Squalene Supplementation as a Novel to Increase PUFA Content in Fish Tissues
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish
2.3. Experimental System
2.4. Diet Formulation & Preparation
2.5. Chemical Analysis
2.6. Growth Measurements
2.7. Hematology
2.8. Biochemistry Indices
2.9. Hepatocyte Measurements
2.10. Statistical Analysis
3. Results
3.1. Fish Growth
3.2. Hematology and Blood Biochemistry Profile
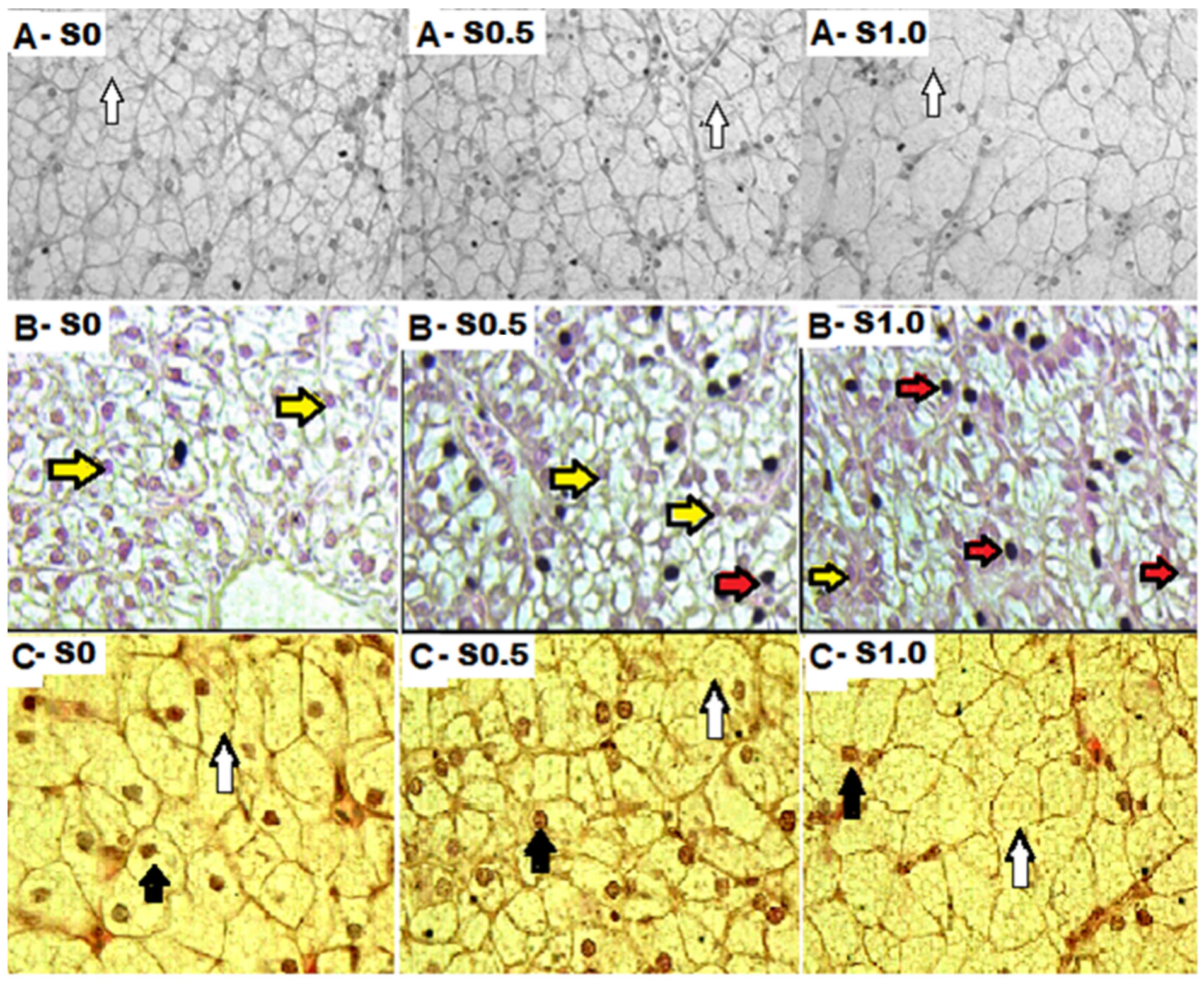
3.3. Hepatocyte Measurements
3.4. Chemical Analysis and Fatty Acids Profile of Fish Muscle and Liver
3.5. Squalene Retention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. North Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed. Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, Å.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Blanchard, G.; Makombu, J.G.; Kestemont, P. Influence of different dietary 18:3n-3/18:2n-6 ratio on growth performance, fatty acid composition and hepatic ultrastructure in Eurasian perch, Perca fluviatilis. Aquaculture 2008, 284, 144–150. [Google Scholar] [CrossRef]
- Tocher, D. Issues surrounding fish as a source of omega-3 long-chain polyunsaturated fatty acids. Lipid Technol. 2009, 21, 13–16. [Google Scholar] [CrossRef]
- Kowalska, A.; Zakęś, Z.; Jankowska, B.; Siwicki, A. Impact of diets with vegetable oils on the growth, histological structure of internal organs, biochemical blood parameters, and proximate composition of pikeperch Sander lucioperca (L.). Aquaculture 2010, 301, 69–77. [Google Scholar] [CrossRef]
- Kelly, G.S. Squalene and Its Potential Clinical Uses. Altern. Med. Rev. A J. Clin. Ther. 1999, 4, 29–36. [Google Scholar]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant Sources, Extraction Methods, and Uses of Squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef] [Green Version]
- Micera, M.; Botto, A.; Geddo, F.; Antoniotti, S.; Bertea, C.M.; Levi, R.; Gallo, M.P.; Querio, G. Squalene: More than a Step toward Sterols. Antioxidants 2020, 9, 688. [Google Scholar] [CrossRef]
- Chen, Y.P.; Gu, Y.F.; Zhao, H.R.; Zhou, Y.M. Dietary squalene supplementa-tion alleviates diquat-induced oxidative stress and liver damage of broiler chickens. Poult. Sci. 2021, 100, 100919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yeung, W.K.; Huang, Y.; Chen, Z.Y. Effect of squalene and shark liver oil on serum cholesterol level in hamsters. Int. J. Food Sci. Nutr. 2002, 53, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Marcos, L.V.; Martínez-Beamonte, R.; Arnal, C.; Barranquero, C.; Puente-Lanzarote, J.J.; Herrero-Continente, T.; Lou-Bonafonte, J.M.; Gonzalo-Romeo, G.; Mocciaro, G.; Jenkins, B.; et al. Dietary squalene supplementation decreases triglyc-eride species and modifies phospholipid lipidomic profile in the liver of a porcine model of non-alcoholic steatohepatitis. J. Nutr. Biochem. 2023, 112, 109207. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Anandan, R.; Hari Senthil Kumar, S.; Shiny, K.S.; Sankar, T.V.; Thankappan, T.K. Effect of squalene on tissue defense system in isopro-terenol-induced myocardial infarction in rats. Pharmacol. Res. 2004, 50, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, X.; Zhou, Y.; Li, Y.; Xiang, D. Dietary supplementation of squalene in-creases the growth performance of early-weaned piglets by improving gut microbiota, intestinal barrier, and blood antioxidant capacity. Front. Vet. Sci. 2022, 9, 995548. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, Y.; Zhao, H.; Zhang, H.; Zhou, Y. Effects of Graded Levels of Dietary Squalene Supplementation on the Growth Performance, Plasma Biochemical Parameters, Antioxidant Capacity, and Meat Quality in Broiler Chickens. Poult. Sci. 2020, 99, 5915–5924. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, Z.; Wang, C.; Feng, Y.; Peng, X.; Gong, Y. Improvement of reproduction performance in AA+ meat-type male chicken by feeding with squalene. J. Anim. Vet. Adv. 2010, 9, 486–490. [Google Scholar] [CrossRef]
- Dessì, M.A.; Deiana, M.; Day, B.W.; Rosa, A.; Banni, S.; Corongiu, F.P. Oxidative stability of polyunsaturated fatty acids: Effect of squalene. Eur. J. Lipid Sci. Technol. 2002, 104, 506–512. [Google Scholar] [CrossRef]
- Gomulka, P.; Wlasow, T.; Velíšek, J.; Svobodová, Z.; Chmielinska, E. Effects of eugenol and MS-222 anesthesia on Siberian sturgeon Acipenser baerii Brandt. Acta Vet. Brno 2008, 77, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Topic Popovic, N.; Strunjak-Perovic, I.; Coz-Rakovac, R.; Barisic, J.; Jadan, M.; Persin Berakovic, A.; Sauerborn Klobucar, R. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J. Appl. Ichthyol. 2012, 28, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Zarski, D.; Krejszeff, S.; Horváth, Á.; Bokor, Z.; Palińska, K.; Szentes, K.; Łuczyńska, J.; Targońska, K.; Kupren, K.; Urbányi, B.; et al. Dynamics of composition and morphology in oocytes of Eurasian perch, Perca fluviatilis L., during induced spawning. Aquaculture 2012, 364–365, 103–110. [Google Scholar] [CrossRef]
