Postmortem Metabolism and Pork Quality Development Are Affected by Electrical Stimulation across Three Genetic Lines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Animals and Experimental Design
2.2. Muscle Sampling
2.3. Temperature and pH
2.4. Color and Water-Holding Capacity
2.5. Metabolite Analysis
2.6. Myosin Heavy Chain Isoform Abundance Analysis
2.6.1. Myosin Extraction
2.6.2. ELISA Protocol
2.7. Statistical Analyses
3. Results
3.1. Myosin Heavy Chain Abundance (MyHC)
3.2. Metabolites
3.3. Meat Quality Traits
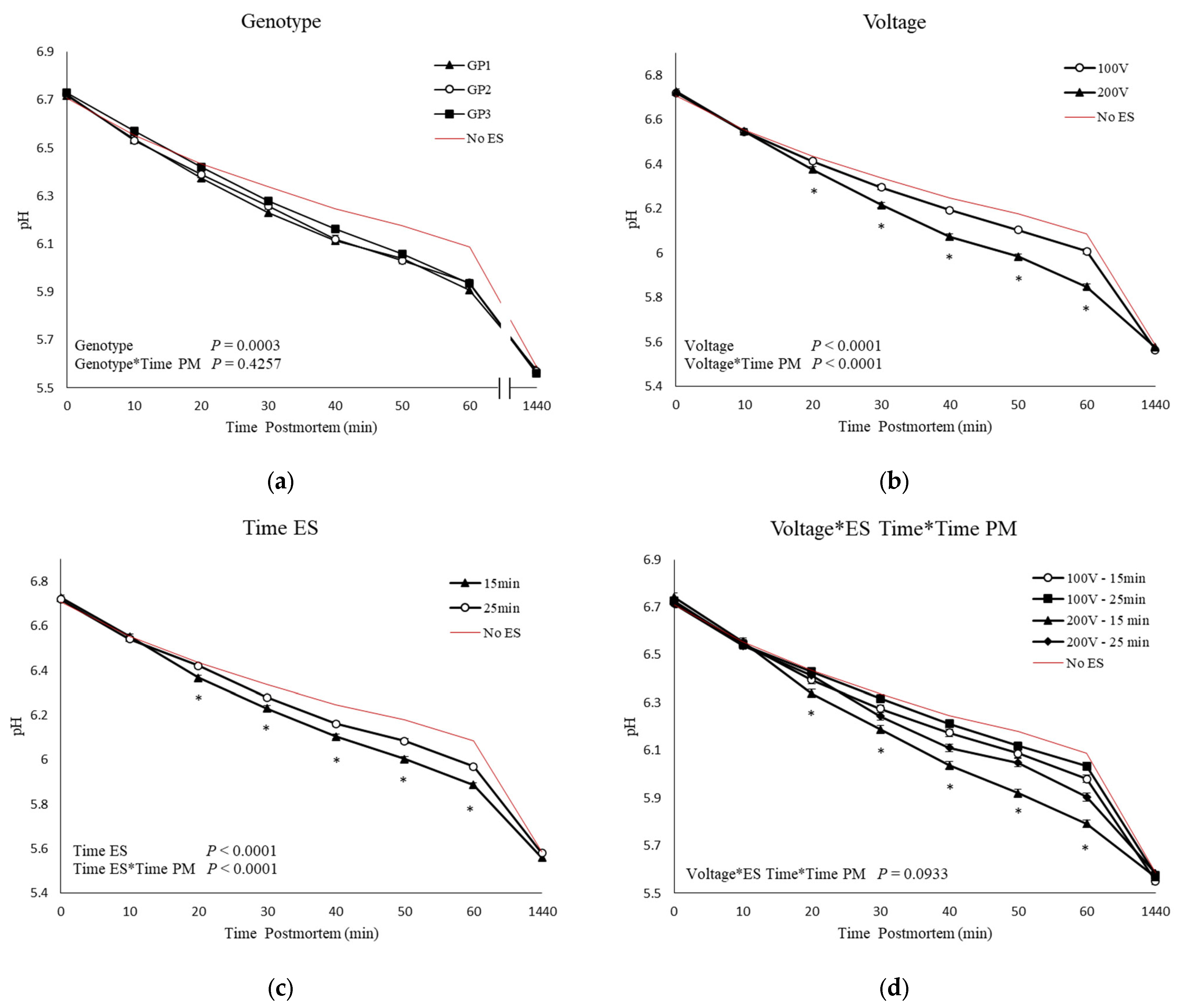
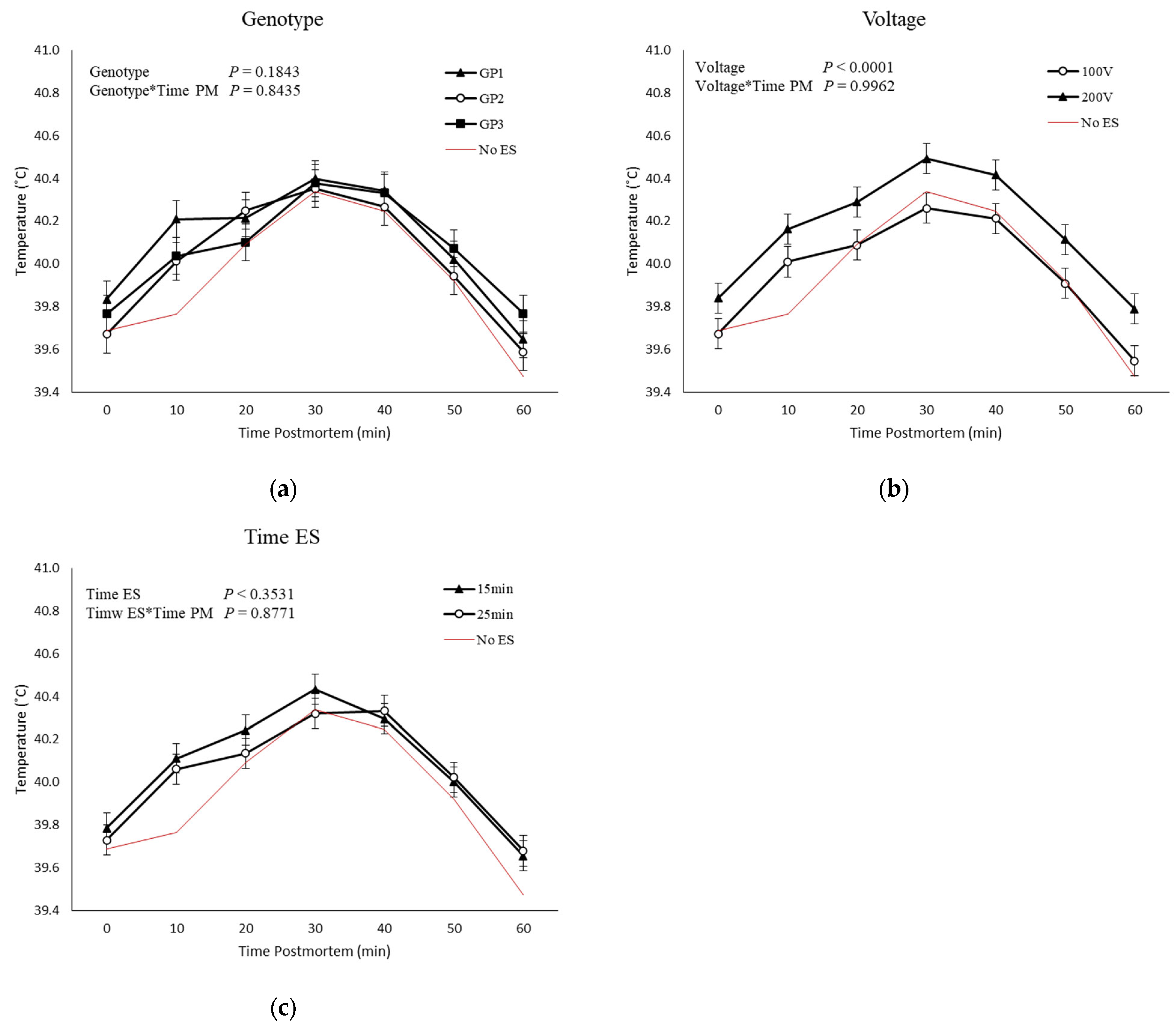
3.4. pH and Temperature
3.5. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duong, C.; Sung, B.; Lee, S.; Easton, J. Assessing Australian Consumer Preferences for Fresh Pork Meat Attributes: A Best-Worst Approach on 46 Attributes. Meat Sci. 2022, 193, 108954. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, S.W.; Weaver, M.A.; Lee, C.Y. Increasing the Pig Market Weight: World Trends, Expected Consequences and Practical Considerations. Asian Aust. J. Anim. Sci. 2005, 18, 590–600. [Google Scholar] [CrossRef]
- Poklukar, K.; Čandek-Potokar, M.; Lukač, N.B.; Tomažin, U.; Škrlep, M. Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review. Animals 2020, 10, 424. [Google Scholar] [CrossRef]
- Bowker, B.C.; Grant, A.L.; Forrest, J.C.; Gerrard, D.E. Muscle Metabolism and PSE Pork. J. Anim. Sci. 2000, 79, 1–8. [Google Scholar] [CrossRef]
- Barbut, S.; Sosnicki, A.A.; Lonergan, S.M.; Knapp, T.; Ciobanu, D.C.; Gatcliffe, L.J.; Huff-Lonergan, E.; Wilson, E.W. Progress in Reducing the Pale, Soft and Exudative (PSE) Problem in Pork and Poultry Meat. Meat Sci. 2008, 79, 46–63. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of Water-Holding Capacity of Meat: The Role of Postmortem Biochemical and Structural Changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Offer, G.; Knight, P. Development in Meat Science; Elsevier Science: London, UK, 1988; Volume 4. [Google Scholar]
- Melody, J.L.; Lonergan, S.M.; Rowe, L.J.; Huiatt, T.W.; Mayes, M.S.; Huff-Lonergan, E. Early Postmortem Biochemical Factors Influence Tenderness and Water-Holding Capacity of Three Porcine Muscles. J. Anim. Sci. 2004, 82, 1195–1205. [Google Scholar] [CrossRef]
- Dalrymple, R.H.; Hamm, R. Postmortem Glycolysis in Ground Skeletal Muscle as Influenced by Prerigor Freezing and Subsequent Thawing. Z. Leb. Unters. Forsch. 1975, 158, 333–339. [Google Scholar] [CrossRef]
- Monin, G.; Sellier, P. Pork of Low Technological Quality with a Normal Rate of Muscle PH Fall in the Immediate Post-Mortem Period: The Case of the Hampshire Breed. Meat Sci. 1985, 13, 49–63. [Google Scholar] [CrossRef]
