Encoding of Arousal and Physical Characteristics in Audible and Ultrasonic Vocalizations of Mongolian Gerbil Pups Testing Common Rules for Mammals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Set Up
2.3. Experimental Procedure
2.4. Acoustic Analysis
2.5. Statistical Analysis
2.5.1. Validation of Different Call Types
2.5.2. Effect of Arousal, Sex, and Age on Vocal Behavior
2.5.3. Vocal Correlates of Ultrasonic and Audible Vocalizations
3. Results
3.1. Distinction of Different Call Types
3.2. Effect of Arousal, Age and Sex on the Vocal Behavior
3.3. Vocal Correlates of Ultrasonic and Audible Vocalizations
3.3.1. Arousal
3.3.2. Developmental Factors
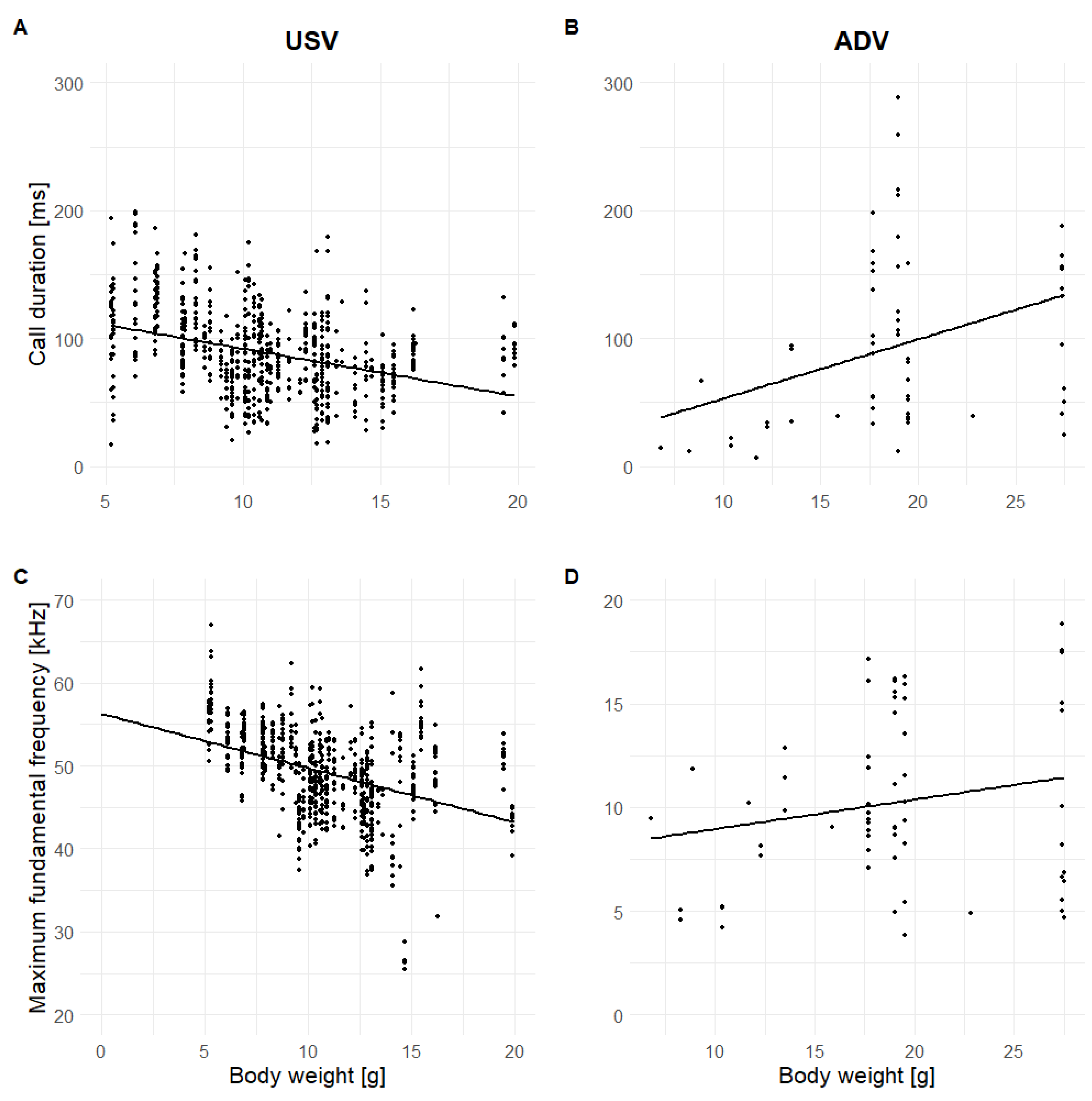
3.3.3. Individual Cues
4. Discussion
4.1. Function of Pup Vocalizations
4.2. Vocal Correlates of Arousal and Physical Characteristics
4.3. How Divergent Encoding Patterns Can Be Explained?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherer, K.R. Vocal Affect Expression: A Review and a Model for Future Research. Psychol. Bull. 1986, 99, 143–165. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P.; Blumstein, S.E. Speech Physiology, Speech Perception, and Acoustic Phonetics; Cambridge University Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Briefer, E.F. Vocal Expression of Emotions in Mammals: Mechanisms of Production and Evidence. J. Zool. 2012, 288, 1–20. [Google Scholar] [CrossRef]
- Briefer, E.F. Coding for ‘Dynamic’ Information: Vocal Expression of Emotional Arousal and Valence in Non-Human Animals. In Coding Strategies in Vertebrate Acoustic Communication. Animal Signals and Communication; Aubin, T., Mathevon, N., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Charlton, B.D.; Pisanski, K.; Raine, J.; Reby, D. Coding of Static Information in Terrestrial Mammal Vocal Signals. In Coding Strategies in Vertebrate Acoustic Communication. Animal Signals and Communication; Aubin, T., Mathevon, N., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Fitch, W.T. The Evolution of Language; Cambridge University Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Taylor, A.M.; Reby, D. The Contribution of Source-Filter Theory to Mammal Vocal Communication Research. J. Zool. 2010, 280, 221–236. [Google Scholar] [CrossRef]
- Mendl, M.; Burman, O.H.P.; Paul, E.-S. An Integrative and Functional Framework for the Study of Animal Emotion and Mood. P. Roy. Soc. B-Biol. Sci. 2010, 277, 2895–2904. [Google Scholar] [CrossRef]
- Anderson, D.J.; Adolphs, R. A Framework for Studying Emotions across Species. Cell 2014, 157, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Rendall, D. Acoustic Correlates of Caller Identity and Affect Intensity in the Vowel-Like Grunt Vocalizations of Baboons. J. Acoust. Soc. Am. 2003, 113, 3390–3402. [Google Scholar] [CrossRef]
- Rendall, D.; Owren, M.J.; Rodman, P.S. The Role of Vocal Tract Filtering in Identity Cueing in Rhesus Monkey (Macaca Mulatta) Vocalizations. J. Acoust. Soc. Am. 1998, 103, 602–614. [Google Scholar] [CrossRef]
