Beyond Canine Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells Transplantation: An Update on Their Secretome Characterization and Applications
Abstract
:Simple Summary
Abstract
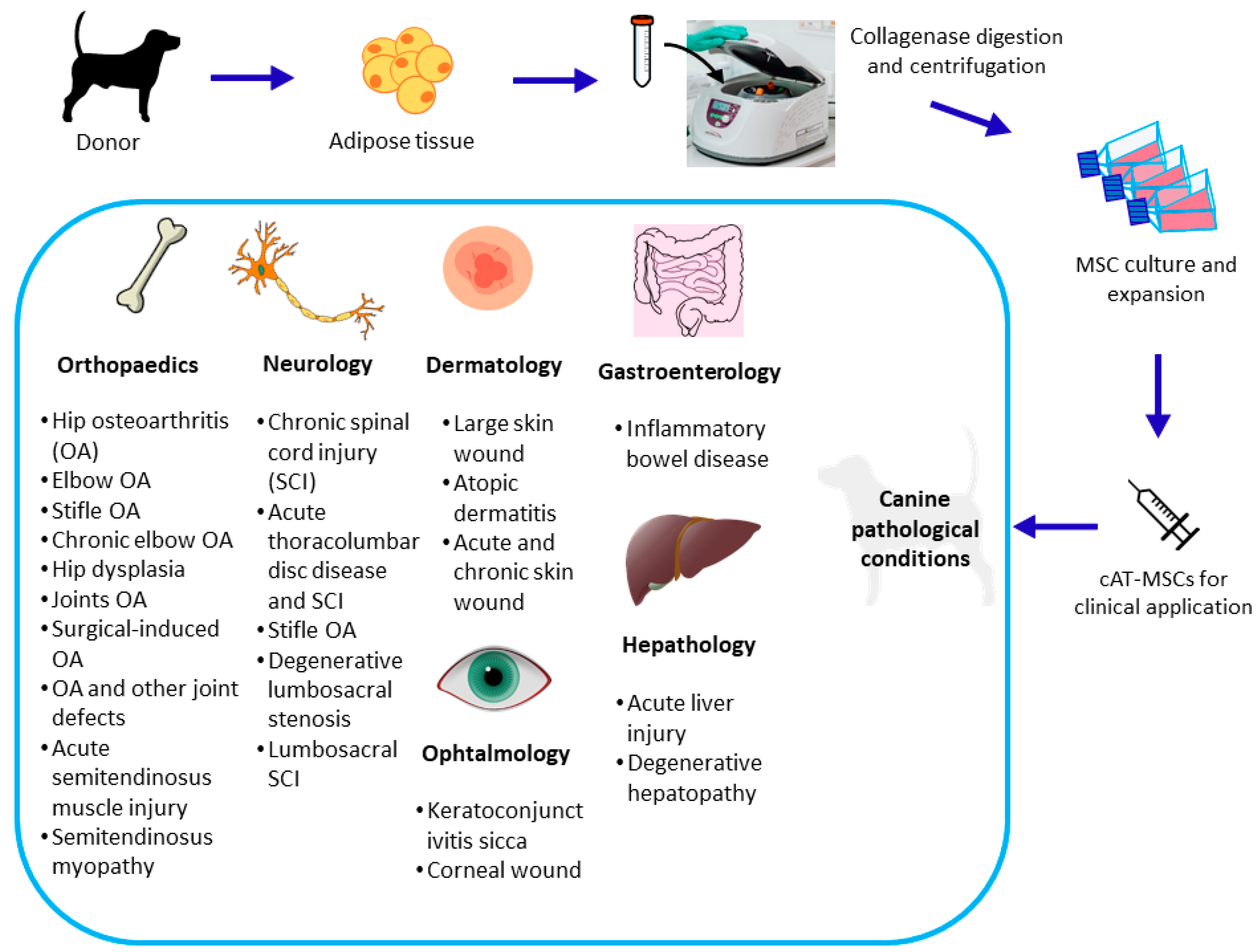
1. Introduction
2. Secretome Characterization
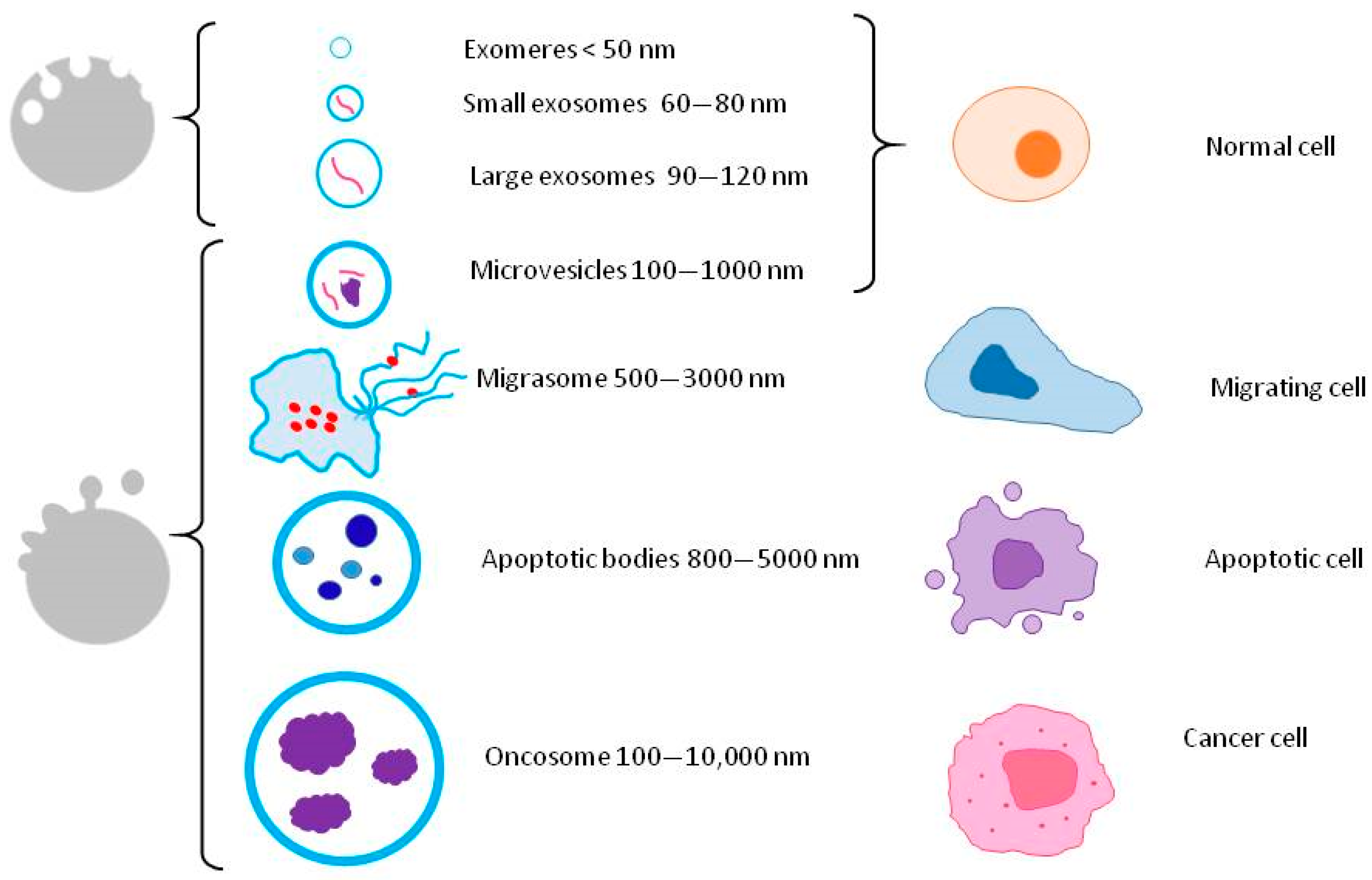
2.1. Extracellular Vesicles
2.2. Soluble Factors
3. Secretome Applications
3.1. Inflammatory and Immuno-Mediated Diseases
3.2. Infections
3.3. Skin and Ear Diseases
3.4. Orthopedic Diseases
3.5. Neurologic Diseases
3.6. Endocrine Diseases
3.7. Tumors
3.8. Other Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| AF | Amniotic fluid |
| AM | Amnion |
| AMPs | Antimicrobial peptides and proteins |
| AT | Adipose tissue |
| bFGF | Basic fibroblast growth factor |
| cAT-MSCs | Canine adipose tissue mesenchymal stem/stromal cells |
| cINF-β | Canine interferon beta |
| CD | Cluster of differentiation |
| CM | Conditioned medium |
| COX-2 | Cyclooxygenase-2 |
| DFO | Deferoxamine |
| DM | Diabetes mellitus |
| DMEM | Dulbecco’s minimum essential medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| DSS | Dextrane sulfate sodium |
| EGF | Epidermal growth factor |
| EM | Epithelial migration |
| EPO | Erythropoietin |
| EV | Ex vivo |
| EVs | Extracellular vesicles |
| F12 | Nutrient mixture F-12 |
| FBS | Fetal bovine serum |
| FC | Flow cytometry |
| FGF-1 | Fibroblast growth factor 1 |
| GFAP | Glial fibrillary acidic protein |
| GLUT4 | Insulin-regulated glucose transporter |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| GMP | Good manufacturing practice |
| GO | Gene Ontology |
| HCC | Hepatocellular carcinoma |
| HGF | Hepatocyte growth factor |
| HGF-R | Hepatocyte growth factor receptor |
| IBD | Inflammatory bowel disease |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN-β | Interferon beta |
| IFN-γ | Interferon gamma |
| IGF-2 | Insulin-like growth factor 2 |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IRS-1 | Insulin receptor substrate 1 |
| ISEVISO | International Society for Extracellular VesiclesInternational Organization for Standardization |
| IV | In vitro |
| IVO | In vivo |
| KGF | Keratinocyte growth factor |
| LPS | Lipopolysaccharide |
| M1 | Pro-inflammatory macrophage phenotype |
| M2 | Anti-inflammatory macrophage phenotype |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MIP-1β | Macrophage inflammatory protein 1 beta |
| miRNA | Micro RNA |
| MISEV | Minimal Information for Studies of Extracellular Vesicles |
| MMP-3 | Matrix metalloproteinase-3 |
| MSCs | Mesenchymal stem/stromal cells |
| NG2 | Neuron–glial antigen 2 |
| NGF-β | Beta-nerve grown factor |
| NGS | Next-generation sequencing |
| NO | Nitric oxide |
| NOAEL | No observed adverse effect level |
| NTA | Nanoparticle tracking analysis |
| OA | Osteoarthritis |
| PBMCs | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PDGF-β | Platelet-derived growth factor beta |
| PGE2 | Prostaglandin E2 |
| RAGE | Receptor for advanced glycation end products |
| rFGF-1 | recombinant fibroblast growth factor 1 |
| ROM | Range of motion |
| SCF | Stem cell factor |
| SCI | Spinal cord injury |
| SCSC | Spinal cord slide culture |
| siRNA | Small interfering RNA |
| STAT3 | Signal transducer and activator of transcription 3 |
| T1DM | Type 1 diabetes mellitus |
| TEM | Transmission electron microscopy |
| TIMP-1 | Tissue inhibitor metallopeptidase 1 |
| TGF-β | Transforming growth factor beta |
| TLR3 | Toll-like receptor 3 |
| TM | Tympanic membrane |
| TNFα | Tumor necrosis factor alpha |
