Effects of Methyl Sulfonyl Methane and Selenium Yeast on Fatty Liver Syndrome in Laying Hens and Their Biological Mechanisms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laying Hens, Treatments, and Sample Collection
2.2. Determination of Production Performance
2.3. Determination of Egg Quality
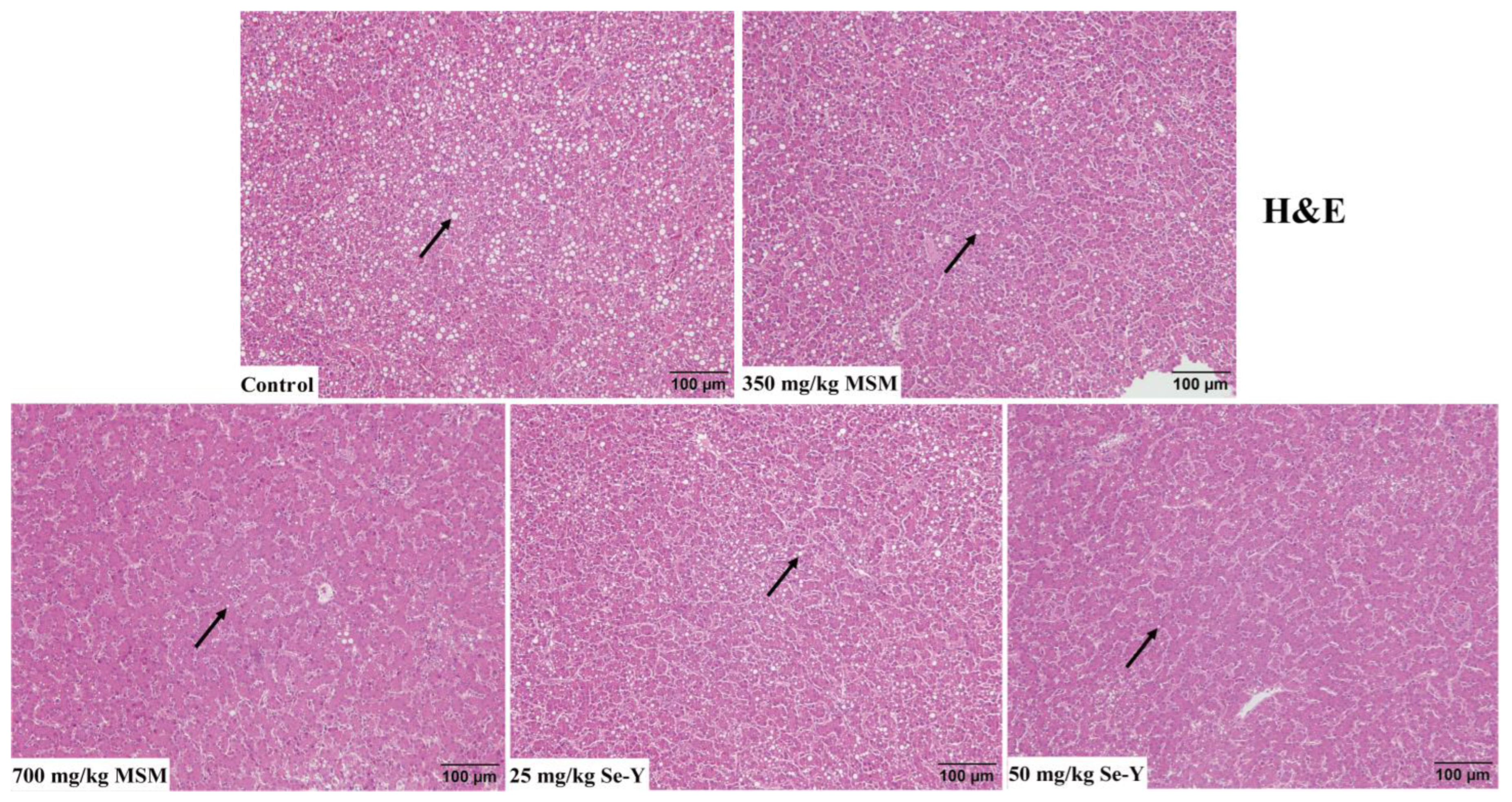
2.4. Liver Histology
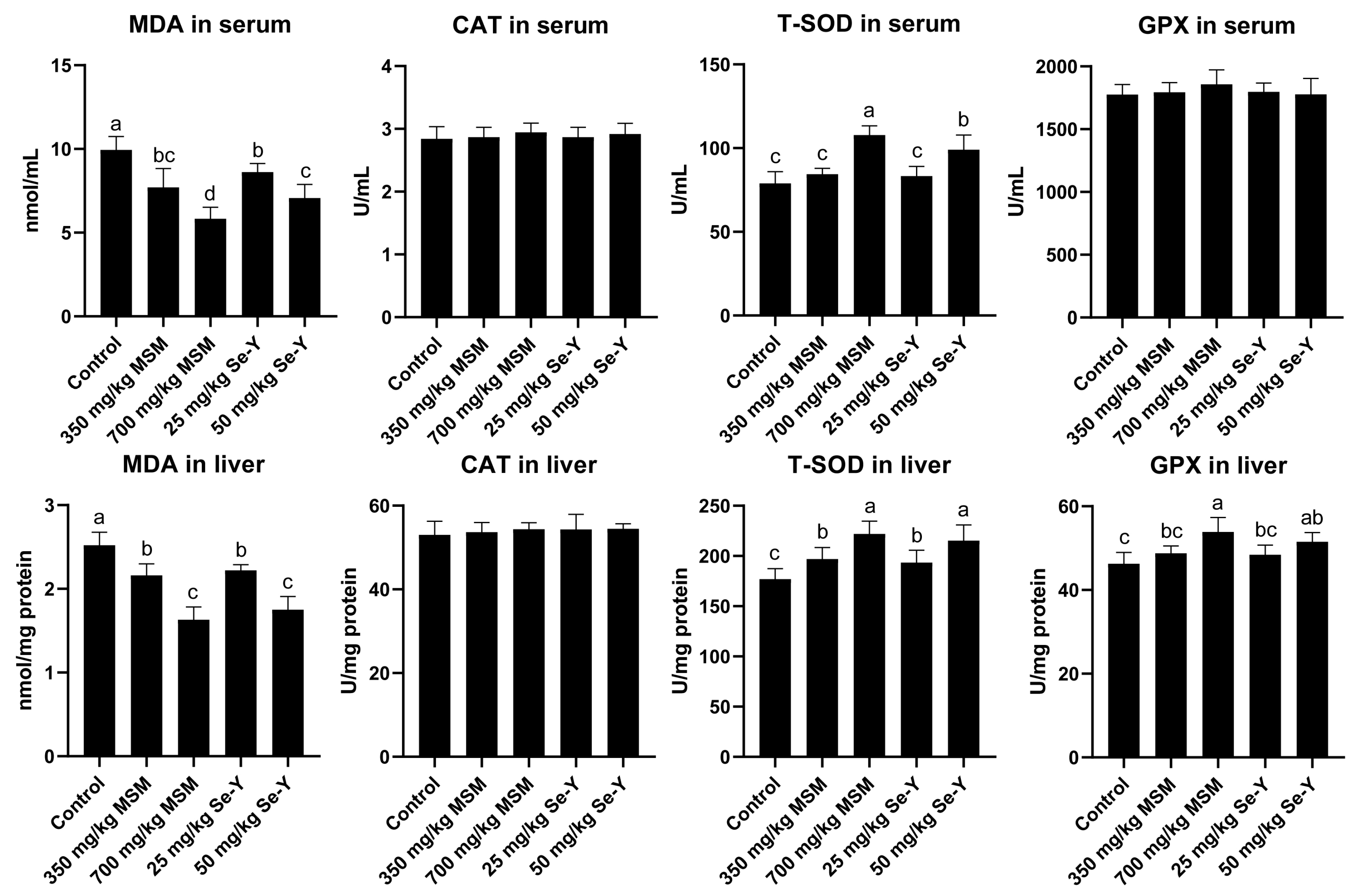
2.5. Determination of Antioxidant Enzyme Activity
2.6. Transcriptome Analysis
2.7. Real-Time q-PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of MSM and Se-Y on Production Performance and Egg Quality
3.2. Results of Liver Histological Analysis
3.3. Effects of MSM and Se-Y on Serum and Liver Antioxidant Index Values of Laying Hens
3.4. Sequencing, De Novo Assembly and Annotation Analysis
3.5. Differential Expression and Functional Analysis of Genes
3.6. Differential Genes in the KEGG Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Squires, E.J.; Leeson, S. Aetiology of fatty liver syndrome in laying hens. Br. Vet. J. 1988, 144, 602–609. [Google Scholar] [CrossRef]
- Song, Y.; Ruan, J.; Luo, J.; Wang, T.; Yang, F.; Cao, H.; Huang, J.; Hu, G. Abnormal histopathology, fat percent and hepatic apolipoprotein A I and apolipoprotein B100 mRNA expression in fatty liver hemorrhagic syndrome and their improvement by soybean lecithin. Poult. Sci. 2017, 96, 3559–3563. [Google Scholar] [CrossRef]
- Shini, A.; Shini, S.; Bryden, W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: Impact of production system. Avian Pathol. 2019, 48, 25–34. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Shini, S.; Shini, A.; Bryden, W.L. Unravelling fatty liver haemorrhagic syndrome: 2. Inflammation and pathophysiology. Avian Pathol. 2020, 49, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Shini, S.; Shini, A.; Bryden, W.L. Unravelling fatty liver haemorrhagic syndrome: 1. Oestrogen and inflammation. Avian Pathol. 2020, 49, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Lee, S.H.; Kim, D.-H.; Lee, K.-W. Effects of dietary methyl sulfonyl methane and selenium on laying performance, egg quality, gut health indicators, and antioxidant capacity of laying hens. Anim. Biosci. 2022, 35, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement. Nutrients 2017, 9, 290. