Assessment of Tilapia (Oreochromis spp.) Welfare in the Semi-Intensive and Intensive Culture Systems in Thailand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tilapia Farms, Data Collection, and Ethics
2.2. Welfare Criteria
2.3. Statistical Analysis
3. Results
3.1. Demographic Information of the Studied Tilapia Farms
3.2. Welfare Indicators
3.2.1. Health Indicators
3.2.2. Environmental Indicators
3.2.3. Behavioural Indicators
3.2.4. Nutritional Indicators
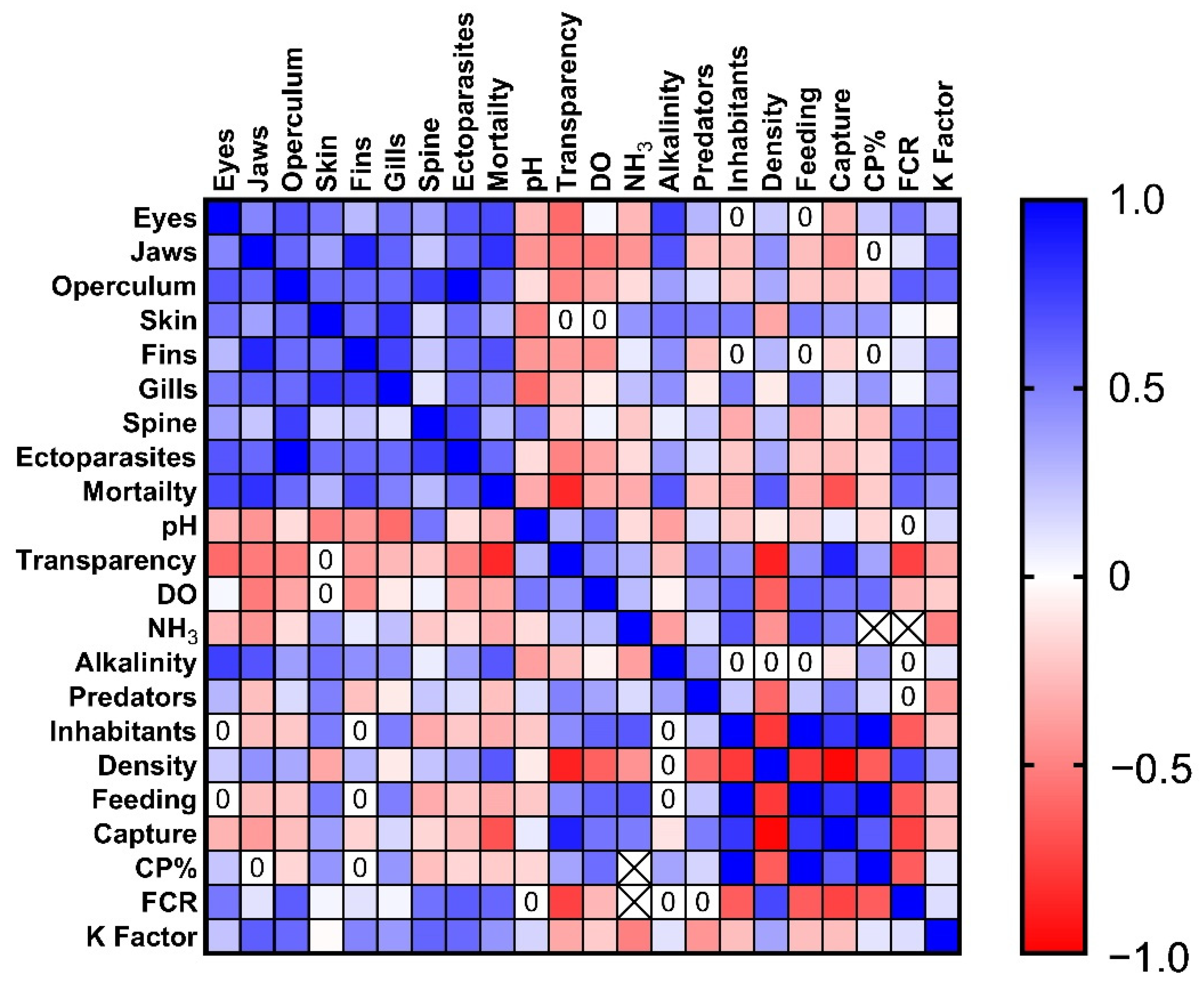
3.3. Correlation between Nutritional, Environmental, and Health Indicators
4. Discussion
4.1. Health Indicators
4.2. Environmental Indicators
4.3. Behavioural Indicators
4.4. Nutrition Criteria
4.5. Welfare Assessments in Thailand: Past, Present, and Future
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agricultural Organization. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; FAO: Rome, Italy, 2022; 266p. [Google Scholar] [CrossRef]
- Noorit, K. Situation of Tilapia and Their Products in 2021 and Trends in 2022 of Thailand. Available online: https://www.fisheries.go.th/strategy/fisheconomic/Monthly%20report/tilapia/3.situation%20of%20tilapia%20Q4%2064.pdf (accessed on 20 July 2023).