- Ey, E.; Pfefferle, D.; Fischer, J. Do Age- and Sex-Related Variations Reliably Reflect Body Size in Non-Human Primate Vocalizations? A Review. Primates 2007, 48, 253–267. [Google Scholar] [CrossRef]
- Bowling, D.L.; Garcia, M.; Dunn, J.C.; Ruprecht, R.; Stewart, A.; Frommolt, K.-H.; Fitch, W.T. Body Size and Vocalization in Primates and Carnivores. Sci. Rep. 2017, 7, 41070. [Google Scholar] [CrossRef] [Green Version]
- Linn, S.N.; Schmidt, S.; Scheumann, M. Individual Distinctiveness across Call Types of the Southern White Rhinoceros (Ceratotherium simum simum). J. Mammal. 2021, 102, 440–456. [Google Scholar] [CrossRef]
- Zimmermann, E.; Leliveld, L.M.C.; Schehka, S. Toward the Evolutionary Roots of Affective Prosody in Human Acoustic Communication: A Comparative Approach to Mammalian Voices. In Evolution of Emotional Communication: From Sounds in Nonhuman Mammals to Speech and Music in Man; Altenmüller, E., Schmidt, S., Zimmermann, E., Eds.; Oxford University Press: Oxford, UK, 2013; pp. 116–132. [Google Scholar]
- Blumstein, D.T.; Munos, O. Individual, Age and Sex-Specific Information Is Contained in Yellow-Bellied Marmot Alarm Calls. Anim. Behav. 2005, 69, 353–361. [Google Scholar] [CrossRef]
- Zaytseva, A.S.; Volodin, I.A.; Ilchenko, O.G.; Volodina, E.V. Audible Calls and Their Ontogenetic Relationship with Ultrasonic Vocalization in a Rodent with a Wide Vocal Range, the Fat-Tailed Gerbil (Pachyuromys duprasi). Behav. Process. 2020, 180, 104241. [Google Scholar] [CrossRef]
- Volodin, I.A.; Yurlova, D.D.; Ilchenko, O.G.; Volodina, E.V. Ontogeny of Audible Squeaks in Yellow Steppe Lemming Eolagurus Luteus: Trend Towards Shorter and Low-Frequency Calls Is Reminiscent of Those in Ultrasonic Vocalization. BMC Zool. 2021, 6, 27. [Google Scholar] [CrossRef]
- Leliveld, L.M.C.; Scheumann, M.; Zimmermann, E. Acoustic Correlates of Individuality in the Vocal Repertoire of a Nocturnal Primate (Microcebus murinus). J. Acoust. Soc. Am. 2011, 129, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; McCowan, B. Barking in Domestic Dogs: Context Specificity and Individual Identification. Anim. Behav. 2004, 68, 343–355. [Google Scholar] [CrossRef]
- Linhart, P.; Ratcliffe, V.F.; Reby, D.; Špinka, M. Expression of Emotional Arousal in Two Different Piglet Call Types. PLoS ONE 2015, 10, e0135414. [Google Scholar] [CrossRef]
- Nowak, R.; Porter, R.H.; Levy, F.; Orgeur, P.; Schaal, B. Role of Mother-Young Interactions in the Survival of Offspring in Domestic Mammals. Rev. Reprod. 2000, 5, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Briefer, E.F.; McElligott, A.G. Indicators of Age, Body Size and Sex in Goat Kid Calls Revealed Using the Source–Filter Theory. Appl. Anim. Behav. Sci. 2011, 133, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Lemasson, A.; Mikus, M.-A.; Blois-Heulin, C.; Lodé, T. Vocal Repertoire, Individual Acoustic Distinctiveness, and Social Networks in a Group of Captive Asian Small-Clawed Otters (Aonyx cinerea). J. Mammal. 