Recovery and Characterization of Spermatozoa in a Neotropical, Terrestrial, Direct-Developing Riparian Frog (Craugastor evanesco) through Hormonal Stimulation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
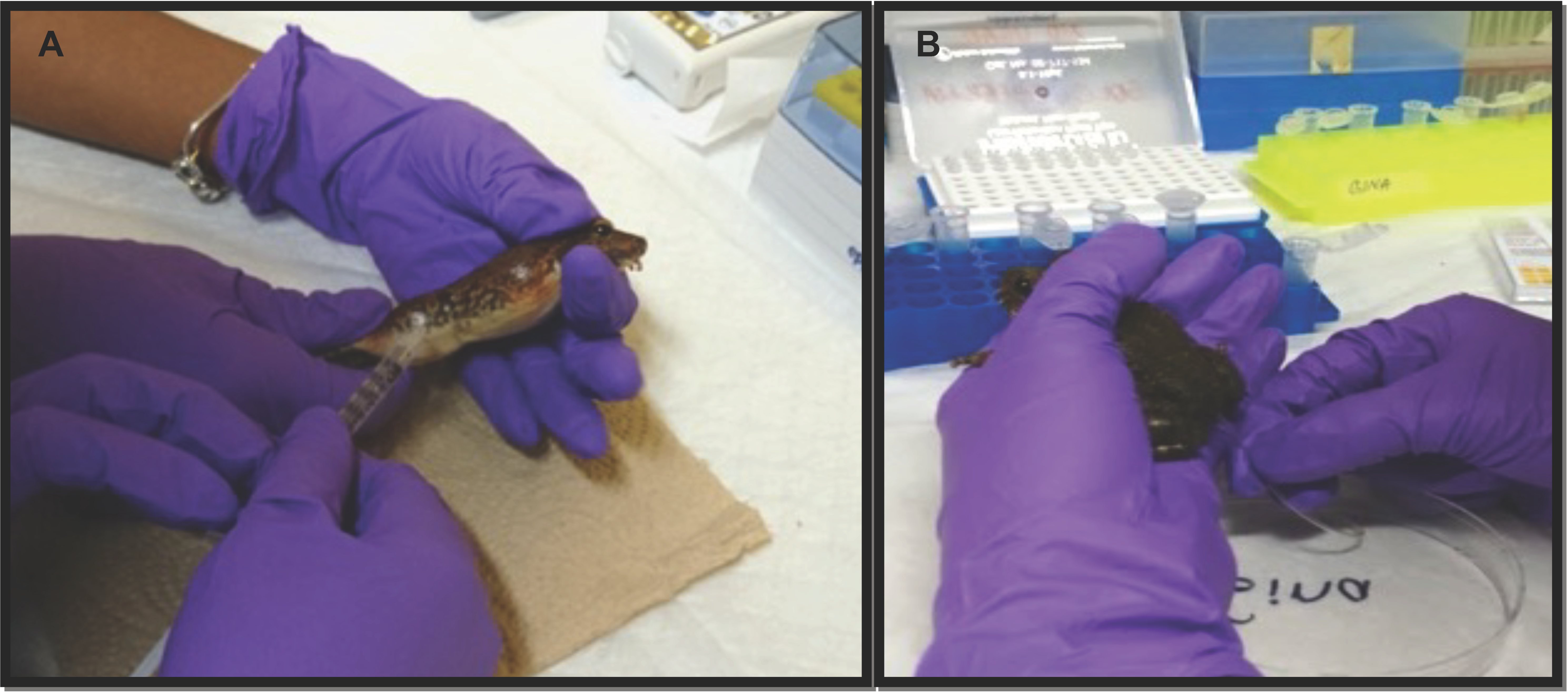