- Sayre, R.N.; Briskey, E.J.; Hoekstra, W.G. Comparison of Muscle Characteristics and Post-Mortem Glycolysis in Three Breeds of Swine. J. Anim. Sci. 1963, 22, 1012–1020. [Google Scholar] [CrossRef]
- Scheffler, T.L.; Matarneh, S.K.; England, E.M.; Gerrard, D.E. Mitochondria Influence Postmortem Metabolism and PH in an in Vitro Model. Meat Sci. 2015, 110, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, T.L.; Gerrard, D.E. Mechanisms Controlling Pork Quality Development: The Biochemistry Controlling Postmortem Energy Metabolism. Meat Sci. 2007, 77, 7–16. [Google Scholar] [CrossRef]
- Bodwell, C.E.; Pearson, A.M.; Wismer-Pedersen, J.; Bratzler, L.J. Post-Mortem Changes in Muscle II. Chemical and Physical Changes in Pork. J. Food Sci. 1966, 31, 1–12. [Google Scholar] [CrossRef]
- Wismer-Pedersen, J.; Briskey, E.J. Rate of Anaerobic Glycolysis versus Structure in Pork Muscle. Nature 1961, 189, 318–320. [Google Scholar] [CrossRef]
- Bendall, J.R.; Wismer-Pedersen, J. Some Properties of the Fibrillar Proteins of Normal and Watery Pork Muscle. J. Food Sci. 1962, 27, 144–159. [Google Scholar] [CrossRef]
- Bowker, B.C.; Wynveen, E.J.; Grant, A.L.; Gerrard, D.E. Effects of Electrical Stimulation on Early Postmortem Muscle PH and Temperature Declines in Pigs from Different Genetic Lines and Halothane Genotypes. Meat Sci. 1999, 53, 125–133. [Google Scholar] [CrossRef]
- Chrystall, B.B.; Devine, G.E. Electrical Stimulation, Muscle Tension and Glycolysis in Bovine Sternomandibularis. Meat Sci. 1978, 2, 49–58. [Google Scholar] [CrossRef]
- NPPC. Procedures to Evaluate Market Hogs, 3rd ed.; National Pork Producers Council: Des Moines, IA, USA, 1991. [Google Scholar]
- CIE. Colorimetry, 2nd ed.; International Commission on Illumination: Vienna, Austria, 1986. [Google Scholar]
- Rasmussen, A.; Stouffer, J.R. New Method for Determination of Drip Loss in Pork Muscles. In Proceedings of the 42nd International Congress of Meat Science and Technology, Lillehammer, Norway, 1–6 September 1996; pp. 286–287. [Google Scholar]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 2nd ed.; Academic Press: New York, NY, USA, 1974; Volume 2. [Google Scholar]
- Bär, A.; Pette, D. Three Fast Myosin Heavy Chains in Adult Rat Skeletal Muscle. FEBS Lett. 1988, 235, 153–155. [Google Scholar] [CrossRef]
- Depreux, F.F.S.; Okamura, C.S.; Swartz, D.R.; Grant, A.L.; Brandstetter, A.M.; Gerrard, D.E. Quantification of Myosin Heavy Chain Isoform in Porcine Muscle Using an Enzyme-Linked Immunosorbent Assay. Meat Sci. 2000, 56, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, T.L.; Scheffler, J.M.; Kasten, S.C.; Sosnicki, A.A.; Gerrard, D.E. High Glycolytic Potential Does Not Predict Low Ultimate PH in Pork. Meat Sci. 2013, 95, 85–91. [Google Scholar] [CrossRef]
