Host Associations of Culicoides Biting Midges in Northeastern Kansas, USA
Abstract
:Simple Summary
Abstract
1. Introduction
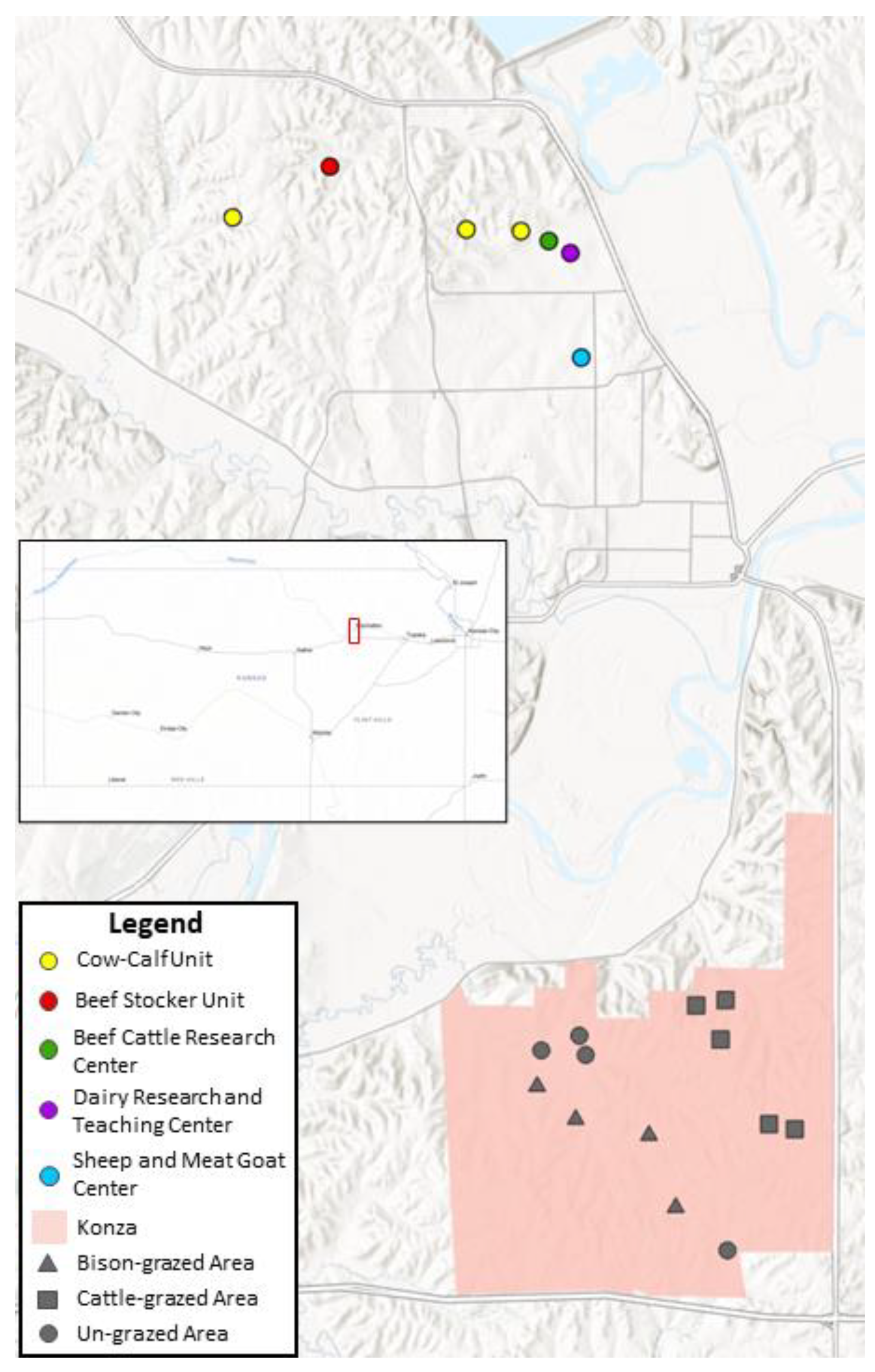
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGregor, B.L.; Shults, P.T.; McDermott, E.G. A review of the vector status of North American Culicoides (Diptera: Ceratopogonidae) for bluetongue virus, epizootic hemorrhagic disease virus, and other arboviruses of concern. Curr. Trop. Med. Rep. 2022, 9, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Martinez-de la Puente, J.; Figuerola, J.; Soriguer, R. Fur or feather? Feeding preferences of species of Culicoides biting midges in Europe. Trends Parasitol. 2015, 31, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopken, M.W.; Ryan, B.M.; Huyvaert, K.P.; Piaggio, A.J. Picky eaters are rare: DNA-based blood meal analysis of Culicoides (Diptera: Ceratopogonidae) species from the United States. Parasites Vectors 2017, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Stenn, T.; Sayler, K.A.; Blosser, E.M.; Blackburn, J.K.; Wisely, S.M.; Burkett-Cadena, N.D. Host use patterns of Culicoides spp. biting midges at a big game preserve in Florida, U.S.A., and implications for the transmission of orbiviruses. Med. Vet. Entomol. 2019, 33, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Kramer, L.D.; Ciota, A.T. Dissecting vectorial capacity for mosquito-borne viruses. Curr. Opin. Virol. 2015, 15, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Paaijmans, K.P.; Blanford, S.; Chan, B.H.K.; Thomas, M.B. Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol. Lett. 2011, 8, 465–468. [Google Scholar] [CrossRef]
- Tempelis, C. Host-feeding patterns of mosquitoes, with a review of advances in analysis of blood meals by serology. J. Med. Entomol. 1975, 11, 635–653. [Google Scholar] [CrossRef]
- Wright, R.E.; DeFoliart, G.R. Associations of Wisconsin mosquitoes and woodland vertebrate hosts. Ann. Entomol. Soc. Am. 1970, 63, 777–786. [Google Scholar] [CrossRef]
- Baum, M.; de Castro, E.A.; Pinto, M.C.; Goulart, T.M.; Baura, W.; Klisiowicz Ddo, R.; Vieira da Costa-Ribeiro, M.C. Molecular detection of the blood meal source of sand flies (Diptera: Psychodidae) in a transmission area of American cutaneous leishmaniasis, Parana State, Brazil. Acta Trop. 2015, 143, 8–12. [Google Scholar] [CrossRef]