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 five domains model: Including human–animal interactions in assessments of animal welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Guidance on the Application on the Application of Thai Agricultural Standard: Good Aquaculture Practices for Food-Aquatic Animals Farm. Available online: https://www.fisheries.go.th/local/file_document/20220531115826_1_file.pdf (accessed on 20 July 2023).
- Aquaculture Stewardship Council (ASC) Variance Request Procedure Version 2.0. Available online: https://www.asc-aqua.org/wp-content/uploads/2020/12/ASC-Standards-related-variance-request-procedure-v1.pdf (accessed on 16 June 2023).
- Aquaculture Facility Certification: Best Aquaculture Practices Certification Standards, Implementation Guidelines. Available online: https://www.bapcertification.org/Downloadables/pdf/GSA%20-%20Farm%20Standard%20-%20Issue%203.1%20-%2007-February-2023.pdf (accessed on 16 June 2023).
- Håstein, T.; Scarfe, A.D.; Lund, V.L. Science-based assessment of welfare: Aquatic animals. Rev. Sci. Tech. 2005, 24, 529–547. [Google Scholar]
- Stien, L.H.; Bracke, M.B.M.; Folkedal, O.; Nilsson, J.; Oppedal, F.; Torgersen, T.; Kittilsen, S.; Midtlyng, P.J.; Vindas, M.A.; Øverli, Ø.; et al. Salmon Welfare Index Model (SWIM 1.0): A semantic model for overall welfare assessment of caged Atlantic salmon: Review of the selected welfare indicators and model presentation. Rev. Aquac. 2013, 5, 33–57. [Google Scholar] [CrossRef]
- Noble, C.; Gismervik, K.; Iversen, M.H.; Kolarevic, J.; Nilsson, J.; Stien, L.H. Welfare Indicators for Farmed Atlantic Salmon: Tools for Assessing Fish Welfare; Turnbull, J.F., Ed.; Nofima: Tromsø, Norway, 2018; p. 351. [Google Scholar]
- Tschirren, L.; Bachmann, D.; Güler, A.C.; Blaser, O.; Rhyner, N.; Seitz, A.; Zbinden, E.; Wahli, T.; Segner, H.; Refardt, D. MyFishCheck: A model to assess fish welfare in aquaculture. Animals 2021, 11, 145. [Google Scholar] [CrossRef]
- Flores-García, L.; Camargo-Castellanos, J.C.; Pascual-Jímenez, C.; Almazán-Rueda, P.; Monroy-López, J.F.; Albertos-Alpuche, P.J.; Martínez-Yáñez, R. Welfare indicators in tilapia: An epidemiological approach. Front. Vet. Sci. 2022, 9, 882567. [Google Scholar] [CrossRef]
- Pedrazzani, A.S.; Quintiliano, M.H.; Bolfe, F.; Sans, E.C.d.O.; Molento, C.F.M. Tilapia on-farm welfare assessment protocol for semi-intensive production systems. Front. Vet. Sci. 2020, 7, 606388. [Google Scholar] [CrossRef]
- McCulloch, S.P. A Critique of FAWC’s Five freedoms as a framework for the analysis of animal welfare. J. Agric. Environ. Ethics 2013, 26, 959–975. [Google Scholar]
- Surachetpong, W.; Roy, S.R.K.; Nicholson, P. Tilapia lake virus: The story so far. J. Fish Dis. 2020, 43, 1115–1132. [Google Scholar] [CrossRef]
- Turner, J.K.; Sakulpolwat, S.; Sukdanon, S.; Lertwanakarn, T.; Waiyamitra, P.; Piewbang, C.; Pierezan, F.; Techangamsuwan, S.; Soto, E.; Surachetpong, W. Tilapia lake virus (TiLV) causes severe anaemia and systemic disease in tilapia. J. Fish Dis. 2023, 46, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Chanagun, C.; Lebel, P.; Whangchai, N.; Promya, J.; Lebel, L. Tilapia diseases and management in river-based cage aquaculture in northern Thailand. J. Appl. Aquac. 2016, 28, 9–16. [Google Scholar]