- England, E.M.; Matarneh, S.K.; Scheffler, T.L.; Wachet, C.; Gerrard, D.E. PH Inactivation of Phosphofructokinase Arrests Postmortem Glycolysis. Meat Sci. 2014, 98, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Rosenvold, K.; Andersen, H.J. Factors of Significance for Pork Quality—A Review. Meat Sci. 2003, 64, 219–237. [Google Scholar] [CrossRef]
- Knox, B.L.; van Laack, R.L.J.M.; Davidson, P.M. Relationships between Ultimate PH and Microbial, Chemical, and Physical Characteristics of Vacuum-Packaged Pork Loins. J. Food Sci. 2008, 73, M104–M110. [Google Scholar] [CrossRef] [PubMed]
- Kastenschimidt, L.L.; Briskey, E.J.; Hoesktra, W.G. Metabolic Intermediates in Skeletal Muscles with Fast and Slow Rates of Post-Mortem Glycolysis. Nature 1966, 212, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Wismer-Pedersen, J.; Briskey, E.J. Relationship of Post-Mortem Acidity and Temperature. Food Technol. 1961, 15, 232. [Google Scholar]
- Warriss, P.D.; Brown, S.N. The Relationships between Initial PH, Reflectance and Exudation in Pig Muscle. Meat Sci. 1987, 20, 65–74. [Google Scholar] [CrossRef]
- Monin, G.; Mejenes-Quijano, A.; Talmant, A.; Sellier, P. Influence of Breed and Muscle Metabolic Type on Muscle Glycolytic Potential and Meat pH in Pigs. Meat Sci. 1987, 20, 149–158. [Google Scholar] [CrossRef]
- Joo, S.T.; Kauffman, R.G.; Kim, B.C.; Park, G.B. The Relationship of Sarcoplasmic and Myofibrillar Protein Solubility to Colour and Water-Holding Capacity in Porcine Longissimus Muscle. Meat Sci. 1999, 52, 291–297. [Google Scholar] [CrossRef]
- Enfält, A.-C.; Lundström, K.; Hansson, I.; Johansen, S.; Nyström, P.-E. Comparison of Non-Carriers and Heterozygous Carriers of the RN− Allele for Carcass Composition, Muscle Distribution and Technological Meat Quality in Hampshire-Sired Pigs. Livest. Prod. Sci. 1997, 47, 221–229. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Myosin Isoforms in Mammalian Skeletal Muscle. J. Appl. Physiol. 1994, 77, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, L.; Hoffman, R.K.; Gerrard, D.E.; Okamura, C.S.; Rubinstein, N.; Kelly, A. Evidence for Three Adult Fast Myosin Heavy Chain Isoforms in Type II Skeletal Muscle Fibers in Pigs. J. Anim. Sci. 1998, 76, 1584. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ahn, C.-H.; Kim, G.-D. Muscle Fiber Typing in Bovine and Porcine Skeletal Muscles Using Immunofluorescence with Monoclonal Antibodies Specific to Myosin Heavy Chain Isoforms. Food Sci. Anim. Resour. 2020, 40, 132–144. [Google Scholar] [CrossRef]
- Chauhan, S.S.; LeMaster, M.N.; Clark, D.L.; Foster, M.K.; Miller, C.E.; England, E.M. Glycolysis and PH Decline Terminate Prematurely in Oxidative Muscles despite the Presence of Excess Glycogen. Meat Muscle Biol. 2019, 3, 254. [Google Scholar] [CrossRef]
