco, O.L. Dentistry Proteomics: From Laboratory Development to Clinical Practice. J. Cell. Physiol. 2013, 228, 2271–2284. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S. Proteome Analysis of Molecular Events in Oral Pathogenesis and Virus: A Review with a Particular Focus on Periodontitis. Int. J. Mol. Sci. 2020, 21, 5184. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.M.F.; Furrow, E.; Souza, C.P.; Granick, J.L.; De Jong, E.P.; Griffin, T.J.; Wang, X. Salivary Proteomics of Healthy Dogs: An in Depth Catalog. PLoS ONE 2018, 13, e0191307. [Google Scholar] [CrossRef]
- Fuentes, L.; Yakob, M.; Wong, D.T.W. Emerging Horizons of Salivary Diagnostics for Periodontal Disease. Br. Dent. J. 2014, 217, 567–573. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, C.J.; Camargo, P.M. Salivary Biomarkers in the Diagnosis of Periodontal Disease. J. Calif. Dent. Assoc. 2013, 41, 119–124. [Google Scholar] [CrossRef]
- Califf, K.J.; Schwarzberg-Lipson, K.; Garg, N.; Gibbons, S.M.; Caporaso, J.G.; Slots, J.; Cohen, C.; Dorrestein, P.C.; Kelley, S.T.; Herman, C. Multi-Omics Analysis of Periodontal Pocket Microbial Communities Pre-and Posttreatment. mSystems 2017, 2, e00016–e00017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taba Jr, M.; de Souza, S.L.S.; Mariguela, V.C. Periodontal Disease: A Genetic Perspective. Braz. Oral Res. 2012, 26, 32–40. [Google Scholar] [CrossRef]
- Michalowicz, B.S.; Diehl, S.R.; Gunsolley, J.C.; Sparks, B.S.; Brooks, C.N.; Koertge, T.E.; Califano, J.V.; Burmeister, J.A.; Schenkein, H.A. Evidence of a Substantial Genetic Basis for Risk of Adult Periodontitis. J. Periodontol. 2000, 71, 1699–1707. [Google Scholar] [CrossRef]
- Zoheir, N.; Kurushima, Y.; Lin, G.H.; Nibali, L. Periodontal Infectogenomics: A Systematic Review Update of Associations between Host Genetic Variants and Subgingival Microbial Detection. Clin. Oral Investig. 2022, 26, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Hart, T.C. Genes and Gene Polymorphisms Associated with Periodontal Disease. Crit. Rev. Oral Biol. Med. 2003, 14, 430–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nibali, L.; D’Aiuto, F.; Donos, N.; Griffiths, G.S.; Parkar, M.; Tonetti, M.S.; Humphries, S.E.; Brett, P.M. Association between Periodontitis and Common Variants in the Promoter of the Interleukin-6 Gene. Cytokine 2009, 45, 50–54. [Google Scholar] [CrossRef]
- Dirschnabel, A.J.; Alvim-Pereira, F.; Alvim-Pereira, C.C.; Bernardino, J.F.; Rosa, E.A.R.; Trevilatto, P.C. Analysis of the Association of IL1B(C-511T) Polymorphism with Dental Implant Loss and the Clusterization Phenomenon. Clin. Oral Implant. Res. 2011, 22, 1235–1241. [Google Scholar] [CrossRef]
- Pontes, C.C.; Gonzales, J.R.; Novaes, A.B.; Taba, M.; Grisi, M.F.M.; Michel, J.; Meyle, J.; Scombatti De Souza, S.L. Interleukin-4 Gene Polymorphism and Its Relation to Periodontal Disease in a Brazilian Population of African Heritage. J. Dent. 2004, 32, 241–246. [Google Scholar] [CrossRef]
- Demmer, R.T.; Behle, J.H.; Wolf, D.L.; Handfield, M.; Kebschull, M.; Celenti, R.; Pavlidis, P.; Papapanou, P.N. Transcriptomes in Healthy and Diseased Gingival Tissues. J. Periodontol. 2008, 79, 2112–2124. [Google Scholar] [CrossRef]
- Page, R.C. The Role of Inflammatory Mediators in the Pathogenesis of Periodontal Disease. J. Periodontal. Res. 1991, 26, 230–242. [Google Scholar] [CrossRef]
- Smith, A.J.P.; Humphries, S.E. Cytokine and Cytokine Receptor Gene Polymorphisms and Their Functionality. Cytokine Growth Factor Rev. 2009, 20, 43–59. [Google Scholar] [CrossRef]