| TNF-RI | Tumor necrosis factor receptor I |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TSG-6 | TNF-α-stimulated gene/protein 6 |
| TSG101 | Tumor susceptibility gene 101 |
| TEWL | Trans epidermal water loss |
| VEGF-A | Vascular endothelial growth factor A |
| WB | Western Blot |
References
- Hoffman, A.M.; Dow, S.W. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells 2016, 34, 1709–1729. [Google Scholar] [CrossRef] [PubMed]
- Harman, R.M.; Marx, C.; Van de Walle, G.R. Translational Animal Models Provide Insight into Mesenchymal Stromal Cell (MSC) Secretome Therapy. Front. Cell Dev. Biol. 2021, 9, 654885. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.C.; Stewart, A.A. Mesenchymal Stem Cells: Characteristics, Sources, and Mechanisms of Action. Vet. Clin. Equine Pract. 2011, 27, 243–261. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tuan, R.S. Transdifferentiation Potential of Human Mesenchymal Stem Cells Derived from Bone Marrow. FASEB J. 2004, 18, 980–982. [Google Scholar] [CrossRef]
- Pezzanite, L.M.; Fortier, L.A.; Antczak, D.F.; Cassano, J.M.; Brosnahan, M.M.; Miller, D.; Schnabel, L.V. Equine Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Elicit Antibody Responses in Vivo. Stem Cell Res. Ther. 2015, 6, 54. [Google Scholar] [CrossRef]
- Neupane, M.; Chang, C.-C.; Kiupel, M.; Yuzbasiyan-Gurkan, V. Isolation and Characterization of Canine Adipose–Derived Mesenchymal Stem Cells. Tissue Eng. Part A 2008, 14, 1007–1015. [Google Scholar] [CrossRef]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal Stem Cells: Amazing Remedies for Bone and Cartilage Defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef]
- Miao, C.; Lei, M.; Hu, W.; Han, S.; Wang, Q. A Brief Review: The Therapeutic Potential of Bone Marrow Mesenchymal Stem Cells in Myocardial Infarction. Stem Cell Res. Ther. 2017, 8, 242. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Cubinkova, V.; Smolek, T.; Murgoci, A.-N.; Danko, J.; Vdoviakova, K.; Humenik, F.; Cizek, M.; Quanico, J.; Fournier, I.; et al. Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 870. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The Secretion Profile of Mesenchymal Stem Cells and Potential Applications in Treating Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Arthaud-Day, M.L.; Weiss, M.L. Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species. Front. Cell Dev. Biol. 2021, 9, 632717. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.G. A Set of Grand Challenges for Veterinary Regenerative Medicine. Front. Vet. Sci. 2016, 3, 20. [Google Scholar] [CrossRef]
- Rizk, M.; Monaghan, M.; Shorr, R.; Kekre, N.; Bredeson, C.N.; Allan, D.S. Heterogeneity in Studies of Mesenchymal Stromal Cells to Treat or Prevent Graft-versus-Host Disease: A Scoping Review of the Evidence. Biol. Blood Marrow Transplant. 2016, 22, 1416–1423. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Bjørge, I.M.; Kim, S.Y.; Mano, J.F.; Kalionis, B.; Chrzanowski, W. Extracellular Vesicles, Exosomes and Shedding Vesicles in Regenerative Medicine—A New Paradigm for Tissue Repair. Biomater. Sci. 2017, 6, 60–78. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tsuchiya, A.; Terai, S. The Development of Mesenchymal Stem Cell Therapy in the Present, and the Perspective of Cell-Free Therapy in the Future. Clin. Mol. Hepatol. 2021, 27, 70–80. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; Rizvanov, A.A. Current Trends in Regenerative Medicine: From Cell to Cell-Free Therapy. BioNanoScience 2017, 7, 240–245. [Google Scholar] [CrossRef]