- Ferguson, D.M.; Gerrard, D.E. Regulation of Post-Mortem Glycolysis in Ruminant Muscle. Anim. Prod. Sci. 2014, 54, 464–481. [Google Scholar] [CrossRef]
- Bowker, B.C.; Grant, A.L.; Swartz, D.R.; Gerrard, D.E. Myosin Heavy Chain Isoforms Influence Myofibrillar ATPase Activity under Simulated Postmortem PH, Calcium, and Temperature Conditions. Meat Sci. 2004, 67, 139–147. [Google Scholar] [CrossRef]
- England, E.M.; Scheffler, T.L.; Kasten, S.C.; Matarneh, S.K.; Gerrard, D.E. Exploring the Unknowns Involved in the Transformation of Muscle to Meat. Meat Sci. 2013, 95, 837–843. [Google Scholar] [CrossRef]
- Choi, Y.M.; Nam, K.W.; Choe, J.H.; Ryu, Y.C.; Wick, M.P.; Lee, K.; Kim, B.C. Growth, Carcass, Fiber Type, and Meat Quality Characteristics in Large White Pigs with Different Live Weights. Livest. Sci. 2013, 155, 123–129. [Google Scholar] [CrossRef]
- Wang, Y.; Thakali, K.; Morse, P.; Shelby, S.; Chen, J.; Apple, J.; Huang, Y. Comparison of Growth Performance and Meat Quality Traits of Commercial Cross-Bred Pigs versus the Large Black Pig Breed. Animals 2021, 11, 200. [Google Scholar] [CrossRef]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of Fresh Meat Quality through Manipulation of Muscle Fiber Characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef]
- Hammelman, J.E.; Bowker, B.C.; Grant, A.L.; Forrest, J.C.; Schinckel, A.P.; Gerrard, D.E. Early Postmortem Electrical Stimulation Simulates PSE Pork Development. Meat Sci. 2003, 63, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wicks, J.C.; Zumbaugh, M.D.; Daniels, R.P.; Matarneh, S.K.; Venhuizen, M.D.; Elgin, J.; Bodmer, J.; Yen, C.N.; Beline, M.; Shi, H.; et al. Time of Dehairing Alters Pork Quality Development. Meat Sci. 2023, 203, 109233. [Google Scholar] [CrossRef] [PubMed]
- Westervelt, R.G.; Stouffer, J.R. Relationships among Spinal Cord Severing, Electrical Stimulation and Postmortem Quality Characteristics of the Porcine Carcass. J. Anim. Sci. 1978, 46, 1206–1211. [Google Scholar] [CrossRef]
- Taylor, A.A.; Martoccia, L. The Effect of Low Voltage and High Voltage electrical Stimulation on Pork Quality. Meat Sci. 1995, 39, 319–326. [Google Scholar] [CrossRef]
- Gigiel, A.J.; James, S.J. Electrical Stimulation and Ultra-Rapid Chilling of Pork. Meat Sci. 1984, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scopes, R.K. Studies with a Reconstituted Muscle Glycolytic System. The Rate and Extent of Glycolysis in Simulated Post Mortem Conditions. Biochem. J. 1974, 142, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Scopes, R.K. Studies with a Reconstituted Muscle Glycolytic System. The Rate and Extent of Creatine Phosphorylation by Anaerobic Glycolysis. Biochem. J. 1973, 134, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kastenschmidt, L.L.; Hoekstra, W.G.; Briskey, E.J. Glycolytic Intermediates and Co-Factors in “Fast-” and “Slow-Glycolyzing” Muscles of the Pig. J. Food Sci. 1968, 33, 151–158. [Google Scholar] [CrossRef]