- Stabholz, A.; Soskolne, W.A.; Shapira, L. Genetic and Environmental Risk Factors for Chronic Periodontitis and Aggressive Periodontitis. Periodontol. 2000 2010, 53, 138–153. [Google Scholar] [CrossRef]
- Bozkurt, F.Y.; Yetkin Ay, Z.; Berker, E.; Tepe, E.; Akkuş, S. Anti-Inflammatory Cytokines in Gingival Crevicular Fluid in Patients with Periodontitis and Rheumatoid Arthritis: A Preliminary Report. Cytokine 2006, 35, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Mercado, F.B.; Marshall, R.I.; Bartold, P.M. Inter-Relationships between Rheumatoid Arthritis and Periodontal Disease A Review. J. Clin. Periodontol. 2003, 30, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Struillou, X.; Boutigny, H.; Soueidan, A.; Layrolle, P. Experimental Animal Models in Periodontology: A Review. Open Dent. J. 2010, 4, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolone, L.; Shin, H.K.; Stefanoni, D.; Baek, J.H.; Gao, Y.; Morrison, E.J.; Nemkov, T.; Thomas, T.; Francis, R.O.; Hod, E.A.; et al. ZOOMICS: Comparative Metabolomics of Red Blood Cells From Old World Monkeys and Humans. Front. Physiol. 2020, 11, 593841. [Google Scholar] [CrossRef] [PubMed]
- Stefanoni, D.; Shin, H.K.H.; Baek, J.H.; Champagne, D.P.; Nemkov, T.; Thomas, T.; Francis, R.O.; Zimring, J.C.; Yoshida, T.; Reisz, J.A.; et al. Red Blood Cell Metabolism in Rhesus Macaques and Humans: Comparative Biology of Blood Storage. Haematologica 2020, 105, 2174–2186. [Google Scholar] [CrossRef] [Green Version]
- Dannan, A.; Dannan, A.; Alkattan Citation Dannan, F.A.; Alkattan, F. Animal Models in Periodontal Research: A Mini-Review of the Literature. Internet J. Vet. Med. 2008, 5, 5. [Google Scholar]
- King, L.J. One World of Veterinary Medicine. Rev. Sci. Tech. 2009, 28, 463–467. [Google Scholar] [CrossRef]
- Clark, J.A.B.J.; Whalen, D.; Marshall, H.D. Genomic Analysis of Gum Disease and Hypertrichosis in Foxes. Genet. Mol. Res. 2016, 15, gmr.15025363. [Google Scholar] [CrossRef]
- Aquino-Martinez, R.; Khosla, S.; Farr, J.N.; Monroe, D.G. Periodontal Disease and Senescent Cells: New Players for an Old Oral Health Problem? Int. J. Mol. Sci. 2020, 21, 7441. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T. Interleukin 6 in Autoimmune and Inflammatory Diseases: A Personal Memoir. Proc. Jpn. Acad. Ser. B 2010, 86, 717–730. [Google Scholar] [CrossRef] [Green Version]
- Dalrymple, S.A.; Slattery, R.; Aud, D.M.; Krishna, M.; Lucian, L.A.; Murray, R. Interleukin-6 Is Required for a Protective Immune Response to Systemic Escherichia Coli Infection. Infect. Immun. 1996, 64, 3231–3235. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Takashiba, S.; Nagai, A.; Takigawa, M.; Myoukai, F.; Kurihara, H.; Murayama, Y.; Kishimoto, T. Assessment of Interleukin-6 in the Pathogenesis of Periodontal Disease. J. Periodontol. 1994, 65, 147–153. [Google Scholar] [CrossRef]
- Matsuki, Y.; Yamamoto, T.; Hara, K. Detection of Inflammatory Cytokine Messenger RNA (MRNA)-Expressing Cells in Human Inflamed Gingiva by Combined in Situ Hybridization and Immunohistochemistry. Immunology 1992, 76, 42–47. [Google Scholar] [PubMed]
- Nibali, L.; Tonetti, M.S.; Ready, D.; Parkar, M.; Brett, P.M.; Donos, N.; D’Aiuto, F. Interleukin-6 Polymorphisms Are Associated With Pathogenic Bacteria in Subjects With Periodontitis. J. Periodontol. 2008, 79, 677–683. [Google Scholar] [CrossRef]
- Schanbacher, F.L.; Goodman, R.E.; Talhouk, R.S. Bovine Mammary Lactoferrin: Implications from Messenger Ribonucleic Acid (MRNA) Sequence and Regulation Contrary to Other Milk Proteins. J. Dairy Sci. 1993, 76, 3812–3831. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Furgang, D. Lactoferrin Iron Levels Affect Attachment of Actinobacillus Actinomycetemcomitans to Buccal Epithelial Cells. J. Periodontol. 2002, 73, 616–623. [Google Scholar] [CrossRef]
