Autologous Platelet Lysate Is an Alternative to Fetal Bovine Serum for Canine Adipose-Derived Mesenchymal Stem Cell Culture and Differentiation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Cell Isolation and Culturing
2.3. Preparation of Autologous Platelet Lysate
2.4. Experimental Design
2.5. Cellular Doubling Time Assay
2.6. Cellular Metabolic Assay
2.7. Cell Migration/Scratch Assay
2.8. Fluorescent-Activated Cell Sorting
2.9. Canine Mesenchymal Stem Cells Bi-Linage Differentiation
2.10. Alkaline Phosphatase Activity
2.11. Gene Expression Analysis
2.12. Statistical Analysis
3. Results
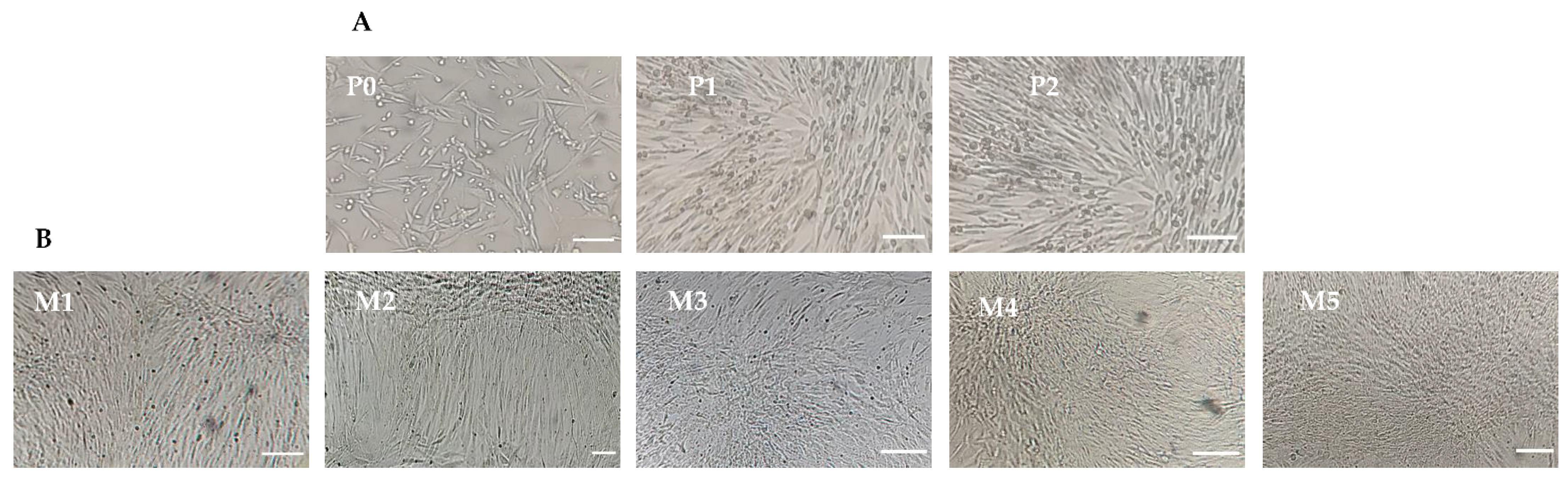
3.1. Isolation and Expansion of Canine Mesenchymal Stem Cells
3.2. Platelets Count and Platelet-Derived Growth Factor-AB Analysis
3.3. Cellular Doubling Time
3.4. Cell Metabolism
3.5. Cell Migration/Scratch Assay
3.6. Immunophenotypic Analysis
3.7. Mesenchymal Stem Cells Bi-Linage Differentiation Efficiency
3.8. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Ryu, H.H.; Park, S.S.; Koyama, Y.; Kikuchi, M.; Woo, H.M.; Kim, W.H.; Kweon, O.K. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton’s jelly for treating bone defects. J. Vet. Sci. 2012, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, A.; Qureshi, A.S.; Sandhu, M.A.; Shahid, R.U.; Naeem, M. Isolation and Characterization of Fetal Adnexa-Derived Mesenchymal Stem Cells from Nili-Ravi Buffalo (Bubalus bubalis). Pak. Vet. J. 2021, 44. [Google Scholar]
- Jurek, S.; Sandhu, M.A.; Trappe, S.; Bermudez-Pena, M.C.; Kolisek, M.; Sponder, G.; Aschenbach, J.R. Optimizing adipogenic transdifferentiation of bovine mesenchymal stem cells: A prominent role of ascorbic acid in FABP4 induction. Adipocyte 2020, 9, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Gaiba, S.; França, L.P.D.; França, J.P.D.; Ferreira, L.M. Characterization of human adipose-derived stem cells. Acta Cir. Bras. 2012, 27, 471–476. [Google Scholar] [CrossRef]
- Bahamondes, F.; Flores, E.; Cattaneo, G.; Bruna, F.; Conget, P. Omental adipose tissue is a more suitable source of canine Mesenchymal stem cells. BMC Vet. Res. 2017, 13, 166. [Google Scholar] [CrossRef]
- Hoffman, A.M.; Dow, S.W. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells 2016, 34, 1709–1729. [Google Scholar] [CrossRef]
- Sasaki, A.; Mizuno, M.; Ozeki, N.; Katano, H.; Otabe, K.; Tsuji, K.; Koga, H.; Mochizuki, M.; Sekiya, I. Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS ONE 2018, 13, e0202922. [Google Scholar] [CrossRef]
- Rashid, U.; Sandhu, M.A.; Yaqoob, M.; Yousaf, A.J.P.V.J.D. Critical bone gap repair using autologous adipose derived canine mesenchymal stem cell graft. Pak. Vet. J. 2021, 10, 513–518. [Google Scholar]
- van der Valk, J.; Mellor, D.; Brands, R.; Fischer, R.; Gruber, F.; Gstraunthaler, G.; Hellebrekers, L.; Hyllner, J.; Jonker, F.H.; Prieto, P.; et al. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol. Vitr. 2004, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.; Hirschi, S.D. Fetal bovine serum: What you should ask your supplier and why. BioProcessing 2014, 13, 1538–8786. [Google Scholar] [CrossRef]
- Xia, H.; Vijayaraghavan, B.; Belak, S.; Liu, L. Detection and identification of the atypical bovine pestiviruses in commercial foetal bovine serum batches. PLoS ONE 2011, 6, e28553. [Google Scholar] [CrossRef]