- Pitzer, J.B.; Kaufman, P.E.; Tenbroeck, S.H.; Maruniak, J.E. Host blood meal identification by multiplex polymerase chain reaction for dispersal evidence of stable flies (Diptera: Muscidae) between livestock facilities. J. Med. Entomol. 2011, 48, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Gaithuma, A.; Yamagishi, J.; Hayashida, K.; Kawai, N.; Namangala, B.; Sugimoto, C. Blood meal sources and bacterial microbiome diversity in wild-caught tsetse flies. Sci. Rep. 2020, 10, 5005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloyer, K.E.; Acevedo, C.; Wisely, S.M.; Burkett-Cadena, N.D. Host associations of biting midges (Diptera: Ceratopogonidae: Culicoides) at deer farms in Florida, USA. J. Med. Entomol. 2023, 60, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Blosser, E.M.; Stenn, T.; Acevedo, C.; Burkett-Cadena, N.D. Host use and seasonality of Culex (Melanoconion) iolambdis (Diptera: Culicidae) from eastern Florida, USA. Acta Trop. 2016, 164, 352–359. [Google Scholar] [CrossRef]
- Kent, R.J.; Norris, D.E. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am. J. Trop. Med. Hyg. 2005, 73, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellekom, B.; Bailey, A.; England, M.; Langlands, Z.; Lewis, O.T.; Hackett, T.D. Effects of storage conditions and digestion time on DNA amplification of biting midge (Culicoides) blood meals. Parasites Vectors 2023, 16, 13. [Google Scholar] [CrossRef]
- Blanton, F.S.; Wirth, W.W. The Sand Flies (Culicoides) of Florida (Diptera: Ceratopogonidae). In Arthropods of Florida and Neighboring Land Areas; Florida Department of Agriculture and Consumer Services: Gainesville, FL, USA, 1979; Volume 10. [Google Scholar]
- Wirth, W.W.; Dyce, A.L.; Peterson, B.V. An atlas of wing photographs, with a summary of the numerical characters of the Nearctic species of Culicoides (Diptera: Ceratopogonidae). Contrib. Am. Entomol. Inst. 1985, 22, 1–72. [Google Scholar]
- Wirth, W.W.; Blanton, F.S. The North American Culicoides of the Guttipennis Group (Diptera: Ceratopogonidae). Fla. Entomol. 1967, 50, 207–232. [Google Scholar] [CrossRef]
- Sanders, C.J.; Harrup, L.E.; Tugwell, L.A.; Brugman, V.A.; England, M.; Carpenter, S. Quantification of within- and between-farm dispersal of Culicoides biting midges using an immunomarking technique. J. Appl. Ecol. 2017, 54, 1429–1439. [Google Scholar] [CrossRef] [Green Version]
- Lassen, S.B.; Nielsen, S.A.; Skovgard, H.; Kristensen, M. Molecular identification of bloodmeals from biting midges (Diptera: Ceratopogonidae: Culicoides Latreille) in Denmark. Parasitol. Res. 2011, 108, 823–829. [Google Scholar] [CrossRef]
- Bartsch, S.; Bauer, B.; Wiemann, A.; Clausen, P.H.; Steuber, S. Feeding patterns of biting midges of the Culicoides obsoletus and Culicoides pulicaris groups on selected farms in Brandenburg, Germany. Parasitol. Res. 2009, 105, 373–380. [Google Scholar] [CrossRef]