- Morgan, H.E.; Parmeggiani, A. Regulation of Glycogenolysis in Muscle. J. Biol. Chem. 1964, 239, 2440–2445. [Google Scholar] [CrossRef] [PubMed]
- Hamm, R. Postmortem breakdown of ATP and glycogen in ground muscle: A review. Meat Sci. 1977, 1, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Maribo, H.; Ertbjerg, P.; Andersson, M.; Barton-Gade, P.; Møller, A.J. Electrical Stimulation of Pigs—Effect on PH Fall, Meat Quality and Cathepsin B+L Activity. Meat Sci. 1999, 52, 179–187. [Google Scholar] [CrossRef] [PubMed]
- van Laack, R.L.; Kauffman, R.G. Glycolytic Potential of Red, Soft, Exudative Pork Longissimus Muscle. J. Anim. Sci. 1999, 77, 2971. [Google Scholar] [CrossRef] [PubMed]
- Crenwelge, D.D.; Terrell, R.N.; Dutson, T.R.; Smith, G.C.; Carpenter, Z.L. Effects of Time Postmortem of Electrical Stimulation and Postmortem Chilling Method on Pork Quality and Palatability Traits. J. Food Sci. 1984, 49, 294–295. [Google Scholar] [CrossRef]
| GPs Characteristics | Genotypes | ||
|---|---|---|---|
| GP1 | GP2 | GP3 | |
| Start wt, kg b | 29.2 ± 0.40 x | 24.0 ± 0.40 y | 20.7 ± 0.40 z |
| Slaughter wt., kg b | 112.7 ± 0.69 y | 114.3 ± 0.68 xy | 115.5 ± 0.69 x |
| Slaughter age, day b | 167 ± 1.36 y | 178 ± 1.35 x | 166 ± 1.36 y |
| Average daily gain, kg/day b | 0.73 ± 0.01 y | 0.75 ± 0.01 y | 0.83 ± 0.01 x |
| 10th rib LMA, cm2 c | 44.8 ± 0.79 x | 43.2 ± 0.88 y | 45.9 ± 0.77 x |
| 10th rib backfat, mm c | 18.8 ± 0.64 y | 22.6 ± 0.72 x | 23.2 ± 0.63 x |
| Last rib fat depth, mm c | 22.9 ± 0.93 y | 24.6 ± 1.0 x | 26.2 ± 0.91 x |
| Calculated % lean c | 54.7 ± 0.43 x | 52.7 ± 0.48 y | 53.3 ± 0.42 y |
| MyHC Isoforms | Genotypes | p Value | ||
|---|---|---|---|---|
| GP1 (n = 10) | GP2 (n = 10) | GP3 (n = 10) | ||
| Type I | 0.62 ± 0.03 | 0.56 ± 0.03 | 0.62 ± 0.03 | 0.2834 |
| Type IIA | 0.53 ± 0.03 y | 0.64 ± 0.03 x | 0.53 ± 0.03 y | 0.0311 |
| Type IIA/X | 0.35 ± 0.15 | 0.77 ± 0.15 | 0.46 ± 0.15 | 0.1638 |
| Type IIB | 1.6 ± 0.10 | 1.4 ± 0.10 | 1.4 ± 0.10 | 0.2665 |
| Traits (µmol/g) | Time PM (min) | Genotypes | p Value | ||||
|---|---|---|---|---|---|---|---|
| GP1 (n = 20) | GP2 (n = 20) | GP3 (n = 20) | Genotype | Time PM | Gen × Time PM | ||
| Glycogen | 1 | 55.7 ± 4.85 | 57.5 ± 5.00 | 63.6 ± 4.85 | 0.0185 | <0.0001 | 0.7957 |
| 30 | 51.8 ± 4.85 | 55.4 ± 4.85 | 64.3 ± 4.85 | ||||
| 60 | 42.2 ± 4.85 | 51.6 ± 4.85 | 59.2 ± 4.85 | ||||
| 1440 | 20.3 ± 4.85 | 20.2 ± 4.85 | 21.6 ± 4.85 | ||||
| Glucose | 1 | 3.9 ± 0.34 | 3.9 ± 0.35 | 3.7 ± 0.34 | 0.7435 | <0.0001 | 0.7524 |