- Jobling, M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2011, 20, 601–602. [Google Scholar] [CrossRef]
- Hung, S.S.O. Recent advances in sturgeon nutrition. Anim. Nutr. 2017, 3, 191–204. [Google Scholar] [CrossRef]
- Hart, S.D.; Brown, B.J.; Gould, N.L.; Robar, M.L.; Witt, E.M.; Brown, P.B. Predicting the optimal dietary essential amino acid profile for growth of juvenile yellow perch with whole body amino acid concentrations. Aquac. Nutr. 2010, 16, 248–253. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 20th ed.; George, W.L., Jr., Ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Shearer, K.D. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 1994, 119, 63–88. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Peisker, K.V. A rapid semi-micro method for preparation of methyl esters from triglycerides using chloroform, methanol, sulphuric acid. J. Am. Oil Chem. Soc. 1964, 41, 87–88. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis. Oily Press 1993, 2, 69–111. [Google Scholar]
- Kopicová, Z.; Vavreinová, S. Occurrence of squalene and cholesterol in various species of Czech freshwater fish. Czech J. Food Sci. 2007, 25, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Jankowska, B.; Zakęś, Z.; Żmijewski, T.; Szczepkowski, M.; Wunderlich, K. The impact of diet on the slaughter yield, proximate composition and fatty acids profile of fillets of tench Tinca tinca (L.). Arch. Pol. Fish. 2006, 14, 195–211. [Google Scholar]
- Bohacenko, I.; Kopicova, Z. Detection of sunflower and soybean oil adulterated with rapeseed oil. Czech J. Food Sci. 1999, 17, 182–187. [Google Scholar]
- Svobodova, Z.; Pravda, D.; Palackova, J. Unified Methods of Haematological Examination of Fish; Research Institute of Fish Culture and Hydrobiology: Vodnany, Czech Republic, 1991. [Google Scholar]
- Zawistowski, S. Technika Histologiczna, Histologia oraz Podstawy Histopatologii; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 1986. [Google Scholar]
- Popa, I.; Băbeanu, N.; Nit, ă.S.; Popa, O. Squalene-Natural Resources and Applications. Farmacia 2014, 62, 840–862. [Google Scholar]
- Moreforsk. No. Available online: https://www.moreforsk.no/download.aspx?object_id=upload_images/6005FD7959B34DCD9FEBF467ECC08A1D.pdf (accessed on 5 April 2023).