2014, 95, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Scheumann, M.; Roser, A.E.; Konerding, W.; Bleich, E.; Hedrich, H.J.; Zimmermann, E. Vocal Correlates of Sender-Identity and Arousal in the Isolation Calls of Domestic Kitten (Felis silvestris catus). Front. Zool. 2012, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Scheumann, M.; Zimmermann, E.; Deichsel, G. Context-Specific Calls Signal Infants’ Needs in a Strepsirrhine Primate, the Gray Mouse Lemur (Microcebus murinus). Dev. Psychobiol. 2007, 49, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Schuchmann, M.; Wiegrebe, L. Localization Dominance and the Effect of Frequency in the Mongolian Gerbil, Meriones unguiculatus. J. Comp. Physiol. A 2010, 196, 463–470. [Google Scholar] [CrossRef]
- Juchter, C.; Beutelmann, R.; Klump, G.M. Speech Sound Discrimination by Mongolian Gerbils. Hear. Res. 2022, 418, 108472. [Google Scholar] [CrossRef]
- Schneider, B.; Döring, D. Gerbils. In Verhaltensberatung Bei Kleinen Heimtieren; Schneider, B., Döring, D., Eds.; Schattauer GmbH: Stuttgard, Germany, 2017. [Google Scholar]
- Ter-Mikaelian, M.; Yapa, W.B.; Rübsamen, R. Vocal Behavior of the Mongolian Gerbil in a Seminatural Enclosure. Behaviour 2012, 149, 461–492. [Google Scholar]
- Kobayasi, K.I.; Riquimaroux, H. Classification of Vocalizations in the Mongolian Gerbil, Meriones unguiculatus. J. Acoust. Soc. Am. 2012, 131, 1622–1631. [Google Scholar] [CrossRef]
- De Ghett, V.J. Developmental Changes in the Rate of Ultrasonic Vocalization in the Mongolian Gerbil. Dev. Psychobiol. 1974, 7, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Holman, S.D.; Seale, W.T.C. Ontogeny of Sexually Dimorphic Ultrasonic Vocalizations in Mongolian Gerbils. Dev. Psychobiol. 1991, 24, 103–115. [Google Scholar] [CrossRef]
- Holman, S.D. Sexually Dimorphic, Ultrasonic Vocalizations of Mongolian Gerbils. Behav. Neural Biol. 1980, 28, 183–192. [Google Scholar] [CrossRef]
- Holman, S.D.; Seale, W.T.; Hutchison, J.B. Ultrasonic Vocalizations in Immature Gerbils: Emission Rate and Structural Changes after Neonatal Exposure to Androgen. Physiol. Behav. 1995, 57, 451–460. [Google Scholar] [CrossRef]
- Zaytseva, A.S.; Volodin, I.A.; Ilchenko, O.G.; Volodina, E.V. Discomfort-Related Changes in Pup Ultrasonic Calls of Fat-Tailed Gerbils Pachyuromys duprasi. Bioacoustics 2017, 26, 1–13. [Google Scholar] [CrossRef]
- Zaytseva, A.S.; Volodin, I.A.; Ilchenko, O.G.; Volodina, E.V. Ultrasonic Vocalization of Pup and Adult Fat-Tailed Gerbils (Pachyuromys duprasi). PLoS ONE 2019, 14, e0219749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Vargas, M.; Riede, T.; Pasch, B. Mechanisms and Constraints Underlying Acoustic Variation in Rodents. Anim. Behav. 2021, 184, 135–147. [Google Scholar] [CrossRef]
- Pasch, B.; Tokuda, I.T.; Riede, T. Grasshopper Mice Employ Distinct Vocal Production Mechanisms in Different Social Contexts. P. Roy. Soc. B-Biol. Sci. 2017, 284, 20171158. [Google Scholar] [CrossRef] [PubMed]