| 30 | 4.3 ± 0.34 | 4.3 ± 0.34 | 4.1 ± 0.34 | ||||
| 60 | 5.2 ± 0.34 | 4.8 ± 0.34 | 4.4 ± 0.34 | ||||
| 1440 | 7.6 ± 0.34 | 7.5 ± 0.34 | 8.0 ± 0.34 | ||||
| G6P | 1 | 11.9 ± 0.49 | 11.9 ± 0.49 | 10.9 ± 0.49 | 0.0040 | <0.0001 | 0.0800 |
| 30 | 9.7 ± 0.50 | 9.2 ± 0.49 | 9.3 ± 0.49 | ||||
| 60 | 7.4 ± 0.49 | 7.3 ± 0.49 | 6.8 ± 0.49 | ||||
| 1440 | 11.4 ± 0.50 | 8.8 ± 0.49 | 8.8 ± 0.50 | ||||
| Lactate | 1 | 43.7 ± 3.08 | 40.2 ± 3.18 | 38.0 ± 3.08 | 0.0003 | <0.0001 | 0.2480 |
| 30 | 57.4 ± 3.08 | 52.6 ± 3.18 | 48.2 ± 3.08 | ||||
| 60 | 78.8 ± 3.08 | 64.1 ± 3.18 | 61.4 ± 3.18 | ||||
| 1440 | 109.6 ± 3.08 | 108.6 ± 3.08 | 106.6 ± 3.08 | ||||
| Glycolytic Potential | 1 | 186.9 ± 8.04 | 186.9 ± 8.29 | 200.8 ± 8.29 | 0.0369 | <0.0001 | 0.7425 |
| 30 | 188.0 ± 8.04 | 194.1 ± 8.04 | 203.6 ± 8.04 | ||||
| 60 | 188.2 ± 8.04 | 179.6 ± 8.29 | 205.4 ± 8.04 | ||||
| 1440 | 188.3 ± 8.04 | 176.5 ± 8.29 | 184.5 ± 8.04 | ||||
| Traits (µmol/g) | Time PM (min) | Voltages | p Value | |||
|---|---|---|---|---|---|---|
| 100 V (n = 30) | 200 V (n = 30) | Voltage | Time PM | Voltage × Time PM | ||
| Glycogen | 1 | 57.7 ± 4.04 | 60.2 ± 3.96 | 0.3264 | <0.0001 | 0.4074 |
| 30 | 61.0 ± 3.96 | 53.3 ± 3.96 | ||||
| 60 | 54.7 ± 3.96 | 47.3 ± 3.96 | ||||
| 1440 | 20.0 ± 3.96 | 21.5 ± 3.96 | ||||
| Glucose | 1 | 4.0 ± 0.28 | 3.7 ± 0.28 | 0.5644 | <0.0001 | 0.6380 |
| 30 | 4.4 ± 0.28 | 4.1 ± 0.28 | ||||
| 60 | 4.9 ± 0.28 | 4.7 ± 0.28 | ||||
| 1440 | 7.5 ± 0.28 | 7.9 ± 0.28 | ||||
| G6P | 1 | 11.8 ± 0.41 | 11.4 ± 0.40 | 0.8359 | <0.0001 | 0.4997 |
| 30 | 9.0 ± 0.41 | 9.7 ± 0.40 | ||||
| 60 | 7.3 ± 0.40 | 7.0 ± 0.40 | ||||
| 1440 | 9.6 ± 0.40 | 9.9 ± 0.41 | ||||
| Lactate | 1 | 38.2 ± 2.57 | 43.1 ± 2.52 | 0.0247 | <0.0001 | 0.9131 |
| 30 | 49.9 ± 2.57 | 55.6 ± 2.52 | ||||
| 60 | 66.7 ± 2.57 | 69.5 ± 2.57 | ||||
| 1440 | 106.9 ± 2.52 | 109.7 ± 2.52 | ||||
| Glycolytic Potential | 1 | 189.3 ± 6.83 | 193.7 ± 6.60 | 0.9209 | <0.0001 | 0.3910 |
| 30 | 200.5 ± 6.60 | 189.9 ± 6.60 | ||||
| 60 | 192.6 ± 6.70 | 189.5 ± 6.60 | ||||
| 1440 | 177.5 ± 6.70 | 188.6 ± 6.60 | ||||
| Traits (µmol/g) | Time PM (min) | Voltages | p Value | |||
|---|---|---|---|---|---|---|
| 15 min (n = 30) | 25 min (n = 30) | Time ES | Time PM | Time ES × Time PM | ||
| Glycogen | 1 | 58.0 ± 4.04 | 59.8 ± 3.96 | 0.7788 | <0.0001 | 0.8803 |
| 30 | 58.6 ± 3.96 | 55.7 ± 3.96 | ||||
| 60 | 49.4 ± 3.96 | 52.6 ± 3.96 | ||||
| 1440 | 20.2 ± 3.96 | 21.2 ± 3.96 | ||||
| Glucose | 1 | 3.7 ± 0.28 | 4.0 ± 0.28 | 0.6144 | <0.0001 | 0.5242 |
| 30 | 4.3 ± 0.28 | 4.2 ± 0.28 | ||||
| 60 | 4.8 ± 0.28 | 4.8 ± 0.28 | ||||
| 1440 | 8.0 ± 0.28 | 7.4 ± 0.28 | ||||
| G6P | 1 | 11.4 ± 0.41 | 11.7 ± 0.40 | 0.7729 | <0.0001 | 0.8610 |
| 30 | 9.2 ± 0.41 | 9.6 ± 0.40 | ||||