- Becker, M.E.; Reeves, W.K.; Dejean, S.K.; Emery, M.P.; Ostlund, E.N.; Foil, L.D. Detection of bluetongue virus RNA in field-collected Culicoides spp. (Diptera: Ceratopogonidae) following the discovery of bluetongue virus serotype 1 in white-tailed deer and cattle in Louisiana. J. Med. Entomol. 2010, 47, 269–273. [Google Scholar] [PubMed]
- Becker, M.; Park, J.-S.; Gentry, G.; Husseneder, C.; Foil, L. Comparison of trapping methods for use in surveys for potential Culicoides vectors of orbiviruses. Parasites Vectors 2021, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Rozo-Lopez, P.; Davis, T.M.; Drolet, B.S. Detection of vesicular stomatitis virus Indiana from insects collected during the 2020 outbreak in Kansas, USA. Pathogen 2021, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Chu, E.; Shults, P.; Golnar, A.; Swanson, D.A.; Benn, J.; Kim, D.; Schneider, P.; Pena, S.; Culver, C.; et al. Culicoides species community composition and infection status with parasites in an urban environment of east central Texas, USA. Parasites Vectors 2019, 12, 39. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; Havelka, P.; Schaefer, H.M.; Segelbacher, G. Bloodmeal analysis reveals avian Plasmodium infections and broad host preferences of Culicoides (Diptera: Ceratopogonidae) vectors. PLoS ONE 2012, 7, e31098. [Google Scholar] [CrossRef] [Green Version]
- Ferraguti, M.; Martínez-de la Puente, J.; Ruiz, S.; Soriguer, R.; Figuerola, J. On the study of the transmisión networks of blood parasites from SW Spain: Diversity of avian haemosporidians in the biting midge Culicoides circumscriptus and wild birds. Parasites Vectors 2013, 6, 208. [Google Scholar] [CrossRef] [Green Version]
- Sunantaraporn, S.; Hortiwakul, T.; Kraivichian, K.; Siriyasatien, P.; Brownell, N. Molecular identification of host blood meals and detection of blood parasites in Culicoides Latreille (Diptera: Ceratopogonidae) collected from Phatthalung Province, Southern Thailand. Insects 2022, 13, 912. [Google Scholar] [CrossRef]
- Sunantaraporn, S.; Thepparat, A.; Phumee, A.; Sor-Suwan, S.; Boonserm, R.; Bellis, G.; Siriyasatien, P. Culicoides Latrielle (Diptera: Ceratopogonidae) as potential vectors for Leishmania martiniquensis and Trypanosoma sp. in northern Thailand. PLoS Neg. Trop. Dis. 2021, 15, e0010014. [Google Scholar] [CrossRef]
- Inumaru, M.; Nakamura, K.; Odagawa, T.; Suzuki, M.; Murata, K.; Sato, Y. The first detection of avian haemosporidia from Culicoides biting midges in Japan, with notes on potential vector species and the transmission cycle. Vet. Parasitol. 2023, 39, 100840. [Google Scholar] [CrossRef]