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Chen, J.; Xiong, D.; Long, M. Detoxification of Selenium Yeast on Mycotoxins and Heavy Metals: A Review. Biol. Trace Element Res. 2023, 1–14. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Gao, X.-J.; Tang, B.; Liang, H.-H.; Yi, L.; Wei, Z.-G. Selenium deficiency inhibits micRNA-146a to promote ROS-induced inflammation via regulation of the MAPK pathway in the head kidney of carp. Fish Shellfish Immunol. 2019, 91, 284–292. [Google Scholar] [CrossRef]
- Wang, H.; Bi, C.; Wang, Y.; Sun, J.; Meng, X.; Li, J. Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-κB and MAPK signaling pathways. BMC Vet. Res. 2018, 14, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, C.; Li, X.; Qin, L.; Luo, J.; Zhang, M.; Ou, Z.; Wang, K. High Selenium Yeast mitigates aluminum-induced cerebral inflammation by increasing oxidative stress and blocking NO production. Biometals 2018, 31, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Liu, Y.-L.; Xie, C.-Y.; Zhang, B.; Huang, Y.-Q.; Zhang, Y.-W.; Yao, Y.; Huang, R.; Wu, X. Effects of Different Selenium Sources on Laying Performance, Egg Selenium Concentration, and Antioxidant Capacity in Laying Hens. Biol. Trace Element Res. 2019, 189, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, Y.; Salih, Y.G. Influence of sumac (Rhus Coriaria L.) and ginger (Zingiber officinale) on egg yolk fatty acid, cholesterol and blood parameters in laying hens. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, K.; Mi, X.; Rajput, S.A.; Qi, D. Effects of 3-Hydroxy-3-methylglutaryl-CoA Reductase Inhibitors on Cholesterol Metabolism in Laying Hens. Animals 2023, 13, 1868. [Google Scholar] [CrossRef]
- Luo, J.-J.; Zhang, Y.; Sun, H.; Wei, J.-T.; Khalil, M.M.; Wang, Y.-W.; Dai, J.-F.; Zhang, N.-Y.; Qi, D.-S.; Sun, L.-H. The response of glandular gastric transcriptome to T-2 toxin in chicks. Food Chem. Toxicol. 2019, 132, 110658. [Google Scholar] [CrossRef]
- Macelline, S.P.; Toghyani, M.; Chrystal, P.V.; Selle, P.H.; Liu, S.Y. Amino acid requirements for laying hens: A comprehensive review. Poult. Sci. 2021, 100, 101036. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Shen, Y.; Liu, H.; Zhao, X.; Li, J.; Ma, N. Protective Effects of Abrus cantoniensis Hance on the Fatty Liver Hemorrhagic Syndrome in Laying Hens Based on Liver Metabolomics and Gut Microbiota. Front. Vet. Sci. 2022, 9, 862006. [Google Scholar] [CrossRef]