- Suwanpakdee, S.; Sriyasak, P.; Pimolrat, P. Risk of climate variability on tilapia cage culture in Songkhram river in Northeastern Thailand. AJSTR 2021, 24, 76–83. [Google Scholar] [CrossRef]
- Yamkasem, J.; Tattiyapong, P.; Gorgoglione, B.; Surachetpong, W. Uncovering the first occurrence of Tilapia parvovirus in Thailand in tilapia during co-infection with Tilapia tilapinevirus. Transbound Emerg. Dis. 2021, 68, 3136–3144. [Google Scholar] [CrossRef]
- Ivan, A.; Zaabwe, T.; Andrew, A.; Kasigwa, H.; Mushabe, N.; Byenkya, G.; Nkambo, M.; Baguma, S.; Hafashimana, D.; Efitre, J. Effect of stocking density on growth and survival of Nile tilapia (Oreochromis niloticus, Linnaeus 1758) under cage culture in Lake Albert, Uganda. Int. J. Fish Aquac. 2020, 12, 26–35. [Google Scholar] [CrossRef]
- Ndashe, K.; Hang’ombe, B.M.; Changula, K.; Yabe, J.; Samutela, M.T.; Songe, M.M.; Kefi, A.S.; Njobvu Chilufya, L.; Sukkel, M. An Assessment of the risk factors associated with disease outbreaks across tilapia farms in Central and Southern Zambia. Fishes 2023, 8, 49. [Google Scholar] [CrossRef]
- Odhiambo, E.; Angienda, P.; Kirsteen, D.O.; Onyango, D. Stocking density induced stress on plasma cortisol and whole blood glucose concentration in Nile tilapia fish (Oreochromis niloticus) of Lake Victoria, Kenya. Int. J. Zool. 2020, 2020, 9395268. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-de-Freitas, E.; Bolognesi, M.C.; Gauy, A.C.d.S.; Brandão, M.L.; Giaquinto, P.C.; Fernandes-Castilho, M. Social behavior and welfare in Nile tilapia. Fishes 2019, 4, 23. [Google Scholar] [CrossRef]
- Salger, S.A.; Reza, J.; Deck, C.A.; Wahab, M.A.; Baltzegar, D.A.; Murr, A.T.; Borski, R.J. Enhanced biodiversity of gut flora and feed efficiency in pond cultured tilapia under reduced frequency feeding strategies. PLoS ONE 2020, 15, e0236100. [Google Scholar] [CrossRef]
- Lopes, T.O.M.; Pinto, E.; Passos, L.S.; Dorr, F.; Vasconcelos, C.M.; Arpini, C.; Silva, M.O.; Pereira, T.M.; Coppo, G.C.; Merçon, J.; et al. Off-flavor detection in tilapia reared in cages in tropical lakes. Aquaculture 2022, 555, 738215. [Google Scholar] [CrossRef]
- De, D.; Cavalcante, H.; Silva, S.; Pinheiro, P.; Masaki, M.; Akao, F.; Vinícius, M.; Carmo-e-Sá, M. Single or paired increase of total alkalinity and hardness of water for cultivation of Nile tilapia juveniles, Oreochromis niloticus. Acta Sci. Technol. 2012, 34, 177–183. [Google Scholar]
- Song, L.; Zhao, Y.; Song, Y.; Zhao, L.; Ma, C.; Zhao, J. Effects of saline-alkaline water on growth performance, nutritional processing, and immunity in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 544, 737036. [Google Scholar] [CrossRef]
- Toni, M.; Manciocco, A.; Angiulli, E.; Alleva, E.; Cioni, C.; Malavasi, S. Review: Assessing fish welfare in research and aquaculture, with a focus on European directives. Animal 2019, 13, 161–170. [Google Scholar] [CrossRef]
- Makori, A.J.; Abuom, P.O.; Kapiyo, R.; Anyona, D.N.; Dida, G.O. Effects of water physico-chemical parameters on tilapia (Oreochromis niloticus) growth in earthen ponds in Teso North Sub-County, Busia County. Fish. Aquatic. Sci. 2017, 20, 30. [Google Scholar] [CrossRef]
- Tsadik, G.G.; Kutty, M.N. Influence of ambient oxygen on feeding and growth of the Tilapia, Oreochromis niloticus (Linnaeus). ARAC 1987. Available online: https://www.fao.org/3/AC168E/AC168E00.htm (accessed on 10 July 2023).