- Doucet, C.; Ernou, I.; Zhang, Y.; Llense, J.R.; Begot, L.; Holy, X.; Lataillade, J.J. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J. Cell Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef]
- Chevallier, N.; Anagnostou, F.; Zilber, S.; Bodivit, G.; Maurin, S.; Barrault, A.; Bierling, P.; Hernigou, P.; Layrolle, P.; Rouard, H. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials 2010, 31, 270–278. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, L.; Santos, G.S.; Huber, S.C.; Setti, T.M.; Setti, T.; Lana, J.F. Human platelet lysate—A potent (and overlooked) orthobiologic. J. Clin. Orthop. Trauma 2021, 21, 101534. [Google Scholar] [CrossRef]
- Suelzu, C.M.; Conti, V.; Khalidy, Y.; Montagna, S.; Strusi, G.; Di Lecce, R.; Berni, P.; Basini, G.; Ramoni, R.; Grolli, S. Xenobiotic-Free Medium Guarantees Expansion of Adipose Tissue-Derived Canine Mesenchymal Stem Cells Both in 3D Fibrin-Based Matrices and in 2D Plastic Surface Cultures. Cells 2020, 9, 2578. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Underwood, W.; Anthony, R. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Available online: https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (accessed on 1 March 2020).
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Rossi, B.; Merlo, B.; Colleoni, S.; Iacono, E.; Tazzari, P.L.; Ricci, F.; Lazzari, G.; Galli, C. Isolation and in vitro characterization of bovine amniotic fluid derived stem cells at different trimesters of pregnancy. Stem Cell Rev. Rep. 2014, 10, 712–724. [Google Scholar] [CrossRef]
- Rodriguez-Menocal, L.; Salgado, M.; Ford, D.; Van Badiavas, E. Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients. Stem Cells Transl. Med. 2012, 1, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Kerpedjieva, S.S. Acceleration of Wound Healing by Multiple Growth Factors and Cytokines Secreted from Multipotential Stromal Cells/Mesenchymal Stem Cells. Adv. Wound Care 2012, 1, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kratchmarova, I.; Blagoev, B.; Haack-Sorensen, M.; Kassem, M.; Mann, M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science 2005, 308, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A.; Dolegowska, B.; Banfi, G. Growth factor content in PRP and their applicability in medicine. J. Biol. Regul. Homeost. Agents 2012, 26, 3S–22S. [Google Scholar] [PubMed]
- Mihaylova, Z.; Tsikandelova, R.; Sanimirov, P.; Gateva, N.; Mitev, V.; Ishkitiev, N. Role of PDGF-BB in proliferation, differentiation and maintaining stem cell properties of PDL cells in vitro. Arch. Oral Biol. 2018, 85, 1–9. [Google Scholar] [CrossRef]
- Bramanti, V.; Grasso, S.; Tibullo, D.; Giallongo, C.; Raciti, G.; Viola, M.; Avola, R. Modulation of extracellular signal-related kinase, cyclin D1, glial fibrillary acidic protein, and vimentin expression in estradiol-pretreated astrocyte cultures treated with competence and progression growth factors. J. Neurosci. Res. 2015, 93, 1378–1387. [Google Scholar] [CrossRef]
- Lai, F.; Kakudo, N.; Morimoto, N.; Taketani, S.; Hara, T.; Ogawa, T.; Kusumoto, K. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res. Ther. 2018, 9, 107. [Google Scholar] [CrossRef]