- Han, X.; Qin, P.; Li, W.; Ma, Q.; Ji, C.; Zhang, J.; Zhao, L. Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult. Sci. 2017, 96, 3973–3980. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Lee, S.-H.; Kim, D.-H.; Lee, H.-G.; Jeon, Y.-S.; Lee, S.-D.; Lee, K.-W. Incorporation of Dietary Methyl Sulfonyl Methane into the Egg Albumens of Laying Hens. Antioxidants 2022, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Shen, C.; Zhang, L.; Wu, X.; Yu, Y.; Yang, X.; Yang, C.; Zhong, C.; Gao, Z.; Miao, W.; et al. Hepatic Krüppel-like factor 16 (KLF16) targets PPARα to improve steatohepatitis and insulin resistance. Gut 2021, 70, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Modulation of hepatic steatosis by dietary fatty acids. World J. Gastroenterol. 2014, 20, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Majdeddin, M.; Degroote, J.; Van Liefferinge, E.; Van Noten, N.; Van Kerschaver, C.; Vandaele, M.; Dorigam, J.C.D.P.; Michiels, J. Effect of supplemental methyl sulfonyl methane on performance, carcass and meat quality and oxidative status in chronic cyclic heat-stressed finishing broilers. Poult. Sci. 2023, 102, 102321. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.; Jensen, L. Reduction of Hepatic Lipid Deposition in Laying Hens by Dietary Selenium-Yeast Interaction. Poult. Sci. 1979, 58, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.; Mandic, A.; Maricic, S.; Srdjenovic, B. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Lee, K.-W. Role of Dietary Methyl Sulfonyl Methane in Poultry. Animals 2023, 13, 351. [Google Scholar] [CrossRef]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wan, A.; Zhou, Z.; Chen, D.; Liang, H.; Liu, C.; Yan, S.; Niu, Y.; Lin, Z.; Zhan, S.; et al. RNA-binding protein RALY reprogrammes mitochondrial metabolism via mediating miRNA processing in colorectal cancer. Gut 2021, 70, 1698–1712. [Google Scholar] [CrossRef]
- Packialakshmi, B.; Stewart, I.J.; Burmeister, D.M.; Feng, Y.; McDaniel, D.P.; Chung, K.K.; Zhou, X. Tourniquet-induced lower limb ischemia/reperfusion reduces mitochondrial function by decreasing mitochondrial biogenesis in acute kidney injury in mice. Physiol. Rep. 2022, 10, e15181. [Google Scholar] [CrossRef]