- van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Strüssmann, C.A.; Takashima, F. Hepatocyte nuclear size and nutritional condition of larval pejerrey, Odontesthes bonariensis (Cuvier et Valenciennes). J. Fish Biol. 1990, 36, 59–65. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Refstie, S.; Fjalestad, K.T.; Storebakken, T.; Rgdbotten, M.; Roem, A.J. Quality parameters of the flesh of Atlantic salmon (Salmo salar) as affected by dietary fat content and full-fat soybean meal as a partial substitute for fish meal in the diet. Aquaculture 1997, 157, 297–309. [Google Scholar] [CrossRef]
- Caballero, M.; Obach, A.; Rosenlund, G.; Montero, D.; Gisvold, M.; Izquierdo, M. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout. Oncorhynchus Mykiss. Aquac. 2002, 214, 253–271. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Dabrowski, K.; Palacios, M.E.; Olejniczak, M.; Wieczorek, M. Growth and morphological changes in the digestive tract of rainbow trout (Oncorhynchus mykiss) and pacu (Piaractus mesopotamicus) due to casein replacement with soybean proteins. Aquaculture 2005, 245, 273–286. [Google Scholar] [CrossRef]
- Hou, L.; Liu, K.; Li, Y.; Ma, S.; Ji, X.; Liu, L. Necrotic pyknosis is a morphologically and biochemically distinct event from apoptotic pyknosis. J. Cell Sci. 2016, 129, 3084–3090. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Makkar, H.P.S.; Becker, K. Nutritional, physiological and haematological responses in rainbow trout (Oncorhynchus mykiss) juveniles fed detoxified Jatropha curcas kernel meal. Aquac. Nutr. 2011, 17, 451–467. [Google Scholar] [CrossRef]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12, 1463–1467. [Google Scholar] [CrossRef] [Green Version]
- Coles, E.H. Veterinary Clinical Pathology, 4th ed.; WB Saunders Company: Philadelphia, PA, USA, 1986. [Google Scholar]
- Mc Donald, D.G.; Milligan, C.L. Chemical Properties of the Blood. In The Cardiovascular System; Hoar, W.S., Randall, D.J., Farrell, A.P., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 12, pp. 55–133. [Google Scholar] [CrossRef]
- Wallaert, C.; Babin, P.J. Frozen storage affects high density lipoproteins and the quantitation of trout (Oncorhynchus mykiss) lipoprotein classes as separated by ultracentrifugation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 110, 439–444. [Google Scholar] [CrossRef]