- Miron, D.d.S.; Moraes, B.; Becker, A.G.; Crestani, M.; Spanevello, R.; Loro, V.L.; Baldisserotto, B. Ammonia and pH effects on some metabolic parameters and gill histology of silver catfish, Rhamdia quelen (Heptapteridae). Aquaculture 2008, 277, 192–196. [Google Scholar] [CrossRef]
- Al-Harbi, A.; Siddiqui, A.Q. Effects of tilapia stocking densities on fish growth and water quality in tanks. Asian. Fish Sci. 2000, 13, 391–396. [Google Scholar] [CrossRef]
- Ntanzi, R. The effects of stocking density on the growth and survival of Nile tilapia (Oreochromis niloticus) fry at Son Fish Farm, Uganda. J. Aquac. Res. Dev. 2014, 5, 2. [Google Scholar] [CrossRef]
- Fitzsimmons, K.M.; Shahkar, E. Tilapia–Shrimp Polyculture. In Tilapia in Intensive Co-Culture; Perschbacher, P.W., Stickney, R.R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 94–113. [Google Scholar]
- Ferreira, J.G.; Falconer, L.; Kittiwanich, J.; Ross, L.; Saurel, C.; Wellman, K.; Zhu, C.B.; Suvanachai, P. Analysis of production and environmental effects of Nile tilapia and white shrimp culture in Thailand. Aquaculture 2015, 447, 23–36. [Google Scholar] [CrossRef]
- Rosales, J.; Ponce-Palafox, J.T.; Alma Delia, R.-G.; Otazo-Sánchez, E.; Pulido-Flores, G.; Vargasmachuca, S.; Lara, A. Effects of white shrimp (Litopenaeus vannamei) and tilapia nilotica (Oreochromis niloticus var. spring) in monoculture and co-culture systems on water quality variables and production in brackish low-salinity water earthen ponds during rainy and dry seasons. Span. J. Agric. Res. 2019, 17, e0605. [Google Scholar] [CrossRef]
- Thiện, P.C.; Yi, Y.; Fitzsimmons, K. Effects of adding shrimp (Penaeus monodon) into intensive culture ponds of Nile Tilapia (Oreochromis niloticus) at different densities. In Proceedings of the 6th International Symposium on Tilapia Aquaculture, Manila, Philippines, 12–16 September 2004. [Google Scholar]
- Ngugi, C.; Bowman, J.; Omolo, B. A New Guide to Fish Farming in Kenya; Oregon State University: Corvallis, OR, USA, 2007; 95p. [Google Scholar]
- De Graaf, G.; Janssen, H. Polyculture of African catfish with Nile tilapia. In Artificial Reproduction and Pond Rearing of the African Catfish Clarias Gariepinus in Sub-Saharan Africa: A Handbook; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1996; Volume 362, p. 73. [Google Scholar]