- Liang, Y.-D.; Zhao, K.; Chen, Y.; Zhang, S.-Q. [Role of CYCS in the cytogenesis and apoptosis of male germ cells and its clinical application]. Zhonghua Nan Ke Xue 2020, 26, 265–270. [Google Scholar] [PubMed]
- Wei, L.; Zhao, C.; Dong, S.; Yao, S.; Ji, B.; Zhao, B.; Liu, Z.; Liu, X.; Wang, Y. Secoisolariciresinol diglucoside alleviates hepatic lipid metabolic misalignment involving the endoplasmic reticulum–mitochondrial axis. Food Funct. 2020, 11, 3952–3963. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Shi, M.; Xiao, H.; Chi, X. WGCNA-Based Identification of Hub Genes and Key Pathways Involved in Nonalcoholic Fatty Liver Disease. BioMed Res. Int. 2021, 2021, 5633211. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD+ Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, B.; Lv, H.; Liu, J.; Xiong, Y.; Hu, L.; Xue, H.; Panayi, A.C.; Liu, G.; Zhou, W. Shared KEGG pathways of icariin-targeted genes and osteoarthritis. J. Cell. Biochem. 2019, 120, 7741–7750. [Google Scholar] [CrossRef]
- Ojha, C.R.; Rodriguez, M.; Karuppan, M.K.M.; Lapierre, J.; Kashanchi, F.; El-Hage, N. Toll-like receptor 3 regulates Zika virus infection and associated host inflammatory response in primary human astrocytes. PLoS ONE 2019, 14, e0208543. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Byeon, S.E.; Yi, Y.-S.; Oh, J.; Yoo, B.C.; Hong, S.; Cho, J.Y. The Role of Src Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm. 2012, 2012, 512926. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Yuan, L.; Li, X.; Yu, J.; Xu, Z. BMP2 inhibits cell proliferation by downregulating EZH2 in gastric cancer. Cell Cycle 2022, 21, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Fukuda, R.; Miyazono, K.; Heldin, C.-H. Tumor Promoting Effect of BMP Signaling in Endometrial Cancer. Int. J. Mol. Sci. 2021, 22, 7882. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-M.; Wu, S.-Z.; Yang, S.-H.; Wu, C.-C.; Wang, C.-Y.; Huang, B.-M. FGF9/FGFR1 promotes cell proliferation, epithelial-mesenchymal transition, M2 macrophage infiltration and liver metastasis of lung cancer. Transl. Oncol. 2021, 14, 101208. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Y.; Yu, W.; Yan, Y.; Qiao, M.; Jiang, R.; Guan, W.; Wang, L. Downregulation of miRNA-214 in cancer-associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J. Exp. Clin. Cancer Res. 2019, 38, 20. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Jia, R.; Zhao, L.; Song, S.; Gu, J.; Zhang, H. LDB2 inhibits proliferation and migration in liver cancer cells by abrogating HEY1 expression. Oncotarget 2017, 8, 94440–94449. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Ingredients | Percentage (%) | Nutrient Level | Content |