- Vieira, N.M.; Brandalise, V.; Zucconi, E.; Secco, M.; Strauss, B.E.; Zatz, M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010, 19, 279–289. [Google Scholar] [CrossRef]
- Sandhu, M.A.; Jurek, S.; Trappe, S.; Kolisek, M.; Sponder, G.; Aschenbach, J.R. Influence of Bovine Serum Lipids and Fetal Bovine Serum on the Expression of Cell Surface Markers in Cultured Bovine Preadipocytes. Cells Tissues Organs 2017, 204, 13–24. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Gao, Z.W.; Dong, K.; Zhang, H.Z. The roles of CD73 in cancer. BioMed Res. Int. 2014, 2014, 460654. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhanja, A.; Bhattacharyya, J.; Jaganathan, B.G. Multiple roles of CD90 in cancer. Tumour Biol. 2016, 37, 11611–11622. [Google Scholar] [CrossRef] [PubMed]
- Requicha, J.F.; Viegas, C.A.; Albuquerque, C.M.; Azevedo, J.M.; Reis, R.L.; Gomes, M.E. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. Rep. 2012, 8, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Rashid, U.; Yousaf, A.; Yaqoob, M.; Saba, E.; Moaeen-Ud-Din, M.; Waseem, S.; Becker, S.K.; Sponder, G.; Aschenbach, J.R.; Sandhu, M.A. Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources. BMC Vet. Res. 2021, 17, 388. [Google Scholar] [CrossRef]
- Kresic, N.; Prislin, M.; Vlahovic, D.; Kostesic, P.; Ljolje, I.; Brnic, D.; Turk, N.; Musulin, A.; Habrun, B. The Expression Pattern of Surface Markers in Canine Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 7476. [Google Scholar] [CrossRef]
- Anderson, P.; Carrillo-Galvez, A.B.; Garcia-Perez, A.; Cobo, M.; Martin, F. CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS ONE 2013, 8, e76979. [Google Scholar] [CrossRef]
- Farahani, R.M.; Xaymardan, M. Platelet-Derived Growth Factor Receptor Alpha as a Marker of Mesenchymal Stem Cells in Development and Stem Cell Biology. Stem Cells Int. 2015, 2015, 362753. [Google Scholar] [CrossRef]
- Gurriaran-Rodriguez, U.; Al-Massadi, O.; Roca-Rivada, A.; Crujeiras, A.B.; Gallego, R.; Pardo, M.; Seoane, L.M.; Pazos, Y.; Casanueva, F.F.; Camina, J.P. Obestatin as a regulator of adipocyte metabolism and adipogenesis. J. Cell Mol. Med. 2011, 15, 1927–1940. [Google Scholar] [CrossRef]
- Cervelli, V.; Scioli, M.G.; Gentile, P.; Doldo, E.; Bonanno, E.; Spagnoli, L.G.; Orlandi, A. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl. Med. 2012, 1, 206–220. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Mandrup, S. Adipogenesis: Forces that tip the scales. Trends Endocrinol. Metab. 2002, 13, 5–11. [Google Scholar] [CrossRef]
- Reddi, S.; Shanmugam, V.P.; Tanedjeu, K.S.; Kapila, S.; Kapila, R. Effect of buffalo casein-derived novel bioactive peptides on osteoblast differentiation. Eur. J. Nutr. 2018, 57, 593–605. [Google Scholar] [CrossRef]