- Schryvers, A.B.; Morris, L.J. Identification and Characterization of the Human Lactoferrin-Binding Protein from Neisseria Meningitidis. Infect. Immun. 1988, 56, 1144–1149. [Google Scholar] [CrossRef]
- Yamauchi, K.; Tomita, M.; Giehl, T.J.; Ellison Iii2, R.T. Antibacterial Activity of Lactoferrin and a Pepsin-Derived Lactoferrin Peptide Fragment. Infect. Immun. 1993, 61, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Erdei, J.; Forsgren, A.; Naidu, A.S. Lactoferrin Binds to Porins OmpF and OmpC in Escherichia Coli. Infect. Immun. 1994, 62, 1236–1240. [Google Scholar] [CrossRef]
- Sojar, H.T.; Hamada, N.; Genco, R.J. Structures Involved in the Interaction of Porphyromonas Gingivalis Fimbriae and Human Lactoferrin. FEBS Lett. 1998, 422, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S. The Role of Crevicular Fluid Iron in Periodontal Disease*. J. Periodontol. 1985, 56, 22–27. [Google Scholar] [CrossRef]
- Bullen, J.J.; Rogers, H.J.; Griffiths, E. Role of Iron in Bacterial Infection. Curr. Top. Microbiol. Immunol. 1978, 56, 22–27. [Google Scholar]
- Alugupalli, K.R.; Kalfas, S.; Edwardsson, S.; Naidu, A.S. Lactoferrin Interaction with Actinobacillus Actinomycetemcomitans. Oral Microbiol. Immunol. 1995, 10, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.A.; Mandelf, I.D.; Herreraf, M.S. Lysozyme and Lactoferrin Quantitation in the Crevicular Fluid. J. Periodontol. 1983, 54, 347–350. [Google Scholar] [CrossRef]
- Wei, P.F.; Ho, K.Y.; Ho, Y.P.; Wu, Y.M.; Yang, Y.H.; Tsai, C.C. The Investigation of Glutathione Peroxidase, Lactoferrin, Myeloperoxidase and Interleukin-1β in Gingival Crevicular Fluid: Implications for Oxidative Stress in Human Periodontal Diseases. J. Periodontal Res. 2004, 39, 287–293. [Google Scholar] [CrossRef]
- Cutler, C.W.; Stanford, T.W.; Abraham, C.; Cederberg, R.A.; Boardman, T.J.; Ross, C. Clinical Benefits of Oral Irrigation for Periodontitis Are Related To. J. Clin. Periodontol. 2000, 27, 134–143. [Google Scholar] [CrossRef]
- Albuquerque, C.M.; Cortinhas, A.J.; Morinha, F.J.; Leitão, J.C.; Viegas, C.A.; Bastos, E.M. Association of the IL-10 Polymorphisms and Periodontitis: A Meta-Analysis. Mol. Biol. Rep. 2012, 39, 9319–9329. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zucker, K.; Fuller, L.; Tzakis, A.; Esquenazi, V.; Miller, J. Cloning and Expression of Canine Interleukin-10. J. Interferon Cytokine Res. 1995, 15, 1103–1109. [Google Scholar] [CrossRef]
- Zucker, C.; Zucker, K.; Asthana, D.; Carreno, M.; Viciana, A.L.; Ruiz, P.; Esquenazi, V.; Nery, J.; Burke, G.; Miller, J. Longitudinal Induced IL-2 MRNA Monitoring in Renal Transplant Patients Immunosuppressed with Cyclosporine and in Unmodified Canine Renal Transplant Rejection. Hum. Immunol. 1996, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Short, A.D.; Catchpole, B.; Kennedy, L.J.; Barnes, A.; Fretwell, N.; Jones, C.; Thomson, W.; Ollier, W.E.R. Analysis of Candidate Susceptibility Genes in Canine Diabetes. J. Hered. 2007, 98, 518–525. [Google Scholar] [CrossRef]