| 60 | 7.1 ± 0.40 | 7.1 ± 0.40 | ||||
| 1440 | 9.8 ± 0.41 | 9.6 ± 0.40 | ||||
| Lactate | 1 | 41.5 ± 2.57 | 39.7 ± 2.52 | 0.6764 | <0.0001 | 0.8604 |
| 30 | 52.2 ± 2.57 | 53.3 ± 2.52 | ||||
| 60 | 66.8 ± 2.57 | 69.3 ± 2.57 | ||||
| 1440 | 107.7 ± 2.52 | 108.8 ± 2.52 | ||||
| Glycolytic Potential | 1 | 192.2 ± 6.83 | 190.9 ± 6.57 | 0.5647 | <0.0001 | 0.9817 |
| 30 | 198.2 ± 6.57 | 192.3 ± 6.57 | ||||
| 60 | 191.6 ± 6.57 | 190.5 ± 6.70 | ||||
| 1440 | 184.3 ± 6.57 | 181.8 ± 7.70 | ||||
| Traits (µmol/g) | Genotypes | Voltage | ES Time | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GP1 | GP2 | GP3 | 100 V | 200 V | 15 min | 25 min | Genotype | Voltage | Time ES | |
| L* | 64.4 ± 0.44 | 63.3 ± 0.45 | 63.9 ± 0.45 | 63.1 ± 0.36 | 64.6 ± 0.36 | 63.7 ± 0.36 | 64.0 ± 0.36 | 0.2382 | 0.0066 | 0.6028 |
| a* | 12.6 ± 0.23 | 12.9 ± 0.23 | 12.8 ± 0.23 | 12.6 ± 0.19 | 12.9 ± 0.19 | 12.8 ± 0.19 | 12.7 ± 0.19 | 0.7622 | 0.3072 | 0.5988 |
| b* | 11.2 ± 0.20 | 11.1 ± 0.20 | 11.4 ± 0.20 | 10.9 ± 0.17 | 11.5 ± 0.17 | 11.2 ± 0.17 | 11.2 ± 0.17 | 0.5015 | 0.0166 | 0.8013 |
| Color a | 1.9 ± 0.07 | 2.0 ± 0.07 | 2.0 ± 0.07 | 2.2 ± 0.05 | 1.8 ± 0.05 | 1.9 ± 0.05 | 2.0 ± 0.05 | 0.6156 | <0.0001 | 0.1236 |
| Firmness b | 1.7 ± 0.07 | 1.8 ± 0.07 | 1.9 ± 0.07 | 2.0 ± 0.06 | 1.6 ± 0.06 | 1.8 ± 0.06 | 1.9 ± 0.06 | 0.3955 | <0.0001 | 0.1897 |
| Drip Loss, % | 6.5 ± 0.29 | 6.9 ± 0.29 | 6.5 ± 0.29 | 6.1 ± 0.23 | 7.2 ± 0.24 | 6.8 ± 0.23 | 6.4 ± 0.24 | 0.5351 | 0.0020 | 0.2520 |
| MyHC | Type IIA | Type IIA/X | Type IIB |
|---|---|---|---|
| Type I | −0.14 | −0.40 | 0.33 |
| Type IIA | 0.56 ** | 0.26 | |
| Type IIA/X | −0.77 *** |
| MyHC | pH 0 | pH 10 | pH 20 | pH 30 | pH 40 | pH 50 | pH 60 | pH 1440 | L* | a* | b* | Color | Firmness | DL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I | −0.40 | −0.04 | −0.11 | −0.28 | −0.13 | −0.04 | 0.20 | −0.11 | 0.20 | 0.04 | 0.38 | 0.58 ** | −0.13 | −0.36 |
| Type IIA | −0.18 | 0.021 | −0.41 | −0.02 | −0.06 | −0.02 | 0.22 | −0.11 | 0.04 | −0.32 | −0.26 | −0.09 | −0.40 | −0.23 |
| Type IIA/X | 0.02 | 0.03 | −0.15 | 0.08 | 0.07 | 0.21 | 0.29 | 0.09 | −0.38 | 0.22 | −0.35 | −0.11 | 0.08 | 0.03 |
| Type IIB | −0.05 | 0.11 | −0.12 | −0.10 | −0.11 | −0.26 | −0.20 | −0.07 | 0.50 * | −0.56 ** | 0.09 | 0.02 | −0.37 | −0.30 |
| MyHC | Lac 0 | Lac 30 | Lac 60 | Lac 1440 | G6P 0 | G6P 30 | G6P 60 | G6P 1440 | Glu 0 | Glu 30 | Glu 60 | Glu 1440 | Gly 0 | Gly 30 | Gly 60 | Gly 1440 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I | 0.64 ** | 0.53 * | 0.02 | 0.10 | 0.14 | 0.35 | 0.12 | −0.65 ** | 0.25 | 0.49 * | 0.22 | −0.65 ** | −0.70 *** | −0.59 ** | −0.62 ** | −0.55 ** |
| Type IIA | 0.40 | 0.50 * | 0.68 ** | −0.02 | 0.09 | −0.02 | 0.12 | −0.22 | 0.69 *** | 0.40 | 0.56 ** | 0.05 | −0.22 | −0.35 | −0.47 | −0.45 |