2.2. Hormone Stimulation and Sperm Collection
2.3. Sperm Processing and Analysis
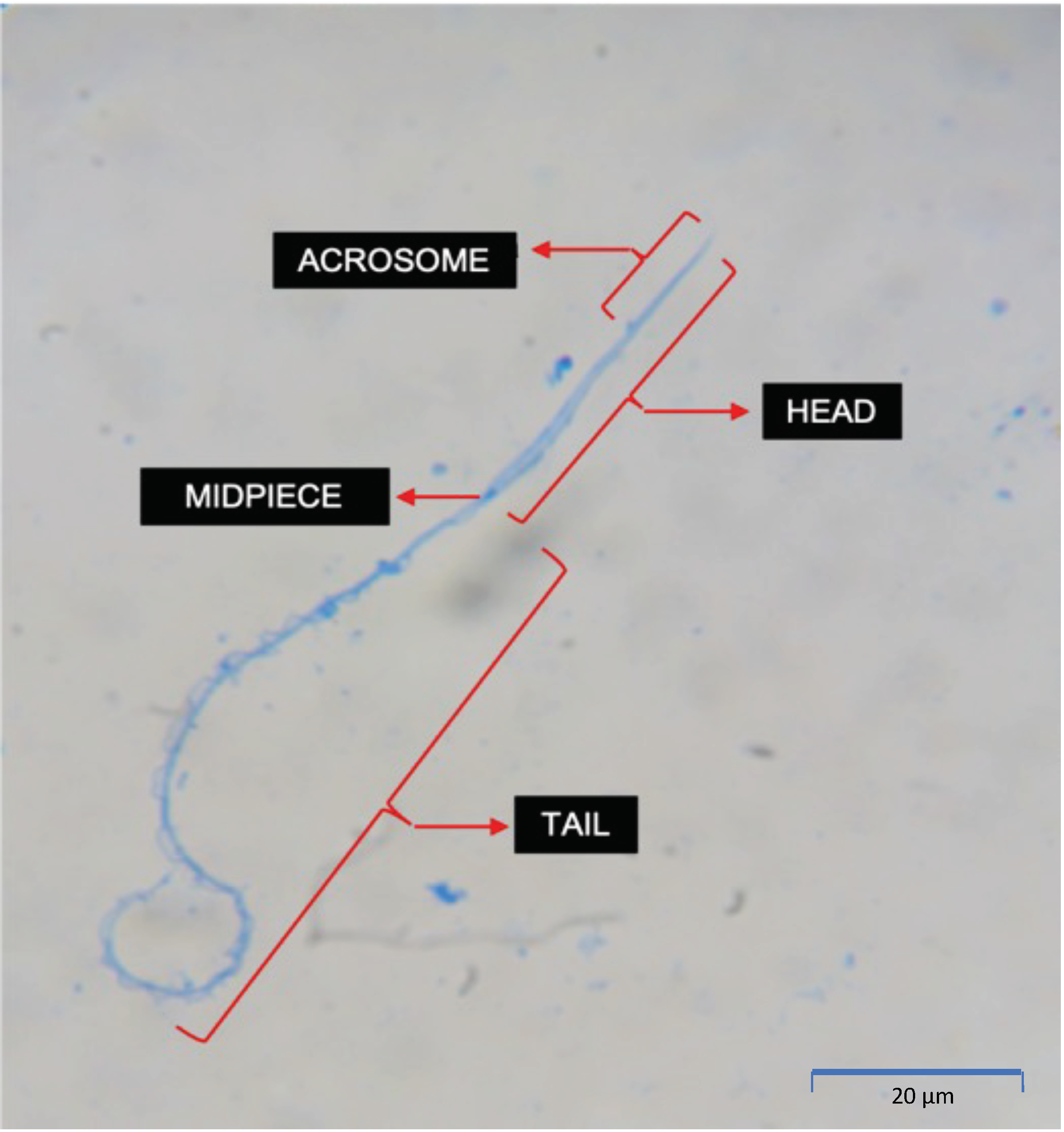
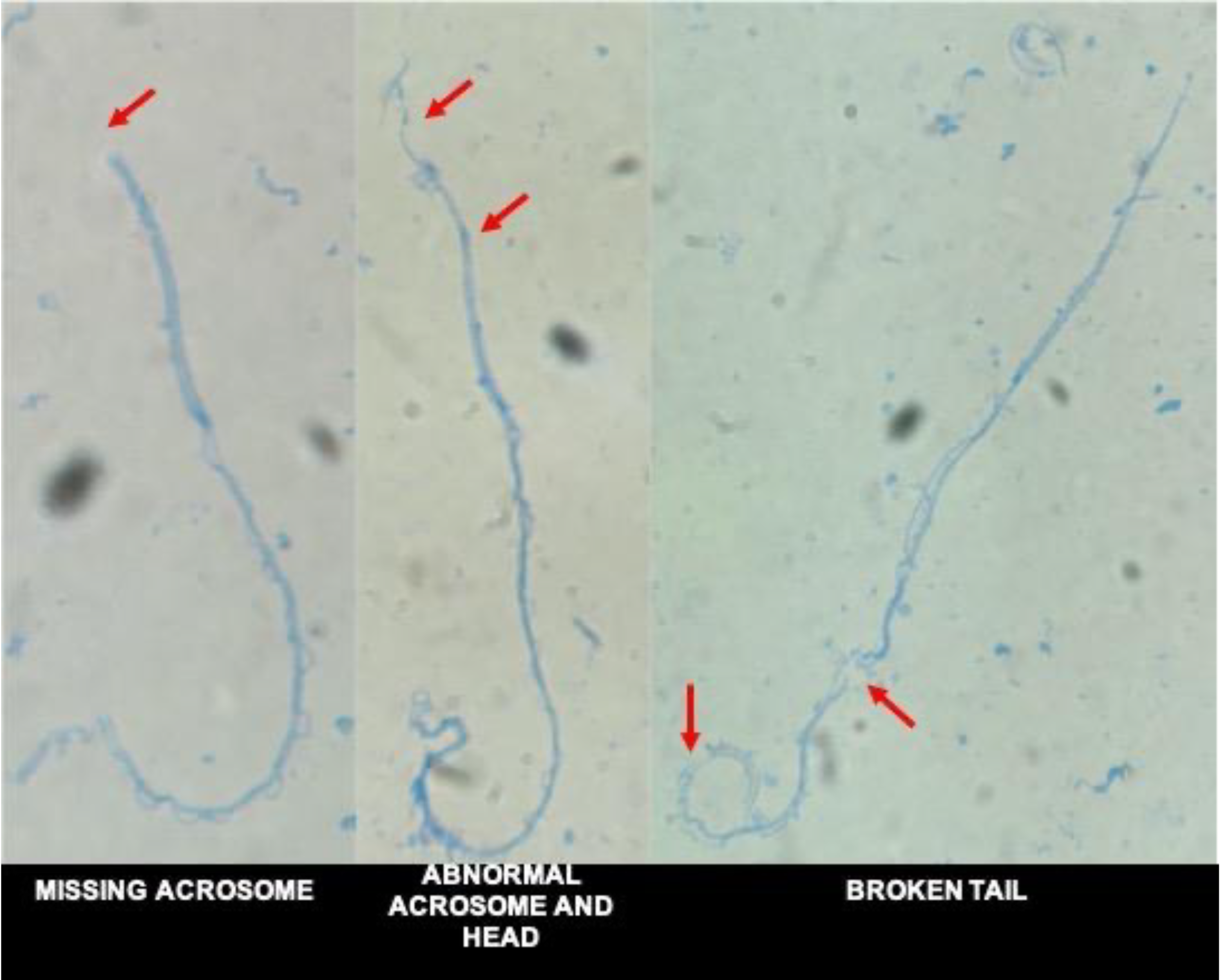