- Murray, K.F.; Hadzic, N.; Wirth, S.; Bassett, M.; Kelly, D. Drug-related Hepatotoxicity and Acute Liver Failure. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Zakęś, Z.; Demska-Zakęś, K.; Szczepkowski, M.; Rożyński, M.; Ziomek, E. Impact of sex and diet on hematological and blood plasma biochemical profiles and liver histology of pikeperch (Sander lucioperca (L.)). Fish. Aquat. Life 2016, 24, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liang, X.-F.; He, S.; Li, J.; Huang, K.; Zhang, Y.-P.; Huang, D. Lipid deposition pattern and adaptive strategy in response to dietary fat in Chinese perch (Siniperca chuatsi). Nutr. Metab. 2018, 15, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrubec, T.; Smith, S. Hematology and Blood Chemistry Reference Intervals for Yellow Perch (Perca flavescens) raised in Recirculation Systems. Int. J. Recirc. Aquac. 2004, 5, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Maita, M.; Maekahwa, J.; Satoh, K.; Futami, K.; Sotoh, S. Disease resistance and hypocholesterolemia in yellowtail Seriola quinqueradiata fed a non-fishmeal diet. Fish. Sci. 2006, 72, 513–519. [Google Scholar] [CrossRef]
- Deng, J.; Bi, B.; Kang, B.; Kong, L.; Wang, Q.; Zhang, X. Improving the growth performance and cholesterol metabolism of rainbow trout (Oncorhynchus mykiss) fed soyabean meal-based diets using dietary cholesterol supplementation. Br. J. Nutr. 2013, 110, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Goedeke, L.; Fernández-Hernando, C. Regulation of cholesterol homeostasis. Cell. Mol. Life Sci. 2012, 69, 915–930. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Nichols, P.D.; Malau-Aduli, A.E.O. Enhancing Omega-3 Long-Chain Polyunsaturated Fatty Acid Content of Dairy-Derived Foods for Human Consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.; Betancor, M.B.; Roo, J.; Montero, D.; Zamorano, M.J.; Izquierdo, M. Selenium levels in early weaning diets for gilthead seabream larvae. Aquaculture 2014, 426–427, 256–263. [Google Scholar] [CrossRef]
- Madsen, L.; Frøyland, L.; Dyrøy, E.; Helland, K.; Berge, R.K. Docosahexaenoic and eicosapentaenoic acids are differently metabolized in rat liver during mitochondria and peroxisome proliferation. J. Lipid Res. 1998, 39, 583–593. [Google Scholar] [CrossRef] [PubMed]



| Formulation, g 100 g−1 | E1 | E2 | E3 |
|---|---|---|---|
| Fishmeal a | 32.00 | 31.00 | 20.00 |
| Soybean protein concentrate b | 30.00 | 30.00 | 30.00 |
| Poultry meal c | 0.00 | 0.00 | 20.00 |
| Wheat flour d | 20.00 | 20.00 | 20.00 |
| Cod-liver oil e | 0.00 | 0.00 | 7.00 |
| Fish oil f | 7.50 | 8.00 | 0.00 |
| Soybean oil g | 7.50 | 8.00 | 1.00 |
| Premix hij | 3.00 | 3.00 | 2.00 |
| Squalene k | 0.00 | 0.00 | 0.00 |
| Ingredients, g 100 g−1 | |||
| Dry matter | 95.85 | 95.67 | 95.50 |
| Crude protein | 41.60 | 41.22 | 45.90 |