|---|---|---|---|
| Wheat | 68.40 | ME (kcal/kg) | 2750 |
| Soybean meal 1 | 15.00 | Crude protein (%) | 16.50 |
| Soybean oil | 1.30 | Calcium (%) | 3.60 |
| DDGS 2 | 3.00 | Available phosphorus (%) | 0.35 |
| Limestone | 8.70 | Ether extract (%) | 5.41 |
| CaHPO4 | 0.80 | Ash (%) | 12.32 |
| Bran 3 | 1.00 | Met (%) | 0.35 |
| Salt | 0.30 | Lys (%) | 0.80 |
| Premix 4 | 1.50 | Met + Cys (%) | 0.70 |
| Sample 1 | Raw Reads Number | Q30 Value 2 | Clean Reads Number | Total Mapped Reads Percentage |
|---|---|---|---|---|
| Control-1 | 51,129,902 | 95.15 | 50,728,872 | 95.32% |
| Control-2 | 55,289,378 | 94.80 | 54,763,456 | 94.28% |
| Control-3 | 61,373,938 | 94.89 | 60,818,000 | 94.13% |
| 700-MSM-1 | 52,676,984 | 95.12 | 52,241,862 | 95.64% |
| 700-MSM-2 | 55,425,860 | 94.67 | 54,862,322 | 92.94% |
| 700-MSM-3 | 58,473,686 | 94.57 | 57,767,186 | 92.06% |
| 700-MSM-4 | 70,086,200 | 94.19 | 69,126,992 | 91.89% |
| 700-MSM-5 | 55,554,098 | 94.44 | 54,884,866 | 92.97% |
| 700-MSM-6 | 54,657,056 | 94.02 | 53,737,510 | 89.96% |
| 50-Se-Y-1 | 52,359,938 | 94.05 | 51,544,302 | 91.58% |
| 50-Se-Y-2 | 53,882,128 | 94.65 | 53,327,412 | 93.85% |
| 50-Se-Y-3 | 52,965,636 | 94.83 | 52,490,900 | 94.14% |
| 50-Se-Y-4 | 59,031,608 | 94.70 | 58,413,278 | 93.38% |
| 50-Se-Y-5 | 56,635,628 | 94.30 | 55,895,582 | 91.86% |
| 50-Se-Y-6 | 50,667,160 | 94.76 | 50,160,676 | 93.57% |
| Gene Name | Accession | Primer Sequence (5′-3′) | Product Size (bp) |
|---|---|---|---|
| ATP5O | XM_416717 | F: CCTGCTTGCTGAGAATGGTC | 210 |
| R: GAGGGATCGGTCTTGGTCTC | |||
| ATP5L | XM_015298211 | F: TGGTACTACGCTAAGGTCGAG | 324 |
| R: GCCTCGTTTGCCTATGATCTC | |||
| COX8A | XM_001235548 | F: AACCAGTGGCAGAGCGATAT | 295 |
| R: CCGCTTCTTGTAGTCCTCGA | |||
| CYCS | NM_001079478 | F: CCAGAAATGTTCCCAGTGCC | 318 |
| R: AGACTTCTTCTTGATACCCGCA | |||
| MAPK10 | NM_001318224 | F: CTGGTGATGGAGCTGATGGA | 294 |
| R: CTTGTAGCCCATTCCCAGGA | |||
| SRC | NM_205457 | F: CTGCTTTGGAGAGGTCTGGA | 242 |
| R: ACTTGCCCATCTCTCCCTTC | |||
| BMP2 | NM_001398170 | F: CAACAGCAGCTACCATCACC | 206 |