- Kober, L.; Zehe, C.; Bode, J. Optimized Signal Peptides for the Development of High Expressing CHO Cell Lines. Biotechnol. Bioeng. 2013, 110, 1164–1173. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimbux, N.Y.; Saraiya, V.M.; Elangovan, S.; Allareddy, V.; Kinnunen, T.; Kornman, K.S.; Duff, G.W. Interleukin-1 Gene Polymorphisms and Chronic Periodontitis in Adult Whites: A Systematic Review and Meta-Analysis. J. Periodontol. 2012, 83, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Korman, K.S.; Crane, A.; Wang, H.-Y.; di Giovine, F.S.; Newman, M.G.; Pirk, F.W.; Wilson, T.G., Jr.; Higginbottom, F.L.; Duff, G.W. The Interleukin-1 Genotype as a Severity Factor in Adult Periodontal Disease. Jounal Clin. Periodontol. 1997, 24, 72–77. [Google Scholar] [CrossRef]
- Nikolopoulos, G.K.; Dimou, N.L.; Hamodrakas, S.J.; Bagos, P.G. Cytokine Gene Polymorphisms Inperiodontal Disease: A Meta-Analysis of 53 Studies Including 4178 Cases and 4590 Controls. J. Clin. Periodontol. 2008, 35, 754–767. [Google Scholar] [CrossRef]
- Hans, M.; Hans, V.M. Toll-like Receptors and Their Dual Role in Periodontitis: A Review. J. Oral Sci 2011, 53, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, P.D.; Xia-Juan, X.; Crump, K.E.; Abe, T.; Hajishengallis, G.; Sahingur, S.E. Toll-like Receptor 9-Mediated Inflammation Triggers Alveolar Bone Loss in Experimental Murine Periodontitis. Infect. Immun. 2015, 83, 2992–3002. [Google Scholar] [CrossRef] [Green Version]
- Sahingur, S.E.; Xia, X.-J.; Voth, S.C.; Yeudall, W.A.; Gunsolley, J.C. Increased Nucleic Acid Receptor Expression in Chronic Periodontitis. J. Periodontol. 2013, 84, e48–e57. [Google Scholar] [CrossRef] [PubMed]
- Holla, L.I.; Vokurka, J.; Hrdlickova, B.; Augustin, P.; Fassmann, A. Association of Toll-like Receptor 9 Haplotypes with Chronic Periodontitis in Czech Population. J. Clin. Periodontol. 2010, 37, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Sojod, B.; Chateau, D.; Mueller, C.G.; Babajko, S.; Berdal, A.; Lézot, F.; Castaneda, B. RANK/RANKL/OPG Signalization Implication in Periodontitis: New Evidence from a RANK Transgenic Mouse Model. Front. Physiol. 2017, 8, 338. [Google Scholar] [CrossRef] [Green Version]
- Albandar, J.M.; Tinoco, E.M.B. Global Epidemiology of Periodontal Diseases in Children and Young Persons. Periodontol. 2000 2002, 29, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Saridomichelakis, M.; Nuttall, T.; Bensignor, E.; Griffin, C.E.; Hill, P.B. Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)-4, a Simplified Severity Scale for Assessing Skin Lesions of Atopic Dermatitis in Dogs. Vet. Dermatol. 2014, 25, 77-e25. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yamamoto-Fukuda, M.; Takizawa, T.; Shimakura, H.; Sakaguchi, M. Association Analysis of Non-Synonymous Polymorphisms of Interleukin-4 Receptor-α and Interleukin-13 Genes in Canine Atopic Dermatitis. J. Vet. Med. Sci. 2020, 82, 1253–1259. [Google Scholar] [CrossRef]
- Wood, S.H.; Ke, X.; Nuttall, T.; McEwan, N.; Ollier, W.E.; Carter, S.D. Genome-Wide Association Analysis of Canine Atopic Dermatitis and Identification of Disease Related SNPs. Immunogenetics 2009, 61, 765–772. [Google Scholar] [CrossRef]
- Short, A.D.; Catchpole, B.; Kennedy, L.J.; Barnes, A.; Lee, A.C.; Jones, C.A.; Fretwell, N.; Ollier, W.E.R. T Cell Cytokine Gene Polymorphisms in Canine Diabetes Mellitus. Vet. Immunol. Immunopathol. 2009, 128, 137–146. [Google Scholar] [CrossRef]
- Short, A.D.; Saleh, N.M.; Catchpole, B.; Kennedy, L.J.; Barnes, A.; Jones, C.A.; Fretwell, N.; Ollier, W.E.R. CTLA4 Promoter Polymorphisms Are Associated with Canine Diabetes Mellitus. Tissue Antigens 2010, 75, 242–252. [Google Scholar] [CrossRef]
- Kathrani, A.; Holder, A.; Catchpole, B.; Alvarez, L.; Simpson, K.; Werling, D.; Allenspach, K. TLR5 Risk-Associated Haplotype for Canine Inflammatory Bowel Disease Confers Hyper-Responsiveness to Flagellin. PLoS ONE 2012, 7, e30117. [Google Scholar] [CrossRef]