- McGregor, B.L.; Reister-Hendricks, L.M.; Nordmeyer, C.; Stapleton, S.; Davis, T.M.; Drolet, B.S. Using zoos as sentinels for re-emerging arboviruses: Vector surveillance during an outbreak of epizootic hemorrhagic disease at the Minnesota Zoo. Pathogen 2023, 12, 140. [Google Scholar] [CrossRef]
- McGregor, B.L.; Sloyer, K.E.; Sayler, K.A.; Goodfriend, O.; Krauer, J.M.C.; Acevedo, C.; Zhang, X.; Mathias, D.; Wisely, S.M.; Burkett-Cadena, N.D. Field data implicating Culicoides stellifer and Culicoides venustus (Diptera: Ceratopogonidae) as vectors of epizootic hemorrhagic disease virus. Parasites Vectors 2019, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Mullen, G.R.; Jones, R.H.; Braverman, Y.; Nusbaum, K.E. Laboratory infections of Culicoides debilipalpis and C. stellifer (Diptera: Ceratopogonidae) with bluetongue virus. Prog. Clin. Biol. Res. 1985, 178, 239–243. [Google Scholar]
- Smith, K.E.; Stallknecht, D.E.; Sewell, C.T.; Rollor, E.A.; Mullen, G.R.; Anderson, R.R. Monitoring of Culicoides spp. at a site enzootic for hemorrhagic disease in white-tailed deer in Georgia, USA. J. Wildl. Dis. 1996, 32, 627–642. [Google Scholar] [CrossRef]
- Martinez-de la Puente, J.; Mendez, M.; Ruiz, S.; Godoy, J.A.; Soriguer, R.C.; Figuerola, J. Individual identification of endangered species using mosquito blood meals: A proof-of-concept study in Iberian lynx. Parasitol. Res. 2015, 114, 1607–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyer, I.J.; Blosser, E.M.; Acevedo, C.; Thompson, A.C.; Reeves, L.E.; Burkett-Cadena, N.D. Mammal decline, linked to invasive Burmese python, shifts host use of vector mosquito towards reservoir hosts of a zoonotic disease. Biol. Lett. 2017, 13, 20170353. [Google Scholar] [CrossRef] [Green Version]
- Burkett-Cadena, N.D.; Blosser, E.M.; Loggins, A.A.; Valente, M.C.; Long, M.T.; Campbell, L.P.; Reeves, L.E.; Bargielowski, I.; McCleery, R.A. Invasive Burmese pythons alter host use and virus infection in the vector of a zoonotic virus. Commun. Biol. 2021, 4, 804. [Google Scholar] [CrossRef]
- Wilson, A.; Mellor, P. Bluetongue in Europe: Vectors, epidemiology, and climate change. Parasitol. Res. 2008, 103, 69–77. [Google Scholar] [CrossRef]
- Gallana, M.; Ryser-Degiorgis, M.-P.; Wahli, T.; Segner, H. Climate change and infectious diseases of wildlife: Altered interactions between pathogens, vectors and hosts. Curr. Zool. 2013, 59, 427–437. [Google Scholar] [CrossRef]
- Tabachnick, W.J. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J. Exp. Biol. 2010, 213, 946–954. [Google Scholar] [CrossRef] [Green Version]