- Prišlin, M.; Vlahović, D.; Kostešić, P.; Ljolje, I.; Brnić, D.; Turk, N.; Lojkić, I.; Kunić, V.; Karadjole, T.; Krešić, N. An Outstanding Role of Adipose Tissue in Canine Stem Cell Therapy. Animals 2022, 12, 1088. [Google Scholar] [CrossRef] [PubMed]
- Humenik, F.; Maloveska, M.; Hudakova, N.; Petrouskova, P.; Hornakova, L.; Domaniza, M.; Mudronova, D.; Bodnarova, S.; Cizkova, D. A Comparative Study of Canine Mesenchymal Stem Cells Isolated from Different Sources. Animals 2022, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Tjalsma, H.; Bolhuis, A.; Jongbloed, J.D.H.; Bron, S.; van Dijl, J.M. Signal Peptide-Dependent Protein Transport in Bacillus Subtilis: A Genome-Based Survey of the Secretome. Microbiol. Mol. Biol. Rev. 2000, 64, 515–547. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.L.; Stahl, P.D.; Raposo, G. Extracellular Vesicles Shuffling Intercellular Messages: For Good or for Bad. Curr. Opin. Cell Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as New Vesicular Lipid Transporters Involved in Cell–Cell Communication and Various Pathophysiologies. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2014, 1841, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Silva, A.M.; Teixeira, J.H.; Almeida, M.I.; Gonçalves, R.M.; Barbosa, M.A.; Santos, S.G. Extracellular Vesicles: Immunomodulatory Messengers in the Context of Tissue Repair/Regeneration. Eur. J. Pharm. Sci. 2017, 98, 86–95. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Villatoro, A.J.; Alcoholado, C.; Martín-Astorga, M.C.; Fernández, V.; Cifuentes, M.; Becerra, J. Comparative Analysis and Characterization of Soluble Factors and Exosomes from Cultured Adipose Tissue and Bone Marrow Mesenchymal Stem Cells in Canine Species. Vet. Immunol. Immunopathol. 2019, 208, 6–15. [Google Scholar] [CrossRef]
- An, J.-H.; Li, Q.; Bhang, D.-H.; Song, W.-J.; Youn, H.-Y. TNF-α and INF-γ Primed Canine Stem Cell-Derived Extracellular Vesicles Alleviate Experimental Murine Colitis. Sci. Rep. 2020, 10, 2115. [Google Scholar] [CrossRef]
- An, J.-H.; Li, Q.; Ryu, M.-O.; Nam, A.-R.; Bhang, D.-H.; Jung, Y.-C.; Song, W.-J.; Youn, H.-Y. TSG-6 in Extracellular Vesicles from Canine Mesenchymal Stem/Stromal Is a Major Factor in Relieving DSS-Induced Colitis. PLoS ONE 2020, 15, e0220756. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; An, J.-H.; Lee, J.-H.; Kim, K.-B.; Chae, H.-K.; Oh, Y.-I.; Song, W.-J.; Youn, H.-Y. Extracellular Vesicles Derived from DFO-Preconditioned Canine AT-MSCs Reprogram Macrophages into M2 Phase. PLoS ONE 2021, 16, e0254657. [Google Scholar] [CrossRef] [PubMed]
- Teshima, T.; Yuchi, Y.; Suzuki, R.; Matsumoto, H.; Koyama, H. Immunomodulatory Effects of Canine Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles on Stimulated CD4+ T Cells Isolated from Peripheral Blood Mononuclear Cells. J. Immunol. Res. 2021, 2021, 2993043. [Google Scholar] [CrossRef] [PubMed]
- Mocchi, M.; Bari, E.; Dotti, S.; Villa, R.; Berni, P.; Conti, V.; Del Bue, M.; Squassino, G.P.; Segale, L.; Ramoni, R.; et al. Canine Mesenchymal Cell Lyosecretome Production and Safety Evaluation after Allogenic Intraarticular Injection in Osteoarthritic Dogs. Animals 2021, 11, 3271. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoon, T.H.; Na, J.; Yi, S.J.; Jin, Y.; Kim, M.; Oh, T.-H.; Chung, T.-W. Mesenchymal Stem Cells and Extracellular Vesicles Derived from Canine Adipose Tissue Ameliorates Inflammation, Skin Barrier Function and Pruritus by Reducing JAK/STAT Signaling in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 4868. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Kim, S.-B.; Kim, S.; Rhee, B.; Yoon, J.; Lee, J.W. Canine Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Atopic Dermatitis. Animals 2023, 13, 2215. [Google Scholar] [CrossRef]