- Kathrani, A.; House, A.; Catchpole, B.; Murphy, A.; German, A.; Werling, D.; Allenspach, K. Polymorphisms in the Tlr4 and Tlr5 Gene Are Significantly Associated with Inflammatory Bowel Disease in German Shepherd Dogs. PLoS ONE 2010, 5, e15740. [Google Scholar] [CrossRef] [Green Version]
- Peiravan, A.; Allenspach, K.; Boag, A.M.; Soutter, F.; Holder, A.; Catchpole, B.; Kennedy, L.J.; Werling, D.; Procoli, F. Single Nucleotide Polymorphisms in Major Histocompatibility Class II Haplotypes Are Associated with Potential Resistance to Inflammatory Bowel Disease in German Shepherd Dogs. Vet. Immunol. Immunopathol. 2016, 182, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Zhang, Z.; Feng, F.; Schweitzer, P.; Phavaphutanon, J.; Vernier-Singer, M.; Corey, E.; Friedenberg, S.; Mateescu, R.; Williams, A.; et al. Single Nucleotide Polymorphisms Refine QTL Intervals for Hip Joint Laxity in Dogs. Anim. Genet. 2008, 39, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Canadas, A.; Santos, M.; Nogueira, A.; Assis, J.; Gomes, M.; Lemos, C.; Medeiros, R.; Dias-Pereira, P. Canine Mammary Tumor Risk Is Associated with Polymorphisms in RAD51 and STK11 Genes. J. Vet. Diagn. Investig. 2018, 30, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Reimann, M.J.; Fredholm, M.; Cremer, S.E.; Christiansen, L.B.; Meurs, K.M.; Møller, J.E.; Häggström, J.; Lykkesfeldt, J.; Olsen, L.H. Polymorphisms in the Serotonin Transporter Gene and Circulating Concentrations of Neurotransmitters in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease. J. Vet. Intern. Med. 2021, 35, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Distl, O. Linkage and Association Analyses of Intragenic SNPs in the Canine B-Crystallin Genes CRYBB1, CRYBB2, CRYBB3, CRYBA1 and CRYBA4 with Primary Cataracts in Wire-Haired Dachshunds. Anim. Genet. 2007, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Brodzikowska, A.; Górski, B.; Bogusławska-Kapała, A. Association between IL-1 Gene Polymorphisms and Stage III Grade B Periodontitis in Polish Population. Int. J. Environ. Res. Public Health 2022, 19, 14687. [Google Scholar] [CrossRef]
- Klein, T. Predisposing Factors and Gross Examination Findings in Periodontal Disease. Clin. Tech. Small Anim. Pr. 2000, 15, 189–196. [Google Scholar] [CrossRef]
- Song, L.; Yao, J.; He, Z.; Xu, B. Genes Related to Inflammation and Bone Loss Process in Periodontitis Suggested by Bioinformatics Methods. BMC Oral Health 2015, 15, 105. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, X.; Zhou, Y.; Acharya, A.; Savkovic, V.; Xu, C.; Wu, N.; Deng, Y.; Hu, X.; Li, H.; et al. Shared Genetic and Epigenetic Mechanisms between Chronic Periodontitis and Oral Squamous Cell Carcinoma. Oral Oncol. 2018, 86, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Whitmore, S.E.; Lamont, R.J. Oral Bacteria and Cancer. PLoS Pathog. 2014, 10, e1003933. [Google Scholar] [CrossRef]
- Zeng, X.T.; Deng, A.P.; Li, C.; Xia, L.Y.; Niu, Y.M.; Leng, W.D. Periodontal Disease and Risk of Head and Neck Cancer: A Meta-Analysis of Observational Studies. PLoS ONE 2013, 8, e79017. [Google Scholar] [CrossRef]
- Barros, S.P.; Fahimipour, F.; Tarran, R.; Kim, S.; Scarel-Caminaga, R.M.; Justice, A.; North, K. Epigenetic Reprogramming in Periodontal Disease: Dynamic Crosstalk with Potential Impact in Oncogenesis. Periodontol. 2000 2020, 82, 157–172. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Study Sample | Study Variable | Objectives | Methodology | Main Results | Genetic Variations Detected | Alteration in the Conformation of Proteins |
|---|---|---|---|---|---|---|---|---|
