1. Introduction
Sheep farming plays a significant role in the agriculture and economy of tropical regions. Sheep serve as an important source of animal protein for the local population, and their meat is valued for its taste and nutritional quality, making it a healthy food option. However, raising lambs, particularly in semi-arid areas, can be challenging due to the limited availability of natural or cultivated pastures. Thus, a major challenge in ruminant production is ensuring a consistent feed supply for animals that can be efficiently utilized. In this context, the production of hay during the rainy season for year-round use in total rations has become an increasingly necessary practice.
Mimosa tenuiflora is a deciduous shrub legume that is notable for its role in the diet of goats, sheep, and cattle. During the rainy season, these animals graze on its green leaves and branches, while during the dry season, they consume its pods, flowers, leaves, and dry branches. Due to these characteristics and its prevalence in the Brazilian semi-arid region, farmers have been using
Mimosa tenuiflora hay as an alternative feed for sheep and goats. In a study conducted by Bandeira et al. [
1], the average values of crude protein (CP), neutral detergent fibers (NDF), and acid detergent fibers (ADF) in
Mimosa tenuiflora hay were found to be 14.4%, 44.5%, and 29.5%, respectively, whereas the digestibility of dry matter (DM) varies between 40% and 60% depending on the physiological phase and plant part, as observed by Pereira Filho et al. [
2] and Cordão et al. [
3].
In contrast, the
Mimosa tenuiflora hay presents varying concentrations of tannins, ranging from 0.67 g/kg [
4] to 1.8 g/kg DM [
5]. These tannins can affect the intake and digestibility of dry matter [
6], crude protein [
7], and carbohydrates [
8]. Due to their astringent properties, the presence of tannins can negatively affect water intake. They interact with salivary glycoproteins [
9], causing them to precipitate, resulting in a loss of lubricating capacity [
10].
Tannins are phenolic compounds that can be classified into hydrolyzable and condensed forms. They have a high molecular weight [
11] and a strong capacity to form complexes with other substances [
12], particularly proteins, through hydrogen bonding [
13] and hydrophobic interactions. When tannins are added to the diet at levels exceeding 50 g condensed tannin/kg DM, they can reduce the efficiency of nitrogen utilization in the rumen [
5,
14,
15], thereby reducing microbial protein production. However, when added in moderate levels, tannins can facilitate the passage of amino acids to the small intestine, thereby providing compensation [
16]. The effects of tannins on sheep can vary depending on their concentration in the diet. Studies have reported positive, negative, or no significant changes in response [
17]. However, at levels between 10 and 50 g tannin/kg DM, the literature highlights their beneficial role as antioxidants [
18,
19].
The intake of tannins by lambs at levels up to 50 g/kg DM has shown promising results. By protecting a portion of the protein from rumen bacteria, tannins can enhance intestinal digestibility [
20]. They have an influence on the intake, digestion, absorption, and excretion of nitrogen [
21], resulting in increased nitrogen retention in the ruminant’s body, [
22,
23] which subsequently contributes to improved meat quality [
24]. However, when consumed in high amounts, tannins can be toxic [
25] and can even lead to animal death [
26,
27]. The presence of condensed tannins >100 g/kg of DM improves the DM digestibility in goats and sheep, which might be associated with the adaptation of the animals to condensed tannins and because of the digestive physiology which appears to be associated with lower retention of ingested feeds [
28]. The prolonged use of condensed tannins from
Mimosa tenuiflora hay in sheep diets reduces body weight gain [
15]. Therefore, it is crucial to understand how natural tannins derived from legume hay, with other factors such as the fiber fraction, may interact with the rumen metabolism of nitrogen, energy, and water balance. This is particularly relevant as animals in tropical regions often face limited availability of both forage and water under natural conditions.
Then, we hypothesized that the inclusion of tannins up to 7% DM in the total diet of lambs can promote protein protection and make it available in the small intestine, reducing mainly energy losses from the nitrogen metabolism and improving digestion and the nitrogen, energy, and water metabolism. In addition, it is crucial for enhancing the N-retention, water, and energy within the body, particularly in regions with limited water availability and vegetation abundant in tannin-rich plants. Therefore, the objective of this study was to assess the effects of increasing levels of tannins from Mimosa tenuiflora hay on the intake, digestibility, and the balance of nitrogen, water, and energy in hair lambs.