- Barthel, B.L.; Cooke, S.J.; Suski, C.D.; Philipp, D.P. Effects of landing net mesh type on injury and mortality in a freshwater recreational fishery. Fish Res. 2003, 63, 275–282. [Google Scholar] [CrossRef]
- Mengistu, S.B.; Mulder, H.A.; Benzie, J.A.H.; Komen, H. A systematic literature review of the major factors causing yield gap by affecting growth, feed conversion ratio and survival in Nile tilapia (Oreochromis niloticus). Rev. Aquac. 2020, 12, 524–541. [Google Scholar] [CrossRef]
- Rahmatullah, R.; Das, M.; Rahmatullah, S.M. Suitable stocking density of tilapia in an aquaponic system. Bangladesh J. Fish. Res. 2010, 14, 29–35. [Google Scholar]
- Allaman, I.; Neto, R.; Freitas, R.; Freato, T.; Lago, A.; Costa, A.; de Lima, R. Weight and morphometric growth of different strains of tilapia (Oreochromis sp.). Rev. Bras. Zootec 2013, 42, 305–311. [Google Scholar] [CrossRef]
- Santos, V.B.d.; Freitas, R.T.F.d.; Logato, P.V.R.; Freato, T.A.; Orfão, L.H.; Millioti, L.C. Rendimento do processamento de linhagens de tilápias (Oreochromis niloticus) em função do peso corporal. Cienc. Agrotec. 2007, 31, 554–562. [Google Scholar] [CrossRef]
- Browning, H. Improving welfare assessment in aquaculture. Front Vet. Sci. 2023, 10, 1060720. [Google Scholar] [CrossRef] [PubMed]

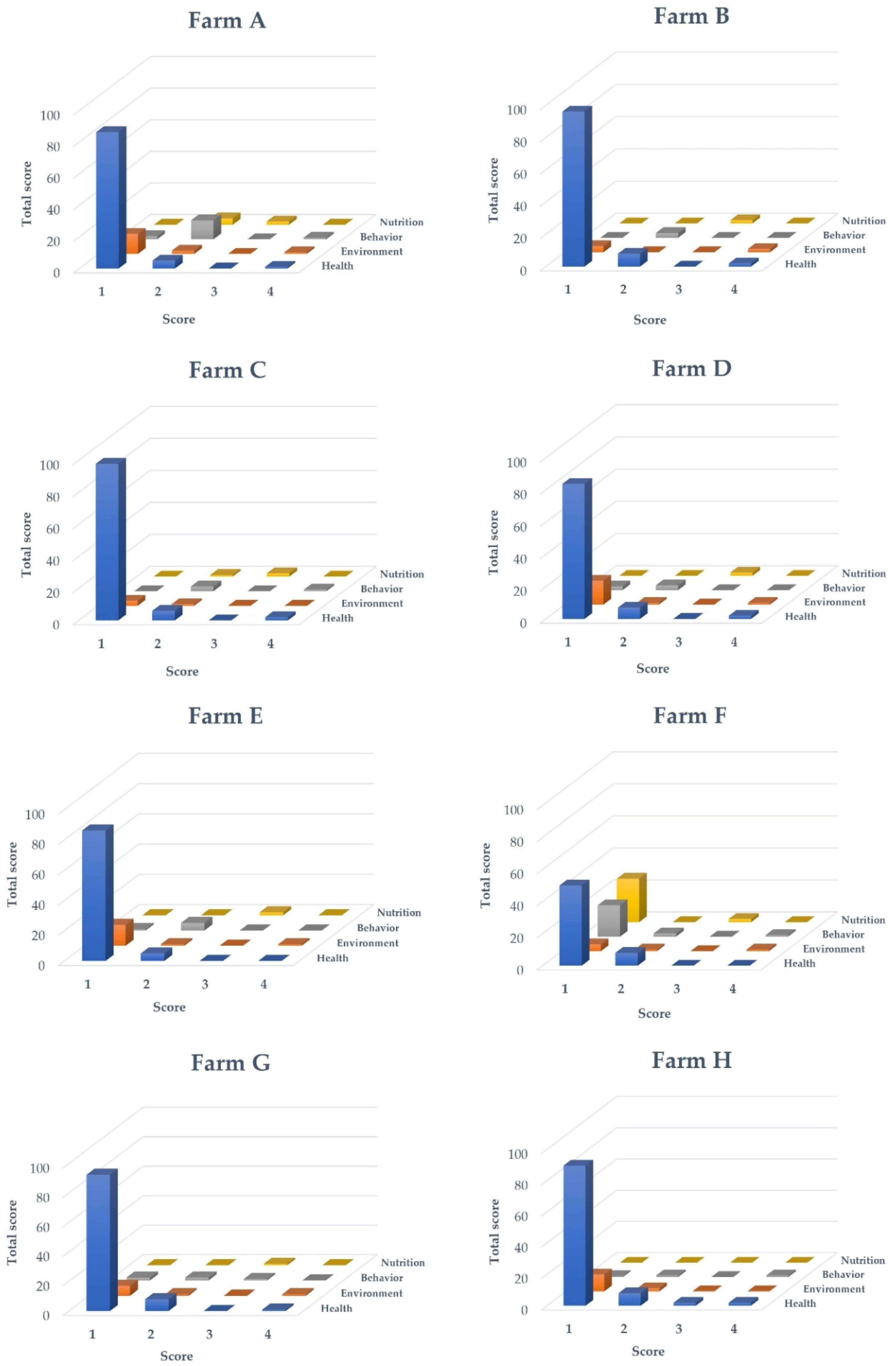
| Farm | Region | Culturing System | Species | Pond Size | Fish Weight (g) (Mean ± SD) | Fish Age (Days) |
|---|---|---|---|---|---|---|
| A | Eastern | Earthen pond | Nile tilapia, white shrimp | 28,800 m2 | 710.6 ± 167.2 | 213 |
| B | Northern | Earthen pond | Nile tilapia | 3200 m2 | 306.2 ± 39.0 | 115 |
| C | Northern | Earthen pond | Nile tilapia | 4800 m2 | 151.5 ± 28.5 | 90 |
| D | Northern | Earthen pond | Nile tilapia | 4800 m2 | 508.6 ± 116.2 | 180 |
| E | Central | Earthen pond | Nile tilapia, white shrimp, catfish | 28,800 m2 | 246.9 ± 27.9 | 186 |
| F | Western | Cage culture | Red tilapia | 25 m2 | 292.0 ± 72.6 | 75 |
| G | Western | Cage culture | Red tilapia | 25 m2 | 106.6 ± 29.6 | 75 |
| H | Eastern | Cage culture | Red tilapia | 20 m2 | 542.8 ± 142.3 | 243 |