| Crude fat | 18.90 | 20.02 | 13.40 |
| Crude fiber | 1.71 | 1.63 | 1.62 |
| NFE | 27.72 | 26.83 | 26.93 |
| Crude ash | 5.92 | 5.97 | 7.65 |
| Gross energy (MJ kg−1) | 17.91 | 15.52 | 16.68 |
| Fatty Acids | Feeds | ||
|---|---|---|---|
| E1 | E2 | E3 | |
| C 10:0 | 0.00 | 0.00 | 0.78 |
| C 12:0 | 0.00 | 0.00 | 1.54 |
| C 14:0 | 9.75 | 7.96 | 4.81 |
| C 15:0 | 0.85 | 0.73 | 9.39 |
| C 16:0 | 19.47 | 21.70 | 20.12 |
| C 17:0 | 0.41 | 0.38 | 0.26 |
| C 18:0 | 3.65 | 3.95 | 7.42 |
| C 20:0 | 0.23 | 0.31 | 0.43 |
| C 22:0 | 0.59 | 0.49 | 0.36 |
| ΣSFA | 34.96 | 35.52 | 45.11 |
| C 14:1 | 0.45 | 0.49 | 0.37 |
| C 16:1 | 6.84 | 5.46 | 4.59 |
| C 17:1 | 0.57 | 0.45 | 2.57 |
| C 18:1 c9 | 20.32 | 21.43 | 12.97 |
| C 18:1 c11 | 3.09 | 2.73 | 4.28 |
| C 20:1 n-9 | 2.58 | 2.06 | 1.61 |
| C 20:1 n-7 | 0.14 | 0.10 | 0.25 |
| C 22:1 n11 | 1.82 | 1.43 | 0.84 |
| C 22:1 n9 | 0.18 | 0.13 | 0.09 |
| ΣMUFA | 35.98 | 35.66 | 27.58 |
| C 18:2 n-6 | 11.24 | 15.23 | 8.84 |
| C 18:3 | 2.18 | 1.84 | 0.69 |
| C 18:4 | 0.99 | 0.70 | 0.78 |
| C 20:2 | 0.16 | 0.11 | 0.11 |
| C 20:3 n6 | 0.02 | 0.01 | 0.06 |
| C 20:4 n6 | 0.18 | 0.13 | 0.07 |
| C 20:3 n3 | 0.02 | 0.01 | 0.04 |
| C 20:4 n3 | 0.16 | 0.11 | 0.09 |
| C 20:5 n3 (EPA) | 1.53 | 1.12 | 0.84 |
| C 22:5 n6 | 0.37 | 0.28 | 0.14 |
| C 22:5 n3 | 0.11 | 0.08 | 0.21 |
| C 22:6 n3 (DHA) | 12.10 | 9.20 | 15.30 |
| ΣPUFA | 29.06 | 28.82 | 27.32 |
| Squalene | 0.16 | 0.11 | 0.19 |
| n-3 | 13.92 | 10,52 | 16.48 |
| n-6 | 11.81 | 5.65 | 9.11 |
| n-3/n-6 | 1.18 | 1.87 | 1.81 |
| Indicate | Siberian Sturgeon | Rainbow Trout | Eurasian Perch | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S0 | S0.5 | S1.0 | S0 | S0.5 | S1.0 | S0 | S0.5 | S1.0 | |
| Initial total length (cm fish−1) | 27.6 ± 1.7 | 27.3 ± 1.7 | 27.8 ± 1.3 | 27.6 ± 1.7 | 27.3 ± 1.7 | 27.8 ± 1.3 | 11.7 ± 0.7 | 11.5 ± 0.8 | 11.6 ± 0.7 |
| Final total length (cm fish−1) | 40.8 ± 5.0 | 40.6 ± 3.8 | 41.0 ± 2.8 | 40.8 ± 5.0 | 40.6 ± 3.9 | 41.0 ± 2.8 | 14.8 ± 1.0 | 14.7 ± 0.9 | 14.9 ± 0.7 |
| Initial weight (g fish−1) | 68.6 ± 15.5 | 65.6 ± 12.3 | 66.7 ± 9.2 | 68.6 ± 15.5 | 66.1 ± 12.7 | 66.7 ± 9.2 | 24.5 ± 4.4 | 23.4 ± 4.6 | 23.4 ± 4.5 |
| Final weight (g fish−1) | 192.3 ± 47.1 | 193.4 ± 46.5 | 209.1 ± 52.7 | 209.1 ± 53.1 | 197.2 ± 51.3 | 193.4 ± 46.5 | 54.2 ± 5.7 | 51.9 ± 6.5 | 52.2 ± 4.1 |
| SGR (%/d) | 1.84 ± 0.05 | 1.87 ± 0.05 | 1.90 ± 0.08 | 1.17 a ± 0.03 | 1.24 b ± 0.04 | 1.24 b ± 0.04 | 1.42 ± 0.09 | 1.42 ± 0.16 | 1.43 ± 0.10 |
| FCR | 1.09 ± 0.02 | 1.07 ± 0.04 | 1.05 ± 0.03 | 1.09 ± 0.02 | 1.07 ± 0.04 | 1.05 ± 0.03 | 1.62 ± 0.04 | 1.58 ± 0.27 | 1.54 ± 0.27 |