- Koeppen, K.; Nymon, A.; Barnaby, R.; Bashor, L.; Li, Z.; Hampton, T.H.; Liefeld, A.E.; Kolling, F.W.; LaCroix, I.S.; Gerber, S.A.; et al. Let-7b-5p in Vesicles Secreted by Human Airway Cells Reduces Biofilm Formation and Increases Antibiotic Sensitivity of P. Aeruginosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2105370118. [Google Scholar] [CrossRef]
- Castro-Leyva, V.; Arenas-Huertero, F.; Espejel-Núñez, A.; Giono Cerezo, S.; Flores-Pliego, A.; Espino y Sosa, S.; Reyes-Muñoz, E.; Vadillo-Ortega, F.; Borboa-Olivares, H.; Camacho-Arroyo, I.; et al. miR-21 Differentially Regulates IL-1β and IL-10 Expression in Human Decidual Cells Infected with Streptococcus B. Reprod. Biol. 2022, 22, 100604. [Google Scholar] [CrossRef]
- Tangtanatakul, P. Down-Regulation of Let-7a and miR-21 in Urine Exosomes from Lupus Nephritis Patients during Disease Flare. Asian Pac. J. Allergy Immunol. 2019, 37, 189–197. [Google Scholar] [CrossRef]
- Chen, G.; Cao, Y.; Zhong, H.; Wang, X.; Li, Y.; Cui, X.; Lu, X.; Bi, X.; Dai, M. Serine/Threonine Kinases Play Important Roles in Regulating Polyunsaturated Fatty Acid Biosynthesis in Synechocystis sp. PCC6803. Front. Bioeng. Biotechnol. 2021, 9, 618969. [Google Scholar] [CrossRef]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The Role of JAK/STAT Signalling in the Pathogenesis, Prognosis and Treatment of Solid Tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Vaka, R.; Parent, S.; Risha, Y.; Khan, S.; Courtman, D.; Stewart, D.J.; Davis, D.R. Extracellular Vesicle microRNA and Protein Cargo Profiling in Three Clinical-Grade Stem Cell Products Reveals Key Functional Pathways. Mol. Ther. Nucleic Acids 2023, 32, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular Vesicle-Based Therapeutics: Natural versus Engineered Targeting and Trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Shelton, D.L.; Reichardt, L.F. Studies on the Expression of the Beta Nerve Growth Factor (NGF) Gene in the Central Nervous System: Level and Regional Distribution of NGF mRNA Suggest That NGF Functions as a Trophic Factor for Several Distinct Populations of Neurons. Proc. Natl. Acad. Sci. USA 1986, 83, 2714–2718. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.T.; Zander, A. Stem cell factor as a survival and growth factor in human normal and malignant hematopoiesis. Acta Haematol. 1996, 95, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mbongue, J.C.; Nicholas, D.A.; Torrez, T.W.; Kim, N.-S.; Firek, A.F.; Langridge, W.H.R. The Role of Indoleamine 2,3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines 2015, 3, 703–729. [Google Scholar] [CrossRef]
- Kang, J.W.; Kang, K.-S.; Koo, H.C.; Park, J.R.; Choi, E.W.; Park, Y.H. Soluble Factors–Mediated Immunomodulatory Effects of Canine Adipose Tissue–Derived Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 681–694. [Google Scholar] [CrossRef]
- Chow, L.; Johnson, V.; Coy, J.; Regan, D.; Dow, S. Mechanisms of Immune Suppression Utilized by Canine Adipose and Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 374–389. [Google Scholar] [CrossRef]
- Teshima, T.; Matsuoka, A.; Shiba, M.; Dairaku, K.; Matsumoto, H.; Suzuki, R.; Koyama, H. Comparison of Properties of Stem Cells Isolated from Adipose Tissue and Lipomas in Dogs. Stem Cells Int. 2019, 2019, e1609876. [Google Scholar] [CrossRef]