- Volodin, I.A.; Zaytseva, A.S.; Ilchenko, O.G.; Volodina, E.V. Small Mammals Ignore Common Rules: A Comparison of Vocal Repertoires and the Acoustics between Pup and Adult Piebald Shrews Diplomesodon Pulchellum. Ethology 2015, 121, 103–115. [Google Scholar] [CrossRef]
- Boersma, P. Praat, a System for Doing Phonetics by Computer. Glot Int. 2001, 5, 341–345. [Google Scholar]
- Owren, M.J. Gsu Praat Tools: Scripts for Modifying and Analyzing Sounds Using Praat Acoustics Software. Behav. Res. Methods 2008, 40, 822–829. [Google Scholar] [CrossRef] [Green Version]
- Beckers, G.J.; Nelson, B.S.; Suthers, R.A. Vocal-Tract Filtering by Lingual Articulation in a Parrot. Curr. Biol. 2004, 14, 1592–1597. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, L.; Gamba, M.; Giacoma, C. The Use of Artificial Neural Networks to Classify Primate Vocalizations: A Pilot Study on Black Lemurs. Am. J. Primatol. 2010, 72, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Romero-Mujalli, D.; Bergmann, T.; Zimmermann, A.; Scheumann, M. Utilizing Deepsqueak for Automatic Detection and Classification of Mammalian Vocalizations: A Case Study on Primate Vocalizations. Sci. Rep. 2021, 11, 24463. [Google Scholar] [CrossRef]
- Valente, D.; De Gregorio, C.; Torti, V.; Miaretsoa, L.; Friard, O.; Randrianarison, R.M.; Giacoma, C.; Gamba, M. Finding Meanings in Low Dimensional Structures: Stochastic Neighbor Embedding Applied to the Analysis of Indri Indri Vocal Repertoire. Animals 2019, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.F.; Leno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Haccou, P.; Melis, E. Statistical Analysis of Behavioural Data; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Mundry, R.; Sommer, C. Discriminant Function Analysis with Nonindependent Data: Consequences and an Alternative. Anim. Behav. 2007, 74, 965–976. [Google Scholar] [CrossRef]
- Hashimoto, H.; Saito, T.R.; Moritani, N.; Komeda, K.; Takahashi, K.W. Comparative Study on Isolation Calls Emitted from Hamster Pups. Exp. Anim. 2001, 50, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Jourjine, N.; Woolfolk, M.L.; Sanguinetti-Scheck, J.I.; Sabatini, J.E.; McFadden, S.; Lindholm, A.K.; Hoekstra, H.E. Two Pup Vocalization Types Are Genetically and Functionally Separable in Deer Mice. Curr. Biol. 2023, 33, 1237–1248.e4. [Google Scholar] [CrossRef]
- Motomura, N.; Shimizu, K.; Shimizu, M.; Aoki-Komori, S.; Taniguchi, K.; Serizawa, I.; Saito, T.R. A Comparative Study of Isolation-Induced Ultrasonic Vocalization in Rodent Pups. Exp. Anim. 2002, 51, 187–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckman, J.; McGuinness, B.; Celikel, T.; Englitz, B. Determinants of the Mouse Ultrasonic Vocal Structure and Repertoire. Neurosci. Biobehav. Rev. 2016, 65, 313–325. [Google Scholar] [CrossRef]
- Eugene, S.M. On the Occurrence and Significance of Motivation-Structural Rules in Some Bird and Mammal Sounds. Am. Nat. 1977, 111, 855–869. [Google Scholar]