2.4. Sperm Morphological Assessment
2.5. Sperm Concentration–Response Curves and Peaks
2.6. Statistical Analysis
3. Results
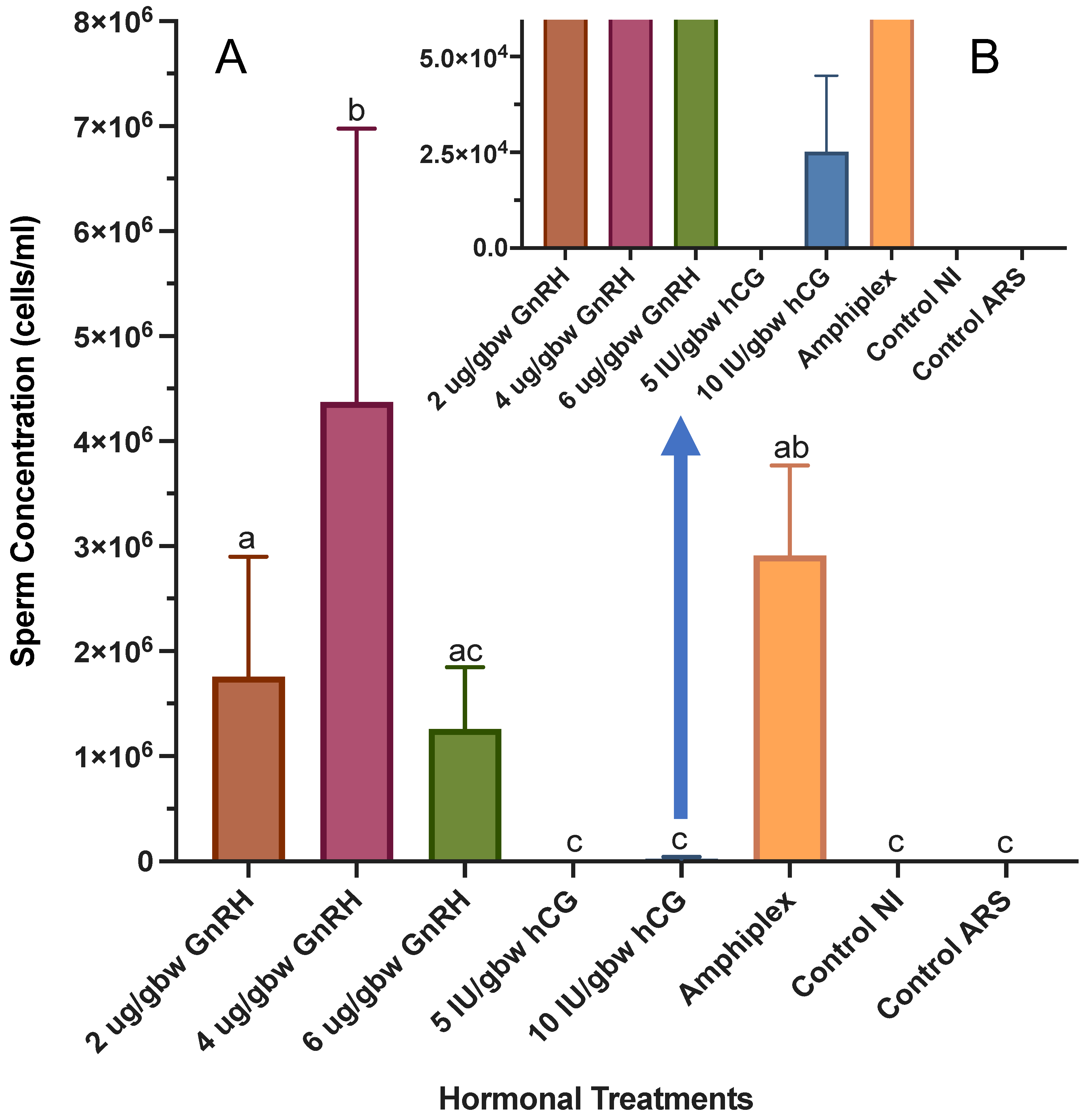
3.1. Sperm Concentration Post Hormone Induction
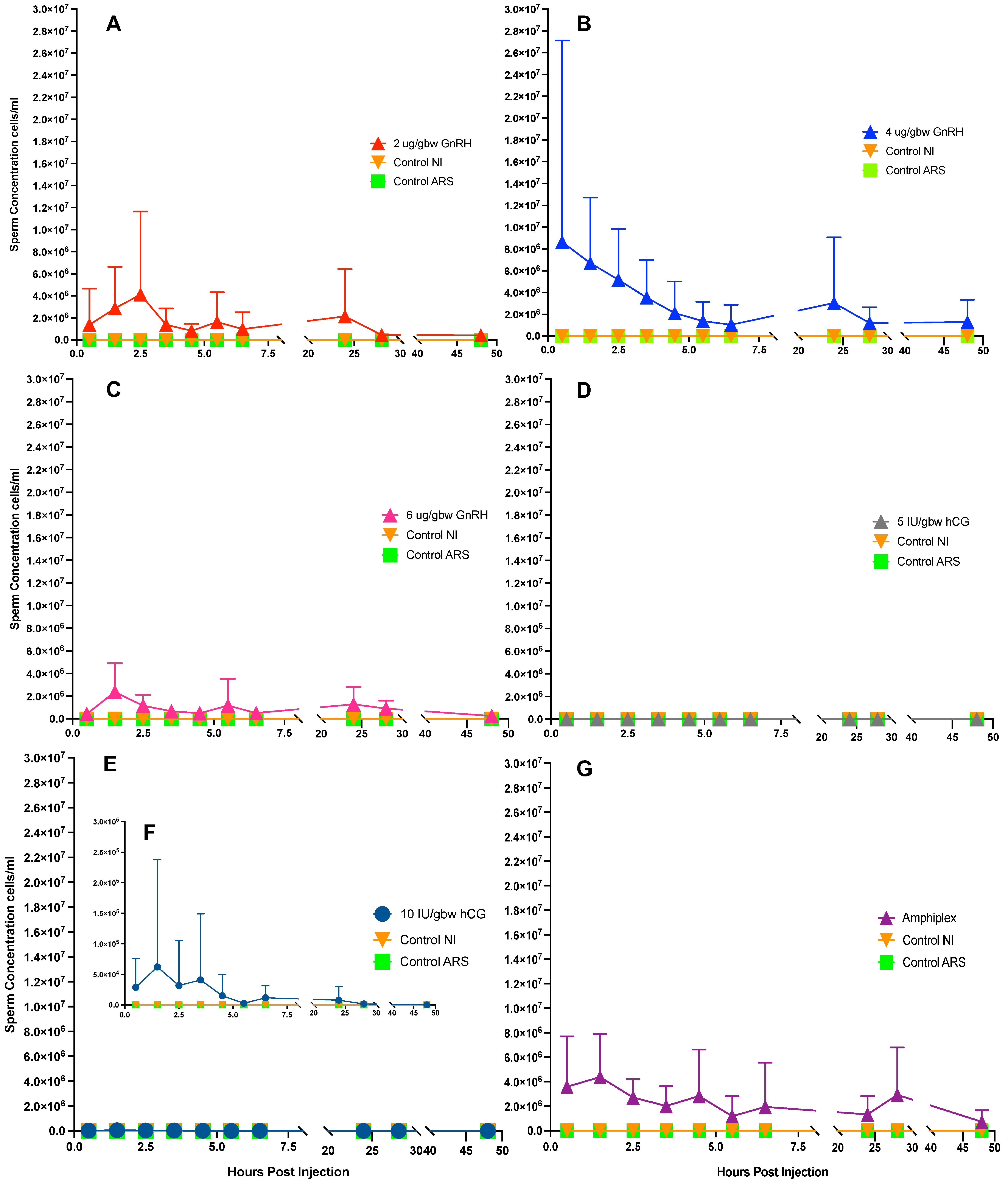