- Han, S.-M.; Park, C.-W.; Ahn, J.-O.; Park, S.-C.; Jung, W.-S.; Seo, K.-W.; Ra, J.-C.; Kang, S.-K.; Lee, H.-W.; Youn, H.-Y. Pro-Apoptotic and Growth-Inhibitory Effect of IFN-β-Overexpressing Canine Adipose Tissue-Derived Mesenchymal Stem Cells Against Melanoma Cells. Anticancer Res. 2015, 35, 4749–4756. [Google Scholar] [PubMed]
- Al Delfi, I.R.; Sheard, J.J.; Wood, C.R.; Vernallis, A.; Innes, J.F.; Myint, P.; Johnson, W.E.B. Canine Mesenchymal Stem Cells Are Neurotrophic and Angiogenic: An in Vitro Assessment of Their Paracrine Activity. Vet. J. 2016, 217, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Li, Q.; Song, W.-J.; Yang, H.-M.; Kim, S.-Y.; Park, S.-C.; Ahn, J.-O.; Youn, H.-Y. Fibroblast Growth Factor-1 as a Mediator of Paracrine Effects of Canine Adipose Tissue-Derived Mesenchymal Stem Cells on in Vitro-Induced Insulin Resistance Models. BMC Vet. Res. 2018, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Teshima, T.; Matsumoto, H.; Koyama, H. Soluble Factors from Adipose Tissue-Derived Mesenchymal Stem Cells Promote Canine Hepatocellular Carcinoma Cell Proliferation and Invasion. PLoS ONE 2018, 13, e0191539. [Google Scholar] [CrossRef] [PubMed]
- Al Delfi, I.R.T.A.; Wood, C.R.; Johnson, L.D.V.; Snow, M.D.; Innes, J.F.; Myint, P.; Johnson, W.E.B. An In Vitro Comparison of the Neurotrophic and Angiogenic Activity of Human and Canine Adipose-Derived Mesenchymal Stem Cells (MSCs): Translating MSC-Based Therapies for Spinal Cord Injury. Biomolecules 2020, 10, 1301. [Google Scholar] [CrossRef] [PubMed]
- Huňáková, K.; Hluchý, M.; Špaková, T.; Matejová, J.; Mudroňová, D.; Kuricová, M.; Rosocha, J.; Ledecký, V. Study of Bilateral Elbow Joint Osteoarthritis Treatment Using Conditioned Medium from Allogeneic Adipose Tissue-Derived MSCs in Labrador Retrievers. Res. Vet. Sci. 2020, 132, 513–520. [Google Scholar] [CrossRef]
- Wood, C.R.; Juárez, E.H.; Ferrini, F.; Myint, P.; Innes, J.; Lossi, L.; Merighi, A.; Johnson, W.E.B. Mesenchymal Stem Cell Conditioned Medium Increases Glial Reactivity and Decreases Neuronal Survival in Spinal Cord Slice Cultures. Biochem. Biophys. Rep. 2021, 26, 100976. [Google Scholar] [CrossRef]
- Johnson, V.; Chow, L.; Harrison, J.; Soontararak, S.; Dow, S. Activated Mesenchymal Stromal Cell Therapy for Treatment of Multi-Drug Resistant Bacterial Infections in Dogs. Front. Vet. Sci. 2022, 9, 925701. [Google Scholar] [CrossRef]
- Pérez-Delgado, S.; Ginel, P.J.; Guerra, R.; Mozos, E.; Alcoholado, C.; del Carmen Martin-Astorga, M.; Becerra, J.; Villatoro, A.J. Effect of Canine Adipose Mesenchymal Stem Cell Secretome on a Model of Second-Intention Wound Healing in the Red-Eared Slider Turtle (Trachemys scripta). J. Wildl. Dis. 2022, 58, 368–372. [Google Scholar] [CrossRef]
- Suh, H.; Kim, S.; Oh, T.; Bae, S. Canine Stem Cell Conditioned Media Accelerates Epithelial Migration in the Canine Tympanic Membrane. Vet. Sci. 2022, 9, 69. [Google Scholar] [CrossRef]
- Malewska, K. Treatment of Inflammatory Bowel Disease (IBD) in Dogs and Cats. Pol. J. Vet. Sci. 2011, 14, 165–171. [Google Scholar] [CrossRef]
- Elhag, D.A.; Kumar, M.; Saadaoui, M.; Akobeng, A.K.; Al-Mudahka, F.; Elawad, M.; Al Khodor, S. Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. Int. J. Mol. Sci. 2022, 23, 6966. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 839. [Google Scholar] [CrossRef]
- Outerbridge, C.A.; Jordan, T.J.M. Current Knowledge on Canine Atopic Dermatitis. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef]
- Munera-Campos, M.; Carrascosa, J.M. Innovation in Atopic Dermatitis: From Pathogenesis to Treatment. Actas Dermo-Sifiliográficas Engl. Ed. 2020, 111, 205–221. [Google Scholar] [CrossRef]