- Elwood, R.W. Ultrasounds and Maternal Behavior in the Mongolian Gerbil. Dev. Psychobiol. 1979, 12, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Kleese, D.; Hull, E. Adult Responsiveness to Ultrasonic Signals from Gerbils of Varying Ages: Parity, Gender, and Housing Effects. Dev. Psychobiol. 1980, 13, 233–241. [Google Scholar] [CrossRef]
- Monticelli, P.F.; Tokumaru, R.S.; Ades, C. Isolation Induced Changes in Guinea Pig Cavia Porcellus Pup Distress Whistles. Acad. Bras. Cienc. 2004, 74, 368–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Roux, A.; Jackson, T.P.; Cherry, M.I. Does Brant’s Whistling Rat, (Parotomys brantsii) Use an Urgency-Based Alarm System in Reaction to Arial and Terrestrial Predators? Behaviour 2001, 138, 757–773. [Google Scholar] [CrossRef]
- Norcross, J.L.; Newman, J.D.; Cofrancesco, L.M. Context and Sex Differences Exist in the Acoustic Structure of Phee Calls by Newly-Paired Common Marmosets (Callithrix jacchus). Am. J. Primatol. 1999, 49, 165–181. [Google Scholar] [CrossRef]
- Schehka, S.; Zimmermann, E. Acoustic Features to Arousal and Identity in Disturbance Calls of Tree Shrews (Tupaia Belangeri). Behav. Brain Res. 2009, 203, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Manser, M.B. The Acoustic Structure of Suricates’ Alarm Calls Varies with Predator Type and the Level of Response Urgency. P. Roy. Soc. B-Biol. Sci. 2001, 268, 2315–2324. [Google Scholar] [CrossRef]
- Sales, G.D.; Smith, J.C. Comparative Studies of the Ultrasonic Calls of Infant Murid Rodents. Dev. Psychobiol. 1978, 11, 595–619. [Google Scholar] [CrossRef] [PubMed]
- Klenova, A.V.; Volodin, I.A.; Ilchenko, O.G.; Volodina, E.V. Discomfort-Related Changes of Call Rate and Acoustic Variables of Ultrasonic Vocalizations in Adult Yellow Steppe Lemmings Eolagurus luteus. Sci. Rep. 2021, 11, 14969. [Google Scholar] [CrossRef]
- Matrosova, V.A.; Volodin, I.A.; Volodina, E.V.; Babitsky, A.F. Pups Crying Bass: Vocal Adaptation for Avoidance of Age-Dependent Predation Risk in Ground Squirrels? Behav. Ecol. Sociobiol. 2007, 62, 181–191. [Google Scholar] [CrossRef]
- Swan, D.C.; Hare, J.F. Signaler and Receiver Ages Do Not Affect Responses to Richardson’s Ground Squirrel Alarm Calls. J. Mammal. 2008, 89, 889–894. [Google Scholar] [CrossRef]
- Weary, D.M.; Fraser, D. Vocal Response of Piglets to Weaning: Effect of Piglet Age. Appl. Anim. Behav. Sci. 1997, 54, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Yurlova, D.D.; Volodin, I.A.; Ilchenko, O.G.; Volodina, E.V. Rapid Development of Mature Vocal Patterns of Ultrasonic Calls in a Fast-Growing Rodent, the Yellow Steppe Lemming (Eolagurus Luteus). PLoS ONE 2020, 15, e0228892. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.J.; Yang, L.L.; Han, J.B.; Yang, Y.; Lu, Z.C.; Li, S.H. Age and Sex Differences in in-Air Vocalization Characteristics of Spotted Seal Pups from Newborn to 1 Year Old in Captivity. Front. Mar. Sci. 2022, 9, 943030. [Google Scholar] [CrossRef]