3.2. Hormonally Induced Sperm Concentration–Response Curves
3.3. Duration of Hormonal Stimulation Response
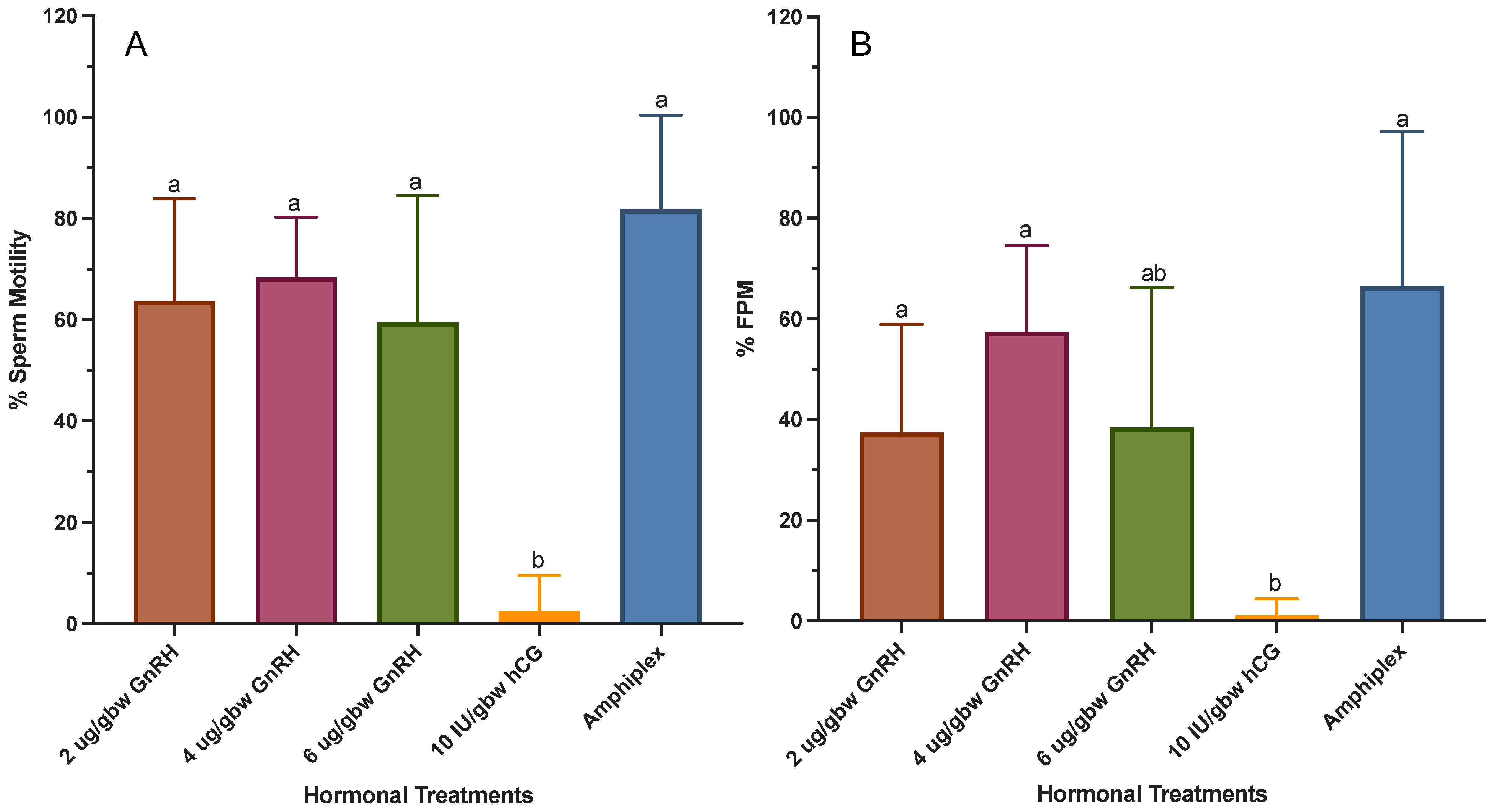