| R: GAACCACCTCCACCACAAAC | |||
| FGF9 | NM_001397365 | F: AGACAGCGGACTCTACCTTG | 253 |
| R: AGGGTCCACTGGTCTAGGTA | |||
| β-actin | NM_205518.2 | F: AGTACCCCATTGAACACGGT | 197 |
| R: ATACATGGCTGGGGTGTTGA |
| Treatment | Control | 25 mg/kg Se-Y | 50 mg/kg Se-Y | 350 mg/kg MSM | 700 mg/kg MSM |
|---|---|---|---|---|---|
| Feed intake (g/bird) | 113.27 ± 2.70 | 112.45 ± 3.22 | 113.12 ± 1.86 | 112.61 ± 2.53 | 112.84 ± 3.15 |
| Egg production (%) | 85.80 ± 5.37 | 84.93 ± 4.62 | 85.27 ± 3.65 | 85.33± 3.79 | 84.65 ± 5.83 |
| Feed conversion ratio | 1.92 ± 0.05 | 1.88 ± 0.04 | 1.91 ± 0.04 | 1.91 ± 0.05 | 1.90 ± 0.04 |
| Egg weight (g) | 59.46 ± 3.61 | 60.27 ± 5.14 | 59.61 ± 4.35 | 59.32 ± 4.15 | 59.55 ± 5.37 |
| Eggshell strength (N) | 36.00 ± 6.50 | 37.11± 5.86 | 38.15 ± 6.43 | 37.62 ± 6.13 | 37.84 ± 6.25 |
| Haugh unit | 81.91 ± 7.13 | 83.09 ± 7.32 | 82.47 ± 6.55 | 81.94 ± 7.36 | 83.27 ± 6.33 |
| Albumen height | 6.86 ± 1.01 | 7.44 ± 1.25 | 7.26 ± 1.03 | 7.14 ± 1.12 | 6.91 ± 1.11 |
| Yolk colour | 13.00 ± 1.35 | 12.57 ± 1.16 | 13.58 ± 1.42 | 12.89 ± 1.35 | 13.24 ± 1.22 |
| Gene ID | Gene Symbol | Log2 FC | p-Value | Gene Description |
|---|---|---|---|---|
| 700 mg/kg MSM VS. Control | ||||
| Mitochondrial energy metabolism | ||||
| 769146 | ATP5I | −0.73 | 0.030 | ATP synthase, H+ transporting, mitochondrial Fo complex subunit E |
| 419992 | ATP5G1 | −0.69 | 0.007 | ATP synthase, H+ transporting, mitochondrial Fo complex subunit C1 (subunit 9) |
| 418508 | ATP5O | −0.59 | 0.025 | ATP synthase, H+ transporting, mitochondrial F1 complex, O subunit |
| 418477 | ATP5J | −0.60 | 0.015 | ATP synthase, H+ transporting, mitochondrial Fo complex subunit F6 |
| 101749042 | ATP5L | −0.72 | 0.009 | ATP synthase, H+ transporting, mitochondrial Fo complex subunit G |
| 770190 | COX17 | −0.90 | 1.36 × 10−5 | COX17, cytochrome c oxidase copper chaperone |
| 420243 | COX6C | −0.79 | 0.022 | cytochrome c oxidase subunit 6C |
| 772260 | COX7A2 | −0.71 | 0.013 | cytochrome c oxidase subunit 7A2 |
| 431629 | COX7C | −0.97 | 0.001 | cytochrome c oxidase subunit 7C |
| 775974 | COX8A | −0.82 | 0.035 | cytochrome c oxidase subunit 8A |
| 771510 | NDUFS1 | −0.61 | 0.032 | NADH:ubiquinone oxidoreductase subunit S1 |
| 63549497 | ND6 | −0.87 | 0.014 | NADH dehydrogenase subunit 6 |
| Nonalcoholic fatty liver disease | ||||