| Morinha, F. et al., 2011 [12] | Case–control | 70 dogs (45 controls and 25 cases) | IL6 gene | Identify genetic variations of IL6 gene and verify its association with PD. Clinical periodontal evaluation of the individuals. | General and oral (odonto-stomatological) examination. Collection of blood samples for subsequent DNA extraction. Amplification and genotyping of 2 regions of the dog IL6 gene (5′UTR-exon 2 and exon 5–3′UTR), using the respective forward and reverse primers. The amplified fragments were purified and sequenced. | In the analyzed population, there was no evidence that the identified variants contributed significantly to PD susceptibility. | Three new single nucleotide polymorphisms: I/2_g.564T>C—exon 2 I/5_g.105G>A—exon 5 I/5_g.440G>A—exon 5 | The sequence variant I/5_g.105 G>A led to an amino acid change (arginine to glutamine). |
| Morinha, F. et al., 2012 [29] | Case–control | 90 dogs (50 controls and 40 cases) | LTF gene | Identify genetic variations of dog LTF gene and verify its association with PD. | General and oral (odonto-stomatological) examination. Collection of blood samples for subsequent DNA extraction. The regions of LTF analyzed were exon 12 and 15. For the polymerase chain reaction (PCR) amplification of these regions were selected specific primers. All amplified fragments were purified and sequenced. | Does not provide evidence that LTF variants contributed to the genetic basis of canine PD. | Eight new single nucleotide polymorphisms were detected: L/2_g.288T>G—intron 2 L/2_g.403G>A—intron 2 L/15_g.213C>T—intron 14 L/15_g.411C>T –exon 15 L/15_g.420G>A—exon 15 L/15_g.445A>G—exon 15 L/15_g.482G>A—exon 15 L/15_g.514A>G—exon 15 | The sequence variant L/15_g.411C>T led to an amino acid change (proline to leucine). |
| Albuquerque, C. et al., 2014 [30] | Case–control | 90 dogs (50 controls and 40 cases) | IL10 gene | Identify IL10 gene variations that can influence IL10 regulatory role, and consequently the canine PD susceptibility. | General and oral (odonto-stomatological) examination. Collection of blood samples for subsequent DNA extraction. Analysis of two fragments, one corresponding to the target region in the 5′ flanking sequence and the other including 5′ untranslated region and exon 1, were obtained using forward and reverse primers. PCR amplifications and subsequent, the fragments were purified and analyzed. | No significant association was found between the IL10 genetic variations and canine PD. | Six new nucleotide variations and a 3-nucleotide deletion: IL10/1_g.276_278delTGA–5′ flanking region IL10/1_g.506A>g—5′ flanking region IL10/2_g.285G>A—exon 1 (signal peptide) IL10/2_g.305C>T—exon 1 IL10/2_g.424C>A—intron 1 IL10/2_g.497C>T—intron 1 IL10/2_g.513C>A—intron 1 | The variation IL10/2_g.285G>A led to an amino acid change (glycine to arginine) in the putative signal peptide. |
| Albuquerque, C. et al., 2015 [31] | Case–control | 90 dogs (50 controls and 40 cases) | IL1-α (IL1A) and IL1-β (IL1B) genes | Identify genetic variations from the IL1A and IL1B genes and evaluate their possible association with PD. | General and oral (odonto-stomatological) examination. Collection of blood samples for subsequent DNA extraction. Two fragments of the IL1A gene were analyzed. Fragment 1 includes exon 2 and partial intron 1 and fragment 2 includes exon 5. Only 1 fragment was analyzed in the IL1B gene, which includes exons 4 and 5. All amplified fragments were purified and sequenced. | Genetic variations in IL1 may play a significant role in canine PD susceptibility. IL1A/1_g.388C allele was associated with a decreased PD risk. IL1A/1_g.521A allele could confer an increased risk. | Eight single nucleotide polymorphisms identified: IL1A/1_g.110A>G—intron 1 IL1A/1_g.113C>A—intron 1 IL1A/1_g.129G>A—intron 1 IL1A/1_g.388A>C—intron 1 IL1A/1_g.521T>A—intron 1 IL1A/1/2_g.153T>A—intron 4 IL1A/2_g.515G>T—exon 5 IL1B_g.525G>A—exon 5 | The genetic variation IL1A/2_g.515G>T resulted in a change of amino acid, glycine to valine. |