- Briefer, E.F.; Vannoni, E.; McElligott, A.G. Quality Prevails over Identity in the Sexually Selected Vocalisations of an Ageing Mammal. BMC Biol. 2010, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, F.; Musolf, K.; Penn, D.J. Spectrographic Analyses Reveal Signals of Individuality and Kinship in the Ultrasonic Courtship Vocalizations of Wild House Mice. Physiol. Behav. 2012, 105, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Holman, S.D.; Hutchison, J.B. Effects of Intracranial Androgen on the Development of Masculine Ultrasonic Vocalizations in the Mongolian Gerbil (Meriones unguiculatus). J. Endocrinol. 1985, 107, 355–363. [Google Scholar] [CrossRef]
- Fernandez-Vargas, M.; Johnston, R.E. Ultrasonic Vocalizations in Golden Hamsters (Mesocricetus Auratus) Reveal Modest Sex Differences and Nonlinear Signals of Sexual Motivation. PLoS ONE 2015, 10, e0116789. [Google Scholar] [CrossRef] [Green Version]
- Lenell, C.; Broadfoot, C.K.; Schaen-Heacock, N.E.; Ciucci, M.R. Biological and Acoustic Sex Differences in Rat Ultrasonic Vocalization. Brain Sci. 2021, 11, 459. [Google Scholar] [CrossRef]
- Kikusui, T.; Sonobe, M.; Yoshida, Y.; Nagasawa, M.; Ey, E.; de Chaumont, F.; Bourgeron, T.; Nomoto, K.; Mogi, K. Testosterone Increases the Emission of Ultrasonic Vocalizations with Different Acoustic Characteristics in Mice. Front. Psychol. 2021, 12, 680176. [Google Scholar] [CrossRef]
- Bell, M.R. Comparing Postnatal Development of Gonadal Hormones and Associated Social Behaviors in Rats, Mice, and Humans. Endocrinology 2018, 159, 2596–2613. [Google Scholar] [CrossRef]
- Vannoni, E.; McElligott, A.G. Low Frequency Groans Indicate Larger and More Dominant Fallow Deer (Dama dama) Males. PloS ONE 2008, 3, e3113. [Google Scholar] [CrossRef] [Green Version]
- Wyman, M.T.; Mooring, M.S.; McCowan, B.; Penedo, M.C.T.; Reby, D.; Hart, L.A. Acoustic Cues to Size and Quality in the Vocalizations of Male North American Bison, Bison Bison. Anim. Behav. 2012, 84, 1381–1391. [Google Scholar] [CrossRef]
- Ritz, J. Sind Drummings in Mongolischen Rennmäusen (Meriones unguiculatus) Kontext-Abhängig? Bachelor’s Thesis, University of Veterinary Medicine Hannover, Hannover, Germany, 2021. [Google Scholar]
- Shapiro, L.E.; Insel, T.R. Infants Response to Social Separation Reflects Adult Differences in Affiliative Behavior—A Comparative Developmental Study in Prairie and Montane Voles. Dev. Psychobiol. 1990, 23, 375–393. [Google Scholar] [CrossRef] [Green Version]
- Stowe, J.R.; Liu, Y.; Curtis, J.T.; Freeman, M.E.; Wang, Z.X. Species Differences in Anxiety-Related Responses in Male Prairie and Meadow Voles: The Effects of Social Isolation. Physiol. Behav. 2005, 86, 369–378. [Google Scholar] [CrossRef]
- Kubke, M.F.; Wild, J. Martin. Anatomy of Vocal Communication and Hearing in Rodents. In Rodent Bioacoustics; Dent Micheal, L., Fay Richard, R., Popper Arthur, N., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 131–164. [Google Scholar]