- Huang, C.-P.; Yang, C.-Y.; Shyr, C.-R. Utilizing Xenogeneic Cells as a Therapeutic Agent for Treating Diseases. Cell Transplant. 2021, 30, 09636897211011995. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Hawke, M. An Ultrastructural Study of the Skin of the Tympanic Membrane and External Ear Canal of the Guinea Pig. J. Otolaryngol. 1985, 14, 357–364. [Google Scholar] [PubMed]
- Gladstone, H.B.; Jackler, R.K.; Varav, K. Tympanic Membrane Wound Healing: An Overview*. Otolaryngol. Clin. N. Am. 1995, 28, 913–932. [Google Scholar] [CrossRef]
- Anand, S.; Danti, S.; Moroni, L.; Mota, C. Regenerative Therapies for Tympanic Membrane. Prog. Mater. Sci. 2022, 127, 100942. [Google Scholar] [CrossRef]
- Sainsbury, E.; do Amaral, R.; Blayney, A.W.; Walsh, R.M.; O’Brien, F.J.; O’Leary, C. Tissue Engineering and Regenerative Medicine Strategies for the Repair of Tympanic Membrane Perforations. Biomater. Biosyst. 2022, 6, 100046. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.L.; Zulch, H.; O’Neill, D.G.; Meeson, R.L.; Collins, L.M. Risk Factors for Canine Osteoarthritis and Its Predisposing Arthropathies: A Systematic Review. Front. Vet. Sci. 2020, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Pye, C.; Bruniges, N.; Peffers, M.; Comerford, E. Advances in the Pharmaceutical Treatment Options for Canine Osteoarthritis. J. Small Anim. Pract. 2022, 63, 721–738. [Google Scholar] [CrossRef]
- Zhang, C.; Kang, J.; Zhang, X.; Zhang, Y.; Huang, N.; Ning, B. Spatiotemporal Dynamics of the Cellular Components Involved in Glial Scar Formation Following Spinal Cord Injury. Biomed. Pharmacother. 2022, 153, 113500. [Google Scholar] [CrossRef]
- McMahill, B.G.; Borjesson, D.L.; Sieber-Blum, M.; Nolta, J.A.; Sturges, B.K. Stem Cells in Canine Spinal Cord Injury—Promise for Regenerative Therapy in a Large Animal Model of Human Disease. Stem Cell Rev. Rep. 2015, 11, 180–193. [Google Scholar] [CrossRef]
- Gao, S.; Guo, X.; Zhao, S.; Jin, Y.; Zhou, F.; Yuan, P.; Cao, L.; Wang, J.; Qiu, Y.; Sun, C.; et al. Differentiation of Human Adipose-Derived Stem Cells into Neuron/Motoneuron-like Cells for Cell Replacement Therapy of Spinal Cord Injury. Cell Death Dis. 2019, 10, 597. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, X.; Mo, B.; Xu, H.; Zhang, L.; Huang, L.; Yao, S.; Huang, Z.; Wang, Y.; Xie, H.; et al. Adipose Mesenchymal Stem Cell Transplantation Alleviates Spinal Cord Injury-Induced Neuroinflammation Partly by Suppressing the Jagged1/Notch Pathway. Stem Cell Res. Ther. 2020, 11, 212. [Google Scholar] [CrossRef]
- Chen, C.-C.; Yang, S.-F.; Wang, I.-K.; Hsieh, S.-Y.; Yu, J.-X.; Wu, T.-L.; Huang, W.-J.; Su, M.-H.; Yang, H.-L.; Chang, P.-C.; et al. The Long-Term Efficacy Study of Multiple Allogeneic Canine Adipose Tissue-Derived Mesenchymal Stem Cells Transplantations Combined with Surgery in Four Dogs with Lumbosacral Spinal Cord Injury. Cell Transplant. 2022, 31, 09636897221081487. [Google Scholar] [CrossRef]
- Stoppini, L.; Buchs, P.-A.; Muller, D. A Simple Method for Organotypic Cultures of Nervous Tissue. J. Neurosci. Methods 1991, 37, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.M.; Jonker, J.W.; Ahmadian, M.; Goetz, R.; Lackey, D.; Osborn, O.; Huang, Z.; Liu, W.; Yoshihara, E.; van Dijk, T.; et al. Endocrinization of FGF1 Produces a Neomorphic and Potent Insulin Sensitizer. Nature 2014, 513, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Aravindhan, S.; Ejam, S.S.; Lafta, M.H.; Markov, A.; Yumashev, A.V.; Ahmadi, M. Mesenchymal Stem Cells and Cancer Therapy: Insights into Targeting the Tumour Vasculature. Cancer Cell Int. 2021, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.J. Canine Oral Melanoma. Clin. Tech. Small Anim. Pract. 2007, 22, 55–60. [Google Scholar] [CrossRef]