4. Discussion
The intake of all nutrients showed a positive quadratic response with the addition of tannins to the diet. The optimal inclusion levels of tannins were between 26.2 and 52.4 g tannin/kg DM, corresponding to an approximately 20 to 40% inclusion of
Mimosa tenuiflora hay in the total diet of the lambs. This can be explained by the binding action of tannins with high-biological-value proteins, protecting them from proteolytic bacteria in the rumen and facilitating their release and absorption in the small intestine [
39]. Additionally, tannins also play a role in controlling protozoa populations and contribute to the microbial balance in the rumen, leading to improved energy and protein utilization [
20].
The maximum intake of DM, CP, and TDN was estimated when the tannin content in the diet reached 29.79, 26.25, and 33.72 g/kg DM, respectively. These findings align with the statements made by Valenti et al. [
40], who suggested that tannin levels of up to 40 g/kg DM can enhance nutrient intake in lambs. Incorporating tannin levels of up to 15 g per kg DM in ruminant diets has been found to increase the flow of amino acids and non-microbial nitrogen into the duodenum, thereby reducing deamination processes that occur in the rumen and resulting in decreased nitrogen excretion in the urine [
41,
42].
It is worth highlighting that the quadratic effect of tannins on the nutrient intake in sheep was characterized by an increase in intake when the tannin level was below 40 g/kg DM. A better balance was observed in the intake of CP and NFC within the range of 26.2 to 52.4 g of tannins per kg DM, and a similar balance was observed for the fiber portion at around 20 to 30 g of tannins per kg DM. This balance ensured a favorable ratio between energy and protein, promoting a balanced interaction between the proteolytic bacteria and those involved in the breakdown of different carbohydrates in the diet. This is evident when considering that the highest estimate of TDN intake occurred when the tannin content of the diet reached 33.72 g/kg DM.
The similar intake can be attributed to a sharp reduction in the intake of all nutrients when the tannin level in the diet exceeded 52.4 g/kg of DM. This observation supports the notion that tannins, when present at appropriate levels, contribute to the microbial balance in the rumen by exerting selective antimicrobial activities that enhance the population of beneficial bacteria in the abomasum and intestine while inhibiting the growth of pathogenic bacteria. However, it is important to note that Serra et al. [
18] have reported that tannin levels above 50 g/kg DM in ruminant diets can initially lead to weight loss and may even result in the intoxication and death of the animals. Ter Braak and Prentice [
38] conducted a study with a maximum level of 80 g/kg DM of tannin extract from
Acacia mearnsii in lambs’ diets and found that levels higher than 40 g of extract were harmful. Additionally, [
43] suggested a limit of approximately 50 g tannins per kg DM for dairy goats, beyond which the efficiency of rumen microbiota in digesting ingested DM and the overall digestive efficiency in the rumen may be reduced.
The quadratic effect of tannins on nutrient intake is only evident in the digestibility of DM, where there is a positive linear response for NFC and a negative for the other nutrients, which explains the observed quadratic effect on DM digestibility. Furthermore, it is important to note that, contrary to the increased intake, the presence of tannins decreased the digestibility of nutrients, except for DM, which remained unchanged, and NFC, which was higher in the treatments with tannins.
The reduction in digestibility of NDF and ADF confirms that phenolic compounds also interfere with cell wall components. According to McSweeney et al. [
44], as the tannin content increases in ruminant diets, the digestibility of the fiber components decreases. This can occur through various mechanisms, including the complexing of polymers such as cellulose and hemicellulose, the inhibition of microorganisms that directly act on NDF and ADF, the formation of chelates with mineral ions necessary for the metabolism of these bacteria, or a combination of these factors. Studies in sheep [
45] and cattle [
46] have also reported the effects of tannins on fatty acid biohydrogenation.