| Acoustic Parameter | Abb. | Definition |
|---|---|---|
| Time-related parameters | ||
| Call duration [ms] | Dur | Time between the onset and the offset of a call. |
| Time of minimum fundamental frequency [ms] | TimeminF0 | Time between the onset and the time point of minimum fundamental frequency of a call. |
| Time of maximum fundamental frequency [ms] | TimemaxF0 | Time between the onset and the time point of maximum fundamental frequency of a call. |
| Source-related parameters | ||
| Minimum fundamental frequency [kHz] | MinF0 | Lowest value of the fundamental frequency across all time frames of a call. |
| Maximum fundamental frequency [kHz] | MaxF0 | Highest value of the fundamental frequency across all time frames of a call. |
| Bandwidth [kHz] | BandF0 | MaxF0–MinF0. |
| Mean fundamental frequency [kHz] | MeanF0 | Mean fundamental frequency of a call calculated across all time frames of a call. |
| Standard deviation of fundamental frequency [kHz] | SDF0 | Standard deviation of the fundamental frequency of a call calculated across all time frames of a call. |
| Meanslope [kHz/s] | SlopeF0 | Mean absolute slope of the fundamental frequency calculated as the sum of the absolute difference of the F0 of two consecutive time frames. |
| Filter-related parameters | ||
| Center of gravity [kHz] | CoG | Mean frequency of the spectrum of a call weighted by the amplitude of a call. |
| Standard deviation of CoG [kHz] | SD | Standard deviation of the CoG measuring the deviation of frequency values from the CoG of a call. |
| Skewness | Ske | Difference between the spectral distribution below and above the CoG of a call. |
| Kurtosis | Kur | Difference between the spectral distribution around the CoG from a Gaussian distribution of a call. |
| Tonality-related parameters | ||
| Voiced percentage [%] | Voiced | Percentage of voiced time frames of a call. |
| Harmonics-to-noise-ratio [dB] | Hnr | Ratio between the periodic (harmonic part) and aperiodic (noise) components of a call. |
| Wiener entropy [dB] | Entropy | Ratio of geometric to arithmetic energy of a call. |
| Acoustic Parameter | Cluster I (USV-ADV) | Cluster II (USV) | Cluster III (ADV) |
|---|---|---|---|
| Time-related parameters | |||
| Dur [ms] | 109.0 ± 31.1 | 89.1 ± 32.3 | 93.6 ± 65.1 |
| TimeminF0 [ms] | 101.0 ± 31.0 | 9.6 ± 12.0 | 77.5 ± 63.7 |
| TimemaxF0 [ms] | 72.8 ± 27.7 | 81.6 ± 32.2 | 14.6 ± 25.5 |
| Source-related parameters | |||
| MinF0 [kHz] | 4.6 ± 4.0 | 36.5 ± 3.3 | 6.1 ± 4.7 |
| MaxF0 [kHz] | 51.9 ± 4.3 | 49.3 ± 5.0 | 11.3 ± 5.8 |
| BandF0 [kHz] | 47.3 ± 5.7 | 12.9 ± 4.4 | 5.2 ± 4.0 |
| MeanF0 [kHz] | 38.3 ± 6.3 | 42.7 ± 3.4 | 8.5 ± 4.8 |
| SDF0 [kHz] | 15.6 ± 3.9 | 3.4 ± 1.3 | 1.6 ± 1.2 |
| SlopeF0 [kHz/s] | 758.7 ± 282.3 | 382.8 ± 177.5 | 98.5 ± 57.3 |
| Filter-related parameters | |||
| CoG [kHz] | 34.7 ± 10.5 | 44.0 ± 3.6 | 10.8 ± 6.5 |
| SD [kHz] | 15.7 ± 5.7 | 5.5 ± 2.4 | 9.1 ± 2.6 |
| Ske | −0.9 ± 1.8 | 4.5 ± 2.7 | 2.8 ± 1.7 |