- Yoshida, J.; Mizuno, M.; Wakabayashi, T. Interferon-Beta Gene Therapy for Cancer: Basic Research to Clinical Application. Cancer Sci. 2004, 95, 858–865. [Google Scholar] [CrossRef]
- Li, G.-C.; Ye, Q.-H.; Xue, Y.-H.; Sun, H.-J.; Zhou, H.-J.; Ren, N.; Jia, H.-L.; Shi, J.; Wu, J.-C.; Dai, C.; et al. Human Mesenchymal Stem Cells Inhibit Metastasis of a Hepatocellular Carcinoma Model Using the MHCC97-H Cell Line. Cancer Sci. 2010, 101, 2546–2553. [Google Scholar] [CrossRef]
- Zhao, W.; Ren, G.; Zhang, L.; Zhang, Z.; Liu, J.; Kuang, P.; Yin, Z.; Wang, X. Efficacy of Mesenchymal Stem Cells Derived from Human Adipose Tissue in Inhibition of Hepatocellular Carcinoma Cells In Vitro. Cancer Biother. Radiopharm. 2012, 27, 606–613. [Google Scholar] [CrossRef]
- Hanson, B.; Johnstone, E.; Dorais, J.; Silver, B.; Peterson, C.M.; Hotaling, J. Female Infertility, Infertility-Associated Diagnoses, and Comorbidities: A Review. J. Assist. Reprod. Genet. 2017, 34, 167–177. [Google Scholar] [CrossRef]
- Saha, S.; Roy, P.; Corbitt, C.; Kakar, S.S. Application of Stem Cell Therapy for Infertility. Cells 2021, 10, 1613. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Capra, E.; Herrera, V.; Lang-Olip, I.; Ponsaerts, P.; Cremonesi, F. Application of Perinatal Derivatives in Ovarian Diseases. Front. Bioeng. Biotechnol. 2022, 10, 818875. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Khalil, W.A.; Alharbi, M.G.; Lee, S.H. The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation. Animals 2020, 10, 2281. [Google Scholar] [CrossRef] [PubMed]
- Mahiddine, F.Y.; Kim, M.-J. Overview on the Antioxidants, Egg Yolk Alternatives, and Mesenchymal Stem Cells and Derivatives Used in Canine Sperm Cryopreservation. Animals 2021, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Improved Post-Thaw Quality of Canine Semen after Treatment with Exosomes from Conditioned Medium of Adipose-Derived Mesenchymal Stem Cells. Animals 2019, 9, 865. [Google Scholar] [CrossRef] [PubMed]

| Means | Isolation | Characterization | Reference |
|---|---|---|---|
| DMEM + 10% FBS exosomes free, 72 h | Centrifugation (13,000× g 30 min) and ultracentrifugation (100,000× g 60 min at 4 °C twice) | TEM, WB, proteomic analysis, and PBMCs proliferation in vitro test | [29] |
| DMEM + 10% FBS exosomes free, 72 h | Centrifugation (300× g 10 min at 4 °C + 2000× g 30 min at 4 °C), filtration (0.22 μm), ultracentrifugation (110,000× g 80 min at 4 °C twice), and filtration (0.22 μm) | Protein concentration, TEM, WB, and LPS-stimulated macrophage in vitro test | [30] |
| DMEM + 10% FBS exosomes free, 48 h | Centrifugation (300× g 10 min at 4 °C + 2000× g 30 min at 4 °C), filtration (0.22 μm), ultracentrifugation (110,000× g 80 min at 4 °C twice), and filtration (0.22 μm) | Protein concentration, WB, and TEM | [31] |
| DMEM + 10% FBS exosomes free, 48 h | Centrifugation (2600× g 20 min) + ExoQuick™(System Biosciences, Palo Alto, CA, USA) isolation according to the manufacturer’s instructions | WB, TEM, and LPS-stimulated macrophage in vitro test | [32] |
| DMEM + 10% FBS exosomes free, 72 h | MagCapture Exosome Isolation Kit PS: centrifugation (300× g 5 min + 1200× g 20 min at 4 °C), EVs blocking reagent + ultrafiltration (Vivaspin), and exosomes isolation according to the manufacturer’s instructions | NTA, TEM, protein concentration, miRNA PCR array, and PBMCs-based in vitro tests | [33] |