| Raising System | Earthen Ponds | Cages | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health Indicators | Farm A (n = 43) | Farm B (n = 26) | Farm C (n = 45) | Farm D (n = 31) | Farm E (n = 30) | Farm F (n = 30) | Farm G (n = 30) | Farm H (n = 25) | ||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Eyes | 97.7 | 2.3 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 50 | 10 | 0 | 40 | 93.3 | 6.7 | 0 | 0 | 100 | 0 | 0 | 0 |
| Jaws | 95.3 | 4.7 | 0 | – | 100 | 0 | 0 | – | 100 | 0 | 0 | – | 90.3 | 9.7 | 0 | – | 100 | 0 | 0 | – | 50 | 0 | 50 | – | 96.7 | 3.3 | 0 | – | 92 | 4 | 0 | – |
| Opercula | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 50 | 10 | 0 | 40 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Skin | 62.8 | 27.9 | 9.3 | 0 | 84.6 | 15.4 | 0 | 0 | 95.6 | 4.4 | 0 | 0 | 61.3 | 32.3 | 6.5 | 0 | 53.3 | 40 | 6.7 | 0 | 50 | 0 | 0 | 50 | 83.3 | 10 | 6.7 | 0 | 96 | 4 | 0 | 0 |
| Fins | 79.1 | 20.9 | 0 | 0 | 92.3 | 7.7 | 0 | 0 | 86.7 | 13.3 | 0 | 0 | 61.3 | 29 | 9.7 | 0 | 73.3 | 26.7 | 0 | 0 | 50 | 10 | 0 | 40 | 80 | 20 | 0 | 0 | 64 | 32 | 4 | 0 |
| Gills | 69.8 | 25.6 | 4.7 | 0 | 96.2 | 3.8 | 0 | 0 | 97.8 | 2.2 | 0 | 0 | 74.2 | 25.8 | 0 | 0 | 70 | 30 | 0 | 0 | 50 | 0 | 30 | 20 | 93.3 | 6.7 | 0 | 0 | 72 | 28 | 0 | 0 |
| Spine | 100 | 0 | 0 | – | 100 | 0 | 0 | – | 97.8 | 2.2 | 0 | – | 100 | 0 | 0 | – | 100 | 0 | 0 | – | 50 | 0 | 50 | – | 100 | 0 | 0 | – | 100 | 0 | 0 | – |
| Ectoparasites | 100 | 0 | 0 | – | 100 | 0 | 0 | – | 100 | 0 | 0 | – | 100 | 0 | 0 | – | 100 | 0 | 0 | – | 40 | 60 | 0 | – | 100 | 0 | 0 | – | 100 | 0 | 0 | – |
| Mortality (%) | 15 | 5 | 10 | 25 | 25 | 50 | 40 | 30 | ||||||||||||||||||||||||
| Raising System | Relative Score | |||||||
|---|---|---|---|---|---|---|---|---|
| Health Indicators | Earthen Ponds | Cages | ||||||
| Farm A (n = 43) | Farm B (n = 26) | Farm C (n = 45) | Farm D (n = 31) | Farm E (n = 30) | Farm F * (n = 30) | Farm G (n = 30) | Farm H (n = 25) | |
| Eyes a | 1.02 | 1.00 | 1.00 | 1.00 | 1.00 | 2.30 | 1.07 | 1.00 |
| Jaws a | 1.05 | 1.00 | 1.00 | 1.10 | 1.00 | 2.00 | 1.03 | 1.08 |
| Opercula a | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 2.40 | 1.00 | 1.00 |
| Skin b | 1.47 | 1.15 | 1.04 | 1.45 | 1.53 | 2.50 | 1.23 | 1.04 |
| Fins b | 1.21 | 1.08 | 1.13 | 1.48 | 1.27 | 2.30 | 1.20 | 1.40 |