| VSI (%) | 11.8 ± 2.0 | 11.8 ± 3.2 | 11.8 ± 2.5 | 14.50 ± 2.10 | 14.50 ± 1.20 | 14.70 ± 1.70 | 14.0 ± 1.6 | 14.8 ± 1.5 | 15.8 ± 3.0 |
| HSI (%) | 3.46 a ± 0.78 | 3.74 a ± 1.04 | 3.88 b ± 0.75 | 3.19 ± 1.07 | 3.74 ± 1.04 | 3.88 ± 0.75 | 2.02 ± 0.67 | 2.09 ± 0.45 | 2.34 ± 0.75 |
| PER (%) | 2.21 ± 0.03 | 2.24 ± 0.08 | 2.27 ± 0.07 | 1.68 a ± 0.10 | 1.81 a ± 0.24 | 1.94 b ± 0.07 | 1.37 ± 0.04 | 1.44 ± 0.24 | 1.46 ± 0.21 |
| RBC (T L −1) | 0.25 ± 0.04 | 0.23 ± 0.04 | 0.21 ± 0.06 | 0.74 a ± 0.09 | 0.84 b ± 0.10 | 0.84 b ± 0.01 | 0.91 ± 0.16 | 0.93 ± 0.22 | 1.10 ± 0.21 |
| PCV (1 L −1) | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.14 ± 0.07 | 0.38 ± 0.02 | 0.36 ± 0.03 | 0.37 ± 0.03 | 0.44 ± 0.11 | 0.42 ± 0.13 | 0.44 ± 0.09 |
| HB (g L −1) | 73.57 a ± 9.79 | 74.36 a ± 8.08 | 71.78 a ± 8.32 | 80.0 a ± 4.7 | 73.4 a ± 11.6 | 72.4 a ± 12.03 | 94.6 a ± 20.7 | 105.2 a ± 19.7 | 106.6 a ± 10.6 |
| MCV (fl) | 107 a ± 19 | 114 a ± 19 | 143 b ± 69 | 521 a ± 65 | 437 b ± 66 | 451 b ± 55 | 492 ± 120 | 480 ± 204 | 407 ± 84 |
| MCH (pg) | 119 a ± 26 | 131 b ± 25 | 141 b ± 28 | 110 a ± 15 | 88 b ± 14 | 88 b ± 20 | 109 ± 43 | 118 ± 38 | 100 ± 24 |
| MCHC (g L−1) | 0.28 ± 0.06 | 0.29 ± 0.06 | 0.29 ± 0.06 | 0.21 ± 0.01 | 0.21 ± 0.05 | 0.20 ± 0.04 | 0.24 ± 0.07 | 0.24 ± 0.05 | 0.25 ± 0.05 |
| GLU (mmol dm−3) | 3.42 ± 0.46 | 3.63 ± 0.32 | 3.77 ± 0.72 | 6.56 ± 2.23 | 6.67 ± 1.68 | 6.56 ± 3.68 | 5.94 ± 2.01 | 6.10 ± 2.35 | 6.57 ± 3.21 |
| TAG (mmol dm−3) | 12.28 ± 4.28 | 14.02 ± 4.45 | 14.04 ± 2.92 | 3.41 a ± 1.25 | 2.65 ab ± 0.52 | 2.46 b ± 0.77 | 8.97 ± 2.53 | 9.80 ± 3.16 | 8.01 ± 2.80 |
| CHOL (mmol dm−3) | 2.79 ± 0.43 | 3.00 ± 0.61 | 3.21 ± 0.64 | 6.51 ± 1.70 | 6.56 ± 2.20 | 6.96 ± 1.27 | 5.51 a ± 0.56 | 6.03 ab ± 0.60 | 6.54 b ± 1.21 |
| TP (g dm−3) | 24.00 ± 5.00 | 25.00 ± 5.00 | 28.00 ± 6.00 | 36.9 ± 4.1 | 36.7 ± 3.9 | 36.7 ± 5.3 | 55.8 ± 7.4 | 51.0 ± 5.5 | 55.4 ± 8.8 |
| ALB (g dm−3) | 10.00 ± 2.00 | 9.00 ± 2.00 | 10.00 ± 2.00 | 11.6 ± 1.0 | 11.3 ± 0.9 | 12.1 ± 2.7 | 16.8 ± 1.9 | 15.8 ± 2.1 | 17.1 ± 3.1 |
| GLOB (g dm−3) | 16.00 ± 6.00 | 15.00 ± 4.00 | 18.00 ± 5.00 | 25.3 ± 3.4 | 25.3 ± 3.3 | 28.00 ± 3.8 | 39.0 ± 7.1 | 35.2 ± 3.8 | 38.4 ± 8.2 |
| AST (U/L) | 210 ± 61 | 250 ± 114 | 270 ± 117 | 237 ± 90 | 232 ± 110 | 220 ± 56 | 127 ± 57 | 146 ± 87 | 174 ± 123 |
| Indicate | Siberian Sturgeon | Rainbow Trout | Eurasian Perch | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S0 | S0.5 | S1.0 | S0 | S0.5 | S1.0 | S0 | S0.5 | S1.0 | |
| HCD (µm) | 23.3 a ± 1.8 | 24.0 a ± 2.4 | 25.5 b ± 1.8 | 13.8 a ± 0.6 | 14.1 ab ± 0.5 | 14.5 b ± 0.6 | 14.2 a ± 0.8 | 14.4 a ± 0.7 | 15.3 b ± 1.2 |