| DMEM/F12 serum free, 48 h | Centrifugation (3500× g 10 min), tangential flow filtration (5 kDa cut-off), diafiltration (with ultrapure water), and freeze-drying (+0.5% mannitol) | NTA | [34] |
| DMEM/F12 serum free, 48 h | Filtration (0.22 μm), tangential flow filtration (500 kDa cut-off), and diafiltration (with PBS) | NTA, WB, and bead-based FC | [35] |
| DMEM/F12 serum free, 48 h | Centrifugation (2000× g 10 min), filtration (0.22 μm), tangential flow filtration (500 kDa cut-off), and diafiltration (with DPBS) | NTA, protein concentration, bead-based FC, calnexin concentration, and miRNA profiling by NGS | [36] |
| Treatment | Disease (Type of Study) | Effect | Reference |
|---|---|---|---|
| CM | Melanoma (IV) | ↑ apoptosis and ↓ growth of canine melanoma cells through IFN-β (overexpressed) | [51] |
| CM | Neurological disorders (IV) | ↑ proliferation of SH-SY5Y neuronal cell line, neurite outgrowth, and immunopositivity for βIII-tubulin; ↑ proliferation and migration of EA.hy926 endothelial cell line; ↑ rate of wound closure in scratch assay | [52] |
| CM | Diabetes (IV) | ↑ IRS-1 and GLUT4 in the insulin resistance model through FGF-1 | [53] |
| CM | Hepatocellular carcinoma (IV) | ↑ proliferation and invasion of canine HCC cells | [54] |
| EVs | Inflammatory bowel disease (IVO) | ↓ DSS-induced colitis in murine model through TNF-α and IFN-γ (primed EVs) | [30] |
| EVs | Inflammatory bowel disease (IVO) | ↓ DSS-induced colitis in murine model through TSG-6 | [31] |
| CM | Spinal cord injury (IV) | ↑ neuronal cell proliferation, neurite outgrowth, and βIII tubulin immunopositivity; ↑ endothelial cell migration, proliferation, and formation of tubule-like structures in Matrigel assay | [55] |
| CM | Osteoarthritis (IVO) | ≠ MMP-3, TIMP-1, IL-6 and TNF-α levels in synovial fluid; ↑ ROM | [56] |
| CM | Osteoarthritis (IVO) | Lyosecretome safety confirmation (no relevant adverse response) | [34] |
| CM | Spinal cord injury (EV) | ↑ glial cell survival and reactivity in SCSC; ↓ neuronal survival | [57] |
| CM | Chronic infections (IV) | ↑ M2 phenotype of macrophage (resting MSC CM); ↑ macrophage bactericidal activity (activated MSC CM) | [58] |
| EVs | Atopic dermatitis (IVO) | Improvement of AD-like dermatitis in a mouse model: ↓ serum IgE, epidermal inflammatory cytokines and chemokines; ↑ skin barrier repair; ↓ pruritus | [35] |
| CM | Wound healing | No effect on second intention wound healing in turtles (Trachemys scripta) | [59] |
| CM | Ear diseases (IVO) | ↑ tympanic membrane epithelial migration | [60] |
| EVs | Atopic dermatitis (IVO) | Improvement of AD-like dermatitis in a mouse model: ↓ serum IgE, inflammatory cytokines and chemokines; ↓ ear thickness | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merlo, B.; Iacono, E. Beyond Canine Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells Transplantation: An Update on Their Secretome Characterization and Applications. Animals 2023, 13, 3571. https://doi.org/10.3390/ani13223571
Merlo B, Iacono E. Beyond Canine Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells Transplantation: An Update on Their Secretome Characterization and Applications. Animals. 2023; 13(22):3571. https://doi.org/10.3390/ani13223571
Chicago/Turabian StyleMerlo, Barbara, and Eleonora Iacono. 2023. "Beyond Canine Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells Transplantation: An Update on Their Secretome Characterization and Applications" Animals 13, no. 22: 3571. https://doi.org/10.3390/ani13223571