| 420624 | CYCS | −0.68 | 0.036 | cytochrome c, somatic |
| 768860 | NDUFA9 | −0.82 | 0.015 | NADH:ubiquinone oxidoreductase subunit A2 |
| 424078 | NDUFB3 | −0.64 | 0.026 | NADH:ubiquinone oxidoreductase subunit B3 |
| 404751 | NDUFC2 | −0.65 | 0.017 | NADH dehydrogenase (ubiquinone) 1, subcomplex unknown, 2 |
| 416336 | UQCRQ | −0.68 | 0.025 | ubiquinol-cytochrome c reductase complex III subunit VII |
| 50 mg/kg Se-Y VS. Control | ||||
| Hepatic inflammatory pathway | ||||
| 422592 | MAPK10 | −1.38 | 0.042 | mitogen-activated protein kinase 10 |
| 417739 | MAPK11 | −0.91 | 0.033 | mitogen-activated protein kinase 11 |
| 396442 | SRC | −0.62 | 0.044 | SRC proto-oncogene, non-receptor tyrosine kinase |
| 418212 | ITPR2 | −2.54 | 0.032 | inositol 1,4,5-trisphosphate receptor type 2 |
| 419910 | ITPR3 | −2.38 | 0.008 | inositol 1,4,5-trisphosphate receptor type 3 |
| Hepatic cancer pathway | ||||
| 378779 | BMP2 | −0.91 | 0.014 | bone morphogenetic protein 2 |
| 419311 | CTSZ | −0.78 | 0.040 | cathepsin Z |
| 378917 | FGF9 | −0.74 | 0.045 | fibroblast growth factor 9 |
| 428365 | HEY1 | −1.09 | 0.025 | hes related family bHLH transcription factor with YRPW motif 1 |
| 427339 | LPAR1 | −1.21 | 0.025 | lysophosphatidic acid receptor 1 |
| 395561 | WNT4 | −1.07 | 0.045 | wnt family member 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, L.; Tian, C.; Rajput, S.A.; Qi, D. Effects of Methyl Sulfonyl Methane and Selenium Yeast on Fatty Liver Syndrome in Laying Hens and Their Biological Mechanisms. Animals 2023, 13, 2466. https://doi.org/10.3390/ani13152466
Wang H, Wang L, Tian C, Rajput SA, Qi D. Effects of Methyl Sulfonyl Methane and Selenium Yeast on Fatty Liver Syndrome in Laying Hens and Their Biological Mechanisms. Animals. 2023; 13(15):2466. https://doi.org/10.3390/ani13152466
Chicago/Turabian StyleWang, Huanbin, Lingfeng Wang, Changyu Tian, Shahid Ali Rajput, and Desheng Qi. 2023. "Effects of Methyl Sulfonyl Methane and Selenium Yeast on Fatty Liver Syndrome in Laying Hens and Their Biological Mechanisms" Animals 13, no. 15: 2466. https://doi.org/10.3390/ani13152466
APA StyleWang, H., Wang, L., Tian, C., Rajput, S. A., & Qi, D. (2023). Effects of Methyl Sulfonyl Methane and Selenium Yeast on Fatty Liver Syndrome in Laying Hens and Their Biological Mechanisms. Animals, 13(15), 2466. https://doi.org/10.3390/ani13152466