Based on the results obtained for intake and nutrient digestibility, it is suggested that the impact of tannins in the rumen environment is primarily due to their interaction with enzymes, proteins, and carbohydrates, rather than their astringency [
47]. This interaction may lead to a longer retention time of the fiber components (NDF and ADF) in the diet, resulting in delayed rumen emptying and potentially influencing feed intake control, which can go from a chemical regulation to a more physical regulation [
48].
The quadratic effect observed of the tannin levels and nitrogen retention (g/day) indicates an increase in N-retained up to a tannin level of 26.2 g per kg DM. This can be explained by the linear reduction in N-urinary excreted, as well as the increased amounts of nitrogen ingested and excreted in the feces. However, at higher tannin concentrations, N-excretion in urine and feces decreased. These findings confirm that tannins, at appropriate levels, promote a favorable nitrogen balance by enhancing its retention in the lambs’ bodies. These findings are confirmed by Mueller-Harvey [
49], who demonstrated that tannins, through the formation of stable complexes with proteins, decrease rumen degradation and significantly influence N-balance. N-urinary, in the form of uric acid, contributes to ammonia pollution in the environment [
50], whereas fecal nitrogen can be converted into organic matter for the soil [
51]. This behavior was observed in our study when comparing the treatments without tannins to those with tannins, wherein N-ingested was similar, but lambs fed tannin-containing diets showed lower N-urinary excretion and higher N-fecal excretion.
The utilization of tannins in ruminant diets is only justified when it leads to an improved production performance and the production outcomes retained in the animal’s body result in high-quality meat and/or milk for human consumption. In this context, the impact of tannins on nitrogen metabolism is noteworthy [
20]. Tannins have been found to reduce proteolytic bacteria in the rumen and inhibit rumen biohydrogenation of fatty acids. As a result, there is an increase in the concentration of unsaturated fatty acids in the abomasum and intestine, leading to an improved muscle nitrogen balance, and contributing to the production of meat [
52] with a higher proportion of unsaturated fats, which reduces human health risks.
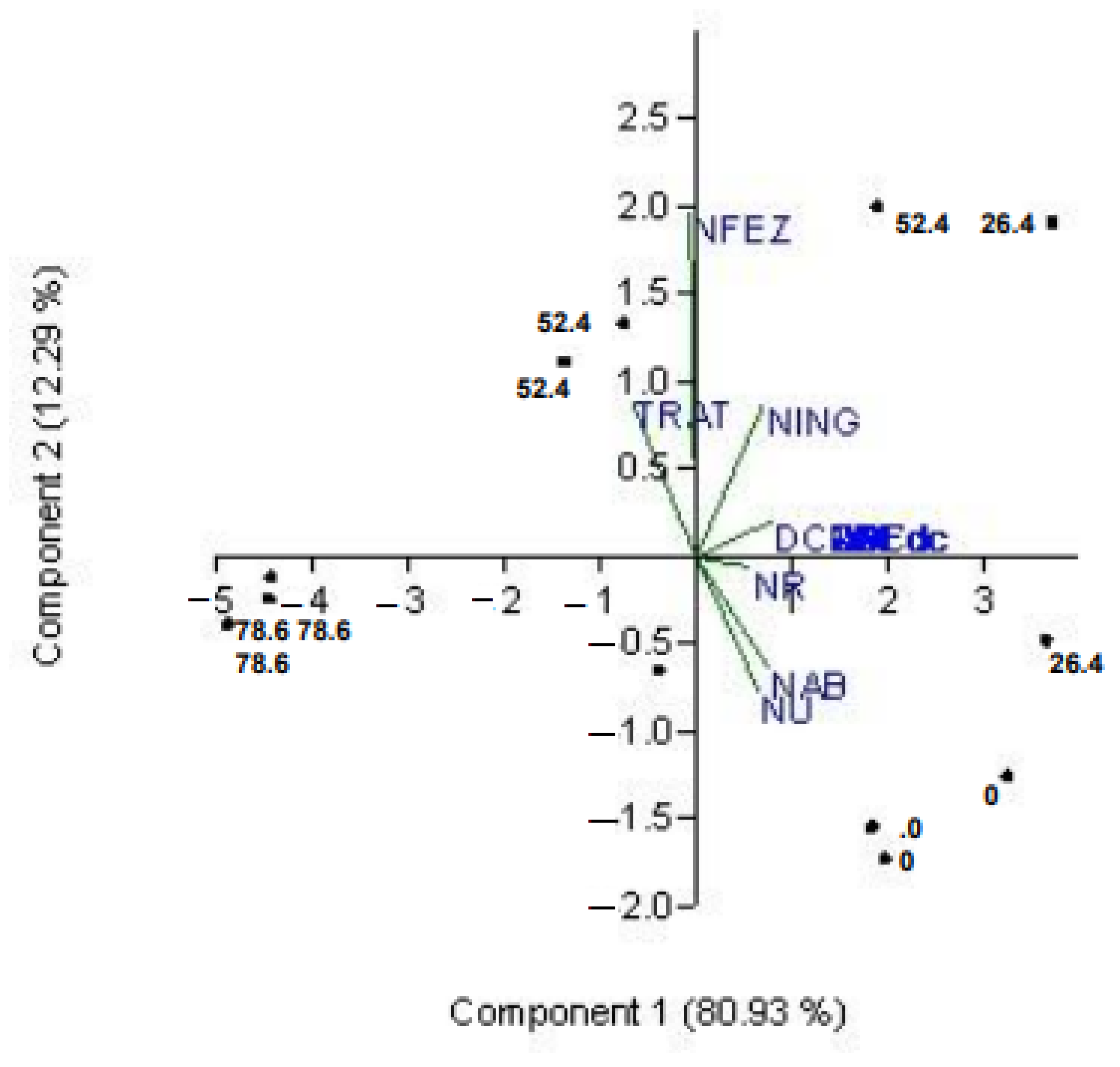
PC1 explained 81% of the variation and showed a strong correlation between digestibility variables and NINT, NU, NAB, and NR, which can be attributed to the effect of tannins on NU (