| Type IIA/X | −0.12 | 0.09 | 0.21 | −0.22 | −0.20 | −0.08 | −0.28 | 0.06 | 0.17 | 0.16 | 0.13 | 0.39 | 0.24 | 0.15 | 0.31 | 0.23 |
| Type IIB | 0.27 | 0.34 | 0.20 | 0.15 | 0.22 | −0.06 | 0.31 | −0.31 | 0.28 | 0.29 | −0.50 * | −0.36 | −0.36 | −0.35 | −0.58 | −0.51 |
| Quality traits | Lac 0 | Lac 30 | Lac 60 | Lac 1440 | G6P 0 | G6P 30 | G6P 60 | G6P 1440 | Glu 0 | Glu 30 | Glu 60 | Glu 1440 | Gly 0 | Gly 30 | Gly 60 | Gly 1440 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | 0.17 | 0.34 *** | 0.36 *** | 0.27 ** | 0.18 | 0.11 | 0.24 ** | 0.09 | −0.06 | −0.06 | 0.25 ** | −0.02 | −0.09 | −0.16 | −0.14 | −0.04 |
| a* | −0.09 | −0.10 | −0.07 | −0.29 ** | −0.11 | 0.01 | −0.03 | −0.16 | 0.07 | 0.19 | 0.08 | −0.20 * | −0.19 | −0.15 | −0.09 | −0.08 |
| b* | 0.04 | 0.01 | −0.08 | −0.04 | −0.06 | 0.10 | −0.12 | 0.10 | 0.18 | 0.14 | −0.16 | 0.02 | 0.08 | 0.09 | 0.19 | 0.23 ** |
| Color | 0.01 | −0.14 | −0.24 ** | −0.12 | −0.23 ** | −0.23 * | −0.11 | −0.25 ** | 0.12 | 0.02 | −0.18 | −0.11 | −0.08 | −0.01 | 0.05 | −0.11 |
| Firmness | −0.19 | −0.22 * | −0.19 * | −0.21 * | −0.29 ** | −0.03 | −0.26 ** | −0.24 ** | −0.05 | 0.02 | −0.10 | −0.06 | 0.06 | −0.02 | 0.02 | −0.06 |
| DL, % | 0.17 | 0.20 * | 0.29 ** | 0.18 | 0.20 * | 0.09 | 0.31 *** | 0.26 ** | −0.17 | −0.02 | 0.14 | 0.15 | 0.13 | 0.16 | 0.08 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spires, M.D.; Bodmer, J.S.; Beline, M.; Wicks, J.C.; Zumbaugh, M.D.; Shi, T.H.; Reichert, B.T.; Schinckel, A.P.; Grant, A.L.; Gerrard, D.E. Postmortem Metabolism and Pork Quality Development Are Affected by Electrical Stimulation across Three Genetic Lines. Animals 2023, 13, 2599. https://doi.org/10.3390/ani13162599
Spires MD, Bodmer JS, Beline M, Wicks JC, Zumbaugh MD, Shi TH, Reichert BT, Schinckel AP, Grant AL, Gerrard DE. Postmortem Metabolism and Pork Quality Development Are Affected by Electrical Stimulation across Three Genetic Lines. Animals. 2023; 13(16):2599. https://doi.org/10.3390/ani13162599
Chicago/Turabian StyleSpires, Matthew D., Jocelyn S. Bodmer, Mariane Beline, Jordan C. Wicks, Morgan D. Zumbaugh, Tim Hao Shi, Brian T. Reichert, Allan P. Schinckel, Alan L. Grant, and David E. Gerrard. 2023. "Postmortem Metabolism and Pork Quality Development Are Affected by Electrical Stimulation across Three Genetic Lines" Animals 13, no. 16: 2599. https://doi.org/10.3390/ani13162599
APA StyleSpires, M. D., Bodmer, J. S., Beline, M., Wicks, J. C., Zumbaugh, M. D., Shi, T. H., Reichert, B. T., Schinckel, A. P., Grant, A. L., & Gerrard, D. E. (2023). Postmortem Metabolism and Pork Quality Development Are Affected by Electrical Stimulation across Three Genetic Lines. Animals, 13(16), 2599. https://doi.org/10.3390/ani13162599