| Gills a | 1.35 | 1.04 | 1.02 | 1.26 | 1.30 | 2.20 | 1.07 | 1.28 |
| Spine a | 1.00 | 1.00 | 1.02 | 1.00 | 1.00 | 2.00 | 1.00 | 1.00 |
| Ectoparasites a | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.60 | 1.00 | 1.00 |
| Mortality c | 2 | 1 | 1 | 2 | 2 | 3 | 3 | 2 |
| Raising System | Earthen Ponds | Cages | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Environmental Indicator | Farm A | Farm B | Farm C | Farm D | Farm E | Farm F | Farm G | Farm H | ||||||||
| Value | Score | Value | Score | Value | Score | Value | Score | Value | Score | Value | Score | Value | Score | Value | Score | |
| Temperature (°C) | 28.4 | 1 | 28.4 | 1 | 30.0 | 1 | 30.4 | 1 | 30.0 | 1 | 27.0 | 1 | 27.8 | 1 | 28.2 | 1 |
| pH | 8.5 | 1 | 8.0 | 1 | 8.7 | 2 | 7.7 | 1 | 6.8 | 1 | 7.5 | 1 | 7.5 | 1 | 7.2 | 1 |
| Transparency (cm) | 20 | 3 | 21 | 3 | 18 | 3 | 24 | 3 | 15 | 3 | 35 | 1 | 30 | 1 | 25 | 1 |
| Dissolved oxygen (mg/L) | 8.25 | 4 | 5.47 | 1 | 8.9 | 4 | 6.39 | 1 | 3.4 | 3 | 6.16 | 1 | 5.2 | 2 | 5.38 | 1 |
| NH3 (mg/L) | 0.00 | 1 | 0.00 | 1 | 0.00 | 1 | 0.01 | 1 | 0.08 | 2 | 0.00 | 1 | 0.00 | 1 | 0.00 | 1 |
| NO2− (mg/L) | 0.00 | 1 | 0.00 | 1 | 0.00 | 1 | 0.10 | 1 | 0.00 | 1 | 0.00 | 1 | 0.00 | 1 | 0.00 | 1 |
| Alkalinity (mg/L of CaCO3) | 102 | 2 | 85 | 1 | 34 | 1 | 102 | 2 | 85 | 1 | 119 | 2 | 119 | 2 | 51 | 1 |
| Shading (%) | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 3 | 60 | 3 |
| Predators | UP | 3 | UP | 3 | UP | 3 | UP | 3 | UP | 3 | UP | 3 | UP | 3 | CP | 2 |
| Inhabitants | UP | 3 | A | 1 | A | 1 | A | 1 | UP | 3 | A | 1 | A | 1 | A | 1 |
| Density (fish/m2) | 1.25 | 1 | 3.13 | 1 | 3.13 | 1 | 3.13 | 1 | 2.08 | 1 | 60.0 | 1 | 60.0 | 1 | 90.0 | 1 |
| Raising System | Earthen Ponds | Cage | ||||||
|---|---|---|---|---|---|---|---|---|
| Behavioural Indicators | Farm A | Farm B | Farm C | Farm D | Farm E | Farm F | Farm G | Farm H |
| Score | Score | Score | Score | Score | Score | Score | Score | |
| Feeding | 4 | 1 | 1 | 1 | 4 | 1 | 1 | 1 |
| Capture | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 1 |
| Raising System | Earthen Ponds | Cages | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutritional Indicators | Farm A | Farm B | Farm C | Farm D | Farm E | Farm F | Farm G | Farm H | ||||||||