- Hagen, A.; Holland, H.; Brandt, V.P.; Doll, C.U.; Haussler, T.C.; Melzer, M.; Moellerberndt, J.; Lehmann, H.; Burk, J. Platelet Lysate for Mesenchymal Stromal Cell Culture in the Canine and Equine Species: Analogous but Not the Same. Animals 2022, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, A.; Duda, G.N.; Wilson, C.J.; Thompson, M.S.; Hunter, M.R.; Simon, P.; Bail, H.J.; van Scherpenzeel, K.M.; Kasper, G. Mechanical stimulation of the pro-angiogenic capacity of human fracture haematoma: Involvement of VEGF mechano-regulation. Bone 2010, 47, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Bi, L.; Zhang, Z.; Huang, Z.; Hou, W.; Lu, X.; Sun, P.; Han, Y. Increased levels of calcitonin gene-related peptide in serum accelerate fracture healing following traumatic brain injury. Mol. Med. Rep. 2012, 5, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.A.; Gibson, T.W.; Chong, A.; Co, C.; Koch, T.G. Canine Platelet Lysate Is Inferior to Fetal Bovine Serum for the Isolation and Propagation of Canine Adipose Tissue- and Bone Marrow-Derived Mesenchymal Stromal Cells. PLoS ONE 2015, 10, e0136621. [Google Scholar] [CrossRef] [PubMed]
- Even, K.M.; Gaesser, A.M.; Ciamillo, S.A.; Linardi, R.L.; Ortved, K.F. Comparing the immunomodulatory properties of equine BM-MSCs culture expanded in autologous platelet lysate, pooled platelet lysate, equine serum and fetal bovine serum supplemented culture media. Front. Vet. Sci. 2022, 9, 958724. [Google Scholar] [CrossRef]






| Media | 1 FBS% | 1 A-PL% |
|---|---|---|
| M1 | 10 | 0 |
| M2 | 7.5 | 2.5 |
| M3 | 5 | 5 |
| M4 | 2.5 | 7.5 |
| M5 | 0 | 10 |
| Gene | Sense 5′-3′ | Antisense 3′-5′ | Amplicon Size (BP) | Annealing Temp. (°C) |
|---|---|---|---|---|
| NT5E | TTTGGGGAAACCTTTGACC | AGAGGCTCGTAACTGGGTACTC | 116 | 54.1 |
| THY1 | CGGCTTCACCACCAAGGACG | TCTGGGCCAGCAGGCTTATG | 140 | 57.6 |
| ENG | CCTCAGTGCAAAGAAGAAT | CTTGGAAGATCAGTTTGGGG | 89 | 51.4 |
| FAS | GGCTGGAGCCGGCTACTGCC | ATTCAGGATGGTAGCGTACA | 94 | 56.7 |
| FABP4 | CACCATTAAATCAGAAAGCACC | CCAGGACACCTCCATCTAAG | 128 | 50.9 |
| PPARγ | TAAAGAGCCTGAGAAAGCC | GCTTCACATTCAGCAAACC | 156 | 51.8 |
| SP7 | TGCTTGAGGAGGAAGCTCAC | TTTGGGGGCTGAAAGGTCAC | 161 | 58.3 |
| BGLAP | TGCAACCTTCGTGTCCAAG | TGGAAGCCAATGTGGTCAG | 171 | 59 |
| SPP1 | TGATTTTCCCACTGACATTCC | TCCATACTCGCACTTTTCAC | 195 | 53 |
| GAPDH | AAGAAGGTAGTGAAGCAGG | GCGTCGAAGGTGGAAGAGTGGG | 212 | 54.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, U.; Saba, E.; Yousaf, A.; Tareen, W.A.; Sarfraz, A.; Rhee, M.H.; Sandhu, M.A. Autologous Platelet Lysate Is an Alternative to Fetal Bovine Serum for Canine Adipose-Derived Mesenchymal Stem Cell Culture and Differentiation. Animals 2023, 13, 2655. https://doi.org/10.3390/ani13162655
Rashid U, Saba E, Yousaf A, Tareen WA, Sarfraz A, Rhee MH, Sandhu MA. Autologous Platelet Lysate Is an Alternative to Fetal Bovine Serum for Canine Adipose-Derived Mesenchymal Stem Cell Culture and Differentiation. Animals. 2023; 13(16):2655. https://doi.org/10.3390/ani13162655
Chicago/Turabian StyleRashid, Usman, Evelyn Saba, Arfan Yousaf, Waleed Ahsan Tareen, Adeel Sarfraz, Man Hee Rhee, and Mansur Abdullah Sandhu. 2023. "Autologous Platelet Lysate Is an Alternative to Fetal Bovine Serum for Canine Adipose-Derived Mesenchymal Stem Cell Culture and Differentiation" Animals 13, no. 16: 2655. https://doi.org/10.3390/ani13162655
APA StyleRashid, U., Saba, E., Yousaf, A., Tareen, W. A., Sarfraz, A., Rhee, M. H., & Sandhu, M. A. (2023). Autologous Platelet Lysate Is an Alternative to Fetal Bovine Serum for Canine Adipose-Derived Mesenchymal Stem Cell Culture and Differentiation. Animals, 13(16), 2655. https://doi.org/10.3390/ani13162655