| HND (µm) | 7.1 ± 0.5 | 7.2 ± 0.6 | 6.9 ± 0.3 | 4.8 ± 0.2 | 4.9 ± 0.2 | 4.9 ± 0.2 | 5.4 ab ± 0.2 | 5.3 a ± 0.2 | 5.7 b ± 0.5 |
| NCPI | 0.31 a ± 0.03 | 0.30 a ± 0.04 | 0.27 b ± 0.02 | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.34 ± 0.01 | 0.39 a ± 0.02 | 0.39 a ± 0.02 | 0.37 b ± 0.04 |
| Composition g 100 g−1 | Muscle | Liver | ||||
|---|---|---|---|---|---|---|
| S0 | S0.5 | S1.0 | S0 | S0.5 | S1.0 | |
| Siberian sturgeon | ||||||
| Dry matter | 21.4 ± 0.1 | 21.3 ± 0.2 | 21.2 ± 0.2 | 22.5 a ± 0.1 | 22.5 ± 0.1 | 22.5 ± 0.1 |
| Crude protein | 13.6 ± 0.3 | 13.6 ± 0.3 | 13.6 ± 0.3 | 4.2 ± 0.03 | 4.2 ± 0.02 | 4.2 ± 0.02 |
| Crude fat | 5.6 ± 0.30 | 5.6 ± 0.4 | 5.6 ± 0.4 | 16.2 ± 0.25 | 16.2 ± 0.25 | 16.3 ± 0.25 |
| Crude ash | 2.2 ± 0.03 | 2.1 ± 0.02 | 2.1 ± 0.02 | 2.2 ± 0.03 | 2.5 ± 0.03 | 2.5 ± 0.03 |
| Rainbow trout | ||||||
| Dry matter | 23.1 ± 0.2 | 23.1 ±0.1 | 23.1 ± 0.1 | 21.2 ± 0.5 | 21.3 ± 0.6 | 21.5 ± 0.5 |
| Crude protein | 17.6 ± 0.4 | 17.6 ± 0.2 | 17.6 ± 0.3 | 6.2 ± 0.3 | 6.3 ± 0.4 | 6.3 ± 0.3 |
| Crude oil | 4.3 ± 0.4 | 4.3 ± 0.2 | 4.3 ± 0.4 | 13.5 ± 0.5 | 13.5 ± 0.6 | 13.7 ± 0.5 |
| Crude ash | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.3 | 1.5 ± 0.4 | 1.5 ± 0.3 |
| Eurasian perch | ||||||
| Dry matter | 22.3 ± 0.1 | 22.3 ± 0.2 | 22.0 ± 0.1 | 22.5 ± 0.1 | 22.5 ± 0.1 | 22.5 ± 0.1 |
| Crude protein | 18.6 ± 0.3 | 18.6 ± 0.2 | 18.5 ± 0.2 | 4.2 ± 0.03 | 4.2 ± 0.02 | 4.2 ± 0.02 |
| Crude oil | 2.8 ± 0.3 | 2.8 ± 0.3 | 2.8 ± 0.2 | 16.2 ± 0.3 | 16.2 ± 0.3 | 16.3 ± 0.3 |
| Crude ash | 0.9 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.1 | 2.5 ± 0.03 | 2.5 ± 0.03 | 2.5 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niewiadomski, P.; Gomułka, P.; Woźniak, M.; Szmyt, M.; Ziomek, E.; Bober, H.; Szczepkowski, M.; Palińska-Żarska, K.; Krejszeff, S.; Żarski, D. Squalene Supplementation as a Novel to Increase PUFA Content in Fish Tissues. Animals 2023, 13, 2600. https://doi.org/10.3390/ani13162600
Niewiadomski P, Gomułka P, Woźniak M, Szmyt M, Ziomek E, Bober H, Szczepkowski M, Palińska-Żarska K, Krejszeff S, Żarski D. Squalene Supplementation as a Novel to Increase PUFA Content in Fish Tissues. Animals. 2023; 13(16):2600. https://doi.org/10.3390/ani13162600
Chicago/Turabian StyleNiewiadomski, Piotr, Piotr Gomułka, Małgorzata Woźniak, Mariusz Szmyt, Elżbieta Ziomek, Helena Bober, Mirosław Szczepkowski, Katarzyna Palińska-Żarska, Sławomir Krejszeff, and Daniel Żarski. 2023. "Squalene Supplementation as a Novel to Increase PUFA Content in Fish Tissues" Animals 13, no. 16: 2600. https://doi.org/10.3390/ani13162600