| Gonçalves-Anjo, N. et al., 2019 [32] | Case–control | 90 dogs (50 controls and 40 cases) | TLR 9 gene | Investigating the role that seven potential polymorphic sites from exon 3 of the gene TLR9 play in predisposing to PD. | General and oral (odonto-stomatological) examination. Collection of blood samples for subsequent DNA extraction. A fragment of exon 3 was obtained with a forward and reverse primer and DNA was amplified. The amplified fragments were purified and subsequently sequenced. | Two of the genetic variants examined (rs375556098 and rs201959275) may serve as biomarkers for the susceptibility of dogs to develop PD. | The selected region of exon 3 has seven hypothetical polymorphic sites: rs851706751 rs22882109 rs852868639 rs852734185 rs851587151 rs851944523 rs22882111 | The rs22882109, rs852734185, rs851587151 and rs851944523 originated a non-conservative amino acid change. |
| Gonçalves-Anjo, N. et al., 2022 [33] | Case–control | 90 dogs (50 controls and 40 cases) | RANK gene | Identification of genetic variations and potential associations between the RANK gene and PD. | General and oral (odonto-stomatological) examination. Collection of blood samples for subsequent DNA extraction. After that, the DNA was amplified by PCR, more specifically the fragment corresponding to exon 7. Finally, the DNA was sequenced and sequence variants were identified. | None of the four variations discovered were thought to have a significant impact on the clinical disease’s severity or in the possibility that the dogs would acquire PD. | Four new genetic variations in the intronic region of the fragment: g.85A>G g.151G>T g.268A>G g.492T>C | Genetic variations in the intronic region could not change the amino acid in the final protein. |
| Author | Selection (4 Items) | Comparability (1 Item) | Exposure (3 Items) | Study Design (4 Items) | Genetic Analysis (8 Items) |
|---|---|---|---|---|---|
| Morinha, F. et al., 2011 [12] | 4/4 | 1/1 | 2/3 | 3/4 | 6/8 |
| Morinha, F. et al., 2012 [29] | 4/4 | 1/1 | 2/3 | 3/4 | 6/8 |
| Albuquerque, C. et al., 2014 [30] | 4/4 | 1/1 | 2/3 | 3/4 | 6/8 |
| Albuquerque, C. et al., 2015 [31] | 4/4 | 1/1 | 2/3 | 3/4 | 6/8 |
| Gonçalves-Anjo, N. et al., 2019 [32] | 4/4 | 1/1 | 2/3 | 3/4 | 6/8 |
| Gonçalves-Anjo, N. et al., 2022 [33] | 4/4 | 1/1 | 2/3 | 3/4 | 6/8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.; Requicha, J.; Dias, I.; Bastos, E.; Viegas, C. Genomic Medicine in Canine Periodontal Disease: A Systematic Review. Animals 2023, 13, 2463. https://doi.org/10.3390/ani13152463
Silva C, Requicha J, Dias I, Bastos E, Viegas C. Genomic Medicine in Canine Periodontal Disease: A Systematic Review. Animals. 2023; 13(15):2463. https://doi.org/10.3390/ani13152463
Chicago/Turabian StyleSilva, Carolina, João Requicha, Isabel Dias, Estela Bastos, and Carlos Viegas. 2023. "Genomic Medicine in Canine Periodontal Disease: A Systematic Review" Animals 13, no. 15: 2463. https://doi.org/10.3390/ani13152463
APA StyleSilva, C., Requicha, J., Dias, I., Bastos, E., & Viegas, C. (2023). Genomic Medicine in Canine Periodontal Disease: A Systematic Review. Animals, 13(15), 2463. https://doi.org/10.3390/ani13152463