Figure 1). PC2, which explained 12% of the variation, was characterized by the lack of correlation between nutrient digestibility and NFEC, as well as the quadratic effect of tannin on N-fecal. The projections in the principal component analysis showed the distinct patterns between tannin-free treatments and the treatment with the highest tannin level (0 and 78.6), as well as the opposing projections observed for NFEC and NU/NAB in different quadrants.
The quadratic effect of tannins on the water intake, without affecting the amount of water excreted in urine and feces, suggests that lambs can maintain adequate levels of water in their bodies by reducing water excretion without compromising body homeostasis. Although the tannin levels influenced the nutrient intake and digestibility, the total water intake expressed as %DM ingested remained unchanged. This finding highlight that those tannins primarily influenced the intake rather than directly impacted the digestibility of the nutrients. This contradicts the assumption that changes in the dry matter intake necessarily led to changes in the water intake. Ruminants have mechanisms to reduce water loss in urine [
53] and feces [
54], as well as the increased production of metabolic water [
55], which collectively contribute to the animal’s water balance [
56].
The contrasting effect of tannins on the total water intake, and the similarity in the total water excretion, can be attributed to the higher intake of free water and dietary water, as well as increased metabolic water production in the diet with a low tannin level. However, at higher tannin levels, these values decrease, which suggests that lambs can regulate water retention by controlling its excretion [
57]. This regulatory mechanism is important for maintaining homeostasis, as the osmolarity of body fluids plays a critical role. The renin–angiotensin–aldosterone system is involved in the control of water intake and excretion [
58].
The quadratic effect of tannin on water retention follows a similar pattern as observed for nutrient intake, DM digestibility, and N retention in grams, always increasing when tannin levels are below 40 g/kg DM and decreasing at higher tannin levels. The decrease in the digestibility of CP, NDF, and NFC with increasing tannin levels may have contributed to the maximum water retention that was estimated at a tannin level of 31.33 g/kg DM, confirming the impact of protein and carbohydrate digestibility on the lambs’ water balance. This is evident when comparing the metabolic water production between the free-tannin diet (402.06 g) and the diet with the highest tannin level (78.6 g of tannin per kg DM), which decreased to 200.04 g, resulting in a 49.75% reduction in metabolic water production.