| Value | Score | Value | Score | Value | Score | Value | Score | Value | Score | Value | Score | Value | Score | Value | Score | |
| Fish weight (g) (mean ± SD) | 710.6 ± 167.2 | – | 306.2 ± 39.0 | – | 151.5 ± 28.5 | – | 508.6 ± 116.2 | – | 246.9 ± 27.9 | – | 292.0 ± 72.6 | – | 106.6 ± 29.6 | – | 542.8 ± 142.3 | – |
| Fish age (days) | 213 | – | 115 | – | 90 | – | 180 | – | 186 | – | 75 | – | 75 | – | 243 | – |
| Use commercial feed | Yes | – | Yes | – | Yes | – | Yes | – | No | – | Yes | – | Yes | – | Yes | – |
| Crude protein ratio (CP%) | 25 | 2 | 30 | 1 | 30 | 1 | 30 | 1 | ND | – | 30 | 1 | 32 | 1 | 30 | 1 |
| Feed conversion ratio (FCR) | 1.1 | 1 | 1.3 | 1 | 1.3 | 1 | 1.2 | 1 | ND | – | 1.55 | 2 | 1.41 | 2 | 1.3 | 1 |
| K factor (mean ± SD) | 2.53 ± 0.27 | 3 | 2.22 ± 0.20 | 2 | 2.53 ± 0.21 | 3 | 2.26 ± 0.21 | 2 | 2.20 ± 0.17 | 2 | 3.7 ± 1.44 | 3 | 2.2 ± 0.34 | 2 | 2.75 ± 0.62 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lertwanakarn, T.; Purimayata, T.; Luengyosluechakul, T.; Grimalt, P.B.; Pedrazzani, A.S.; Quintiliano, M.H.; Surachetpong, W. Assessment of Tilapia (Oreochromis spp.) Welfare in the Semi-Intensive and Intensive Culture Systems in Thailand. Animals 2023, 13, 2498. https://doi.org/10.3390/ani13152498
Lertwanakarn T, Purimayata T, Luengyosluechakul T, Grimalt PB, Pedrazzani AS, Quintiliano MH, Surachetpong W. Assessment of Tilapia (Oreochromis spp.) Welfare in the Semi-Intensive and Intensive Culture Systems in Thailand. Animals. 2023; 13(15):2498. https://doi.org/10.3390/ani13152498
Chicago/Turabian StyleLertwanakarn, Tuchakorn, Thitima Purimayata, Thnapol Luengyosluechakul, Pau Badia Grimalt, Ana Silvia Pedrazzani, Murilo Henrique Quintiliano, and Win Surachetpong. 2023. "Assessment of Tilapia (Oreochromis spp.) Welfare in the Semi-Intensive and Intensive Culture Systems in Thailand" Animals 13, no. 15: 2498. https://doi.org/10.3390/ani13152498
APA StyleLertwanakarn, T., Purimayata, T., Luengyosluechakul, T., Grimalt, P. B., Pedrazzani, A. S., Quintiliano, M. H., & Surachetpong, W. (2023). Assessment of Tilapia (Oreochromis spp.) Welfare in the Semi-Intensive and Intensive Culture Systems in Thailand. Animals, 13(15), 2498. https://doi.org/10.3390/ani13152498