| Site | Host Class | Host | crepus. | haemat. | sonor. | stell. | varii. |
|---|---|---|---|---|---|---|---|
| Konza | Avian | American crow | 2 | 0 | 0 | 0 | 0 |
| Eastern bluebird | 1 | 0 | 0 | 0 | 0 | ||
| Orchard oriole | 1 | 0 | 0 | 0 | 0 | ||
| Summer tanager | 0 | 0 | 0 | 0 | 0 | ||
| Turkey vulture | 1 | 0 | 0 | 0 | 0 | ||
| Wild turkey | 0 | 2 | 0 | 2 | 0 | ||
| Yellow-billed cuckoo | 0 | 3 | 0 | 1 | 0 | ||
| Mammalian | Cow | 0 | 1 | 0 | 14 | 0 | |
| White-tailed deer | 0 | 0 | 0 | 2 | 1 | ||
| KSU | Avian | Barred owl | 0 | 5 | 1 | 1 | 0 |
| Blue jay | 1 | 1 | 0 | 0 | 0 | ||
| Brown-headed cowbird | 1 | 0 | 0 | 0 | 0 | ||
| Common grackle | 1 | 0 | 0 | 0 | 0 | ||
| Cooper’s hawk | 1 | 0 | 0 | 0 | 0 | ||
| Eastern bluebird | 0 | 3 | 1 | 1 | 0 | ||
| Eastern meadowlark | 1 | 0 | 0 | 0 | 0 | ||
| Great horned owl | 0 | 1 | 0 | 0 | 0 | ||
| House finch | 1 | 1 | 0 | 1 | 0 | ||
| Mourning dove | 4 | 0 | 0 | 0 | 0 | ||
| Northern cardinal | 0 | 1 | 0 | 0 | 0 | ||
| Orchard oriole | 1 | 0 | 0 | 0 | 0 | ||
| Summer tanager | 1 | 0 | 0 | 0 | 0 | ||
| Tufted titmouse | 1 | 2 | 0 | 0 | 0 | ||
| Wild turkey | 4 | 12 | 2 | 4 | 1 | ||
| Yellow-billed cuckoo | 1 | 5 | 0 | 2 | 2 | ||
| Mammalian | Cow | 1 | 2 | 39 | 28 | 113 | |
| Dog | 1 | 0 | 0 | 0 | 0 | ||
| Elk | 0 | 0 | 0 | 1 | 0 | ||
| Horse | 0 | 1 | 9 | 2 | 5 | ||
| Sheep | 0 | 0 | 0 | 1 | 0 | ||
| White-tailed deer | 0 | 0 | 3 | 9 | 1 |
| Location | Site | arbor. | bergi | crepus. | haemat. | nanus | sonor. | stell. | varii. |
|---|---|---|---|---|---|---|---|---|---|
| Konza | Bison | 0 | 0 | 1 | 5 | 0 | 0 | 2 | 1 |
| Cattle | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | |
| Ungrazed | 0 | 0 | 4 | 1 | 1 | 0 | 3 | 0 | |
| KSU | Beef Stocker Unit | 2 | 0 | 2 | 27 | 0 | 1 | 19 | 7 |
| Dairy | 0 | 0 | 2 | 0 | 0 | 8 | 1 | 13 | |
| BCRC | 0 | 1 | 0 | 3 | 0 | 41 | 3 | 101 | |
| Cow–Calf Unit | 0 | 0 | 13 | 2 | 0 | 2 | 23 | 1 | |
| Sheep/Meat Goat | 0 | 0 | 3 | 2 | 0 | 3 | 4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGregor, B.L.; Lewis, A. Host Associations of Culicoides Biting Midges in Northeastern Kansas, USA. Animals 2023, 13, 2504. https://doi.org/10.3390/ani13152504
McGregor BL, Lewis A. Host Associations of Culicoides Biting Midges in Northeastern Kansas, USA. Animals. 2023; 13(15):2504. https://doi.org/10.3390/ani13152504
Chicago/Turabian StyleMcGregor, Bethany L., and Aaron Lewis. 2023. "Host Associations of Culicoides Biting Midges in Northeastern Kansas, USA" Animals 13, no. 15: 2504. https://doi.org/10.3390/ani13152504