In ruminants, the variation in the nutrient intake in diets with different tannin concentrations is generally associated with astringency, which also affects water retention in the body. In this study, it was found that water balance was primarily influenced by nutrient intake rather than nutrient digestibility. It is worth noting that the effect of tannins on water intake in mass unit (g and/or kg), regardless of its source (free water or from the diet), did not have a similar effect on water excretion in feces, urine, or total, which suggests that lambs in semi-arid regions employ defense mechanisms to conserve water, as observed in other studies [
59,
60], where lambs retained or eliminated water to maintain their body fluids and ensure proper physiological functions. Similar observations were made by Magalhães et al. [
61] in their evaluation of diets with different proportions of bean residues combined with cactus pear in hair lambs.
According to Karimizadeh et al. [
62], a significant portion (60 to 90%) of the variations in nutrient retention in the animal’s body is attributed to nutrient ingestion, while nutrient digestibility accounts for a smaller percentage (10 to 40%), even when high-moisture diets are used [
53]. However, regardless of astringency, the crucial factor is the amount of tannins ingested. At low levels, tannin can have beneficial effects, but at high levels, it has the potential to influence the water balance of ruminants, affecting both water intake and urine excretion.
It is crucial to determine the appropriate level of tannin for each specific ruminant species and category to ensure optimal animal performance without compromising organic functions. Studies conducted by Cordão et al. [
3] and Bandeira et al. [
1] with hair lambs recommend tannin levels of up to 40 g per kg of dry matter (DM) in the diet. Similarly, [
42] observed that hydration and milk production in dairy cows can be maintained by incorporating up to 4.3 g of tannins per kg of DM in the diet.
The positive quadratic response of energy intake as a function of tannin content in the diet is consistent with the findings for intake and digestibility. This indicates that the inclusion of appropriate levels of tannins from
Mimosa tenuiflora can influence intake, digestibility, and nutrient balance, potentially leading to improved animal performance and enhanced meat quality in lambs. According to Vasta et al. [
63] tannins can affect the energy balance of ruminants, including their impact on rumen microbiology, such as reducing the biohydrogenation of unsaturated fatty acids. This effect has been observed by Gama et al. [
64] in sheep and goats that were finished on native pastures enriched with buffel grass, where the presence of tannin-rich plants such as
Mimosa tenuiflora in the grazing area was prominent.
When comparing the balance, intake, and excretion of energy between tannin-free diets and those with tannins (0 × Tan), the results further highlight the potential of Mimosa tenuiflora tannins to enhance or inhibit functions in a sheep’s body. The presence of tannins in the diet leads to a reduction in the digestible energy (Mcal/day and % of GEI) in lambs, likely due to an increased energy excretion in the feces, especially in animals receiving higher levels of tannins (52.4 and 78.6 g per kg of DM). Additionally, there is a linear decrease in the digestibility of apNDF and a linear increase in the digestibility of NFC, indicating that the effects of tannins on intake and digestibility may differ depending on the tannin level in the diet. It is worth noting that the astringency and bitter taste caused by tannins have a primary effect on intake, while the impact on digestibility is secondary.
The selectivity behavior of lambs compensates for the negative effects of tannins on intake and digestibility. Lambs tend to prefer more palatable components that have a lower tannin content, and when that occurs, the effects on both intake and digestibility become similar, thereby promoting a better nutritional balance. This is beneficial because tannins reduce hydrogen (H
2) production in the rumen, which inhibits the growth of methanogenic bacteria [
9] and fermenters of cellulose and hemicellulose, decreasing the digestibility of fiber carbohydrates [
63], leading to reduced methane production [
65] and the release of energy in the form of pollutant gases into the environment [
66].
However, at high levels of tannins, surpassing the natural selection capacity of sheep, they can disrupt the population balance of bacteria [
67] and protozoa [
68], and their prolonged use even leads to toxicity [
20] and, potentially, cause the death of animals. In this study, the results of the energy balance analysis indicated that the optimal levels of tannins for maximizing digestible energy and metabolized energy were estimated to be 16.1 and 16.3 g per kg of dry matter, respectively. These values represent an average reduction of approximately 40% and 58% compared to the estimated levels of 27 and 39 g tannins, respectively, required to maximize energy intake and minimize energy excretion in the feces of lambs.