| Kur | 5.4 ± 11.6 | 46.7 ± 53.4 | 10.8 ± 13.6 |
| Tonality-related parameters | |||
| Voiced [%] | 89.2 ± 13.8 | 94.5 ± 6.8 | 85.6 ± 19.2 |
| Hnr [dB] | 8.2 ± 5.9 | 12.5 ± 3.0 | 8.7 ± 4.5 |
| Entropy [dB] | −4.7 ± 1.3 | −0.8 ± 0.9 | −6.2 ± 1.4 |
| Call Occurrence | Call Rate | |||||
|---|---|---|---|---|---|---|
| Predictors | χ2 | df | p-Value | χ2 | df | p-Value |
| USV | ||||||
| Age group | 35.36 | 3 | <0.001 | 100.57 | 3 | <0.001 |
| Arousal | 3.59 | 1 | 0.058 | 0.71 | 1 | 0.399 |
| Sex | 0.07 | 1 | 0.788 | 0.22 | 1 | 0.641 |
| Order | 0.02 | 1 | 0.883 | 0.02 | 1 | 0.887 |
| Age group * Sex | 10.68 | 3 | 0.014 | |||
| ADV | ||||||
| Age group | 6.48 | 3 | 0.090 | 17.43 | 3 | <0.001 |
| Arousal | 10.33 | 1 | 0.001 | 6.24 | 1 | 0.013 |
| Sex | 0.55 | 1 | 0.458 | 1.51 | 1 | 0.220 |
| Order | 0.33 | 1 | 0.566 | 0.31 | 1 | 0.577 |
| Age group * Arousal | 14.48 | 3 | 0.002 | |||
| USV-ADV | ||||||
| Age group | 10.82 | 3 | 0.013 | 11.33 | 3 | 0.010 |
| Arousal | 5.43 | 1 | 0.020 | 0.003 | 1 | 0.960 |
| Sex | 1.26 | 1 | 0.263 | 1.22 | 1 | 0.269 |
| Order | 0.06 | 1 | 0.809 | 1.32 | 1 | 0.250 |
| Age group * Sex | 7.92 | 3 | 0.048 | |||
| Parameters | Arousal | Body Weight | Age Group | Identity | Sex | ||||
|---|---|---|---|---|---|---|---|---|---|
| USV | USV | ADV | USV | ADV | USV | ADV | USV | ADV | |
| Time-related parameters | |||||||||
| Duration | L > H | ↓ | ↑ | ↓ | ↑ | + | + | ||
| TimeminF0 | L > H | ↑ | ↑ | + | + | ||||
| TimemaxF0 | ↓ | ↓ | + | + | F > M | ||||
| Source-related parameters | |||||||||
| MinF0 | L < H | ↓ | ↓ | + | + | F > M | |||
| MaxF0 | L < H | ↓ | ↓↑ | ↑ | + | + | |||
| BandF0 | ↓↑ | ↑ | + | + | F < M | ||||
| MeanF0 | L < H | ↓ | ↓ | + | + | F > M | |||
| SDF0 | L < H | ↑ | ↓↑ | ↑ | + | + | |||
| SlopeF0 | ↑ | ↑ | + | + | |||||
| Filter-related parameters | |||||||||
| CoG | L < H | ↓ | ↓ | + | + | F > M | |||
| SD | L > H | ↑ | + | + | |||||
| Ske | L > H | ↓ | ↓ | ↓ | + | + | |||
| Kur | + | + | |||||||
| Tonality-related parameters | |||||||||
| Voiced | ↓ | ↑ | ↓ | + | + | ||||
| Hnr | L < H | ↑ | + | + | |||||
| Entropy | L > H | + | + | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silberstein, Y.; Felmy, F.; Scheumann, M. Encoding of Arousal and Physical Characteristics in Audible and Ultrasonic Vocalizations of Mongolian Gerbil Pups Testing Common Rules for Mammals. Animals 2023, 13, 2553. https://doi.org/10.3390/ani13162553
Silberstein Y, Felmy F, Scheumann M. Encoding of Arousal and Physical Characteristics in Audible and Ultrasonic Vocalizations of Mongolian Gerbil Pups Testing Common Rules for Mammals. Animals. 2023; 13(16):2553. https://doi.org/10.3390/ani13162553
Chicago/Turabian StyleSilberstein, Yara, Felix Felmy, and Marina Scheumann. 2023. "Encoding of Arousal and Physical Characteristics in Audible and Ultrasonic Vocalizations of Mongolian Gerbil Pups Testing Common Rules for Mammals" Animals 13, no. 16: 2553. https://doi.org/10.3390/ani13162553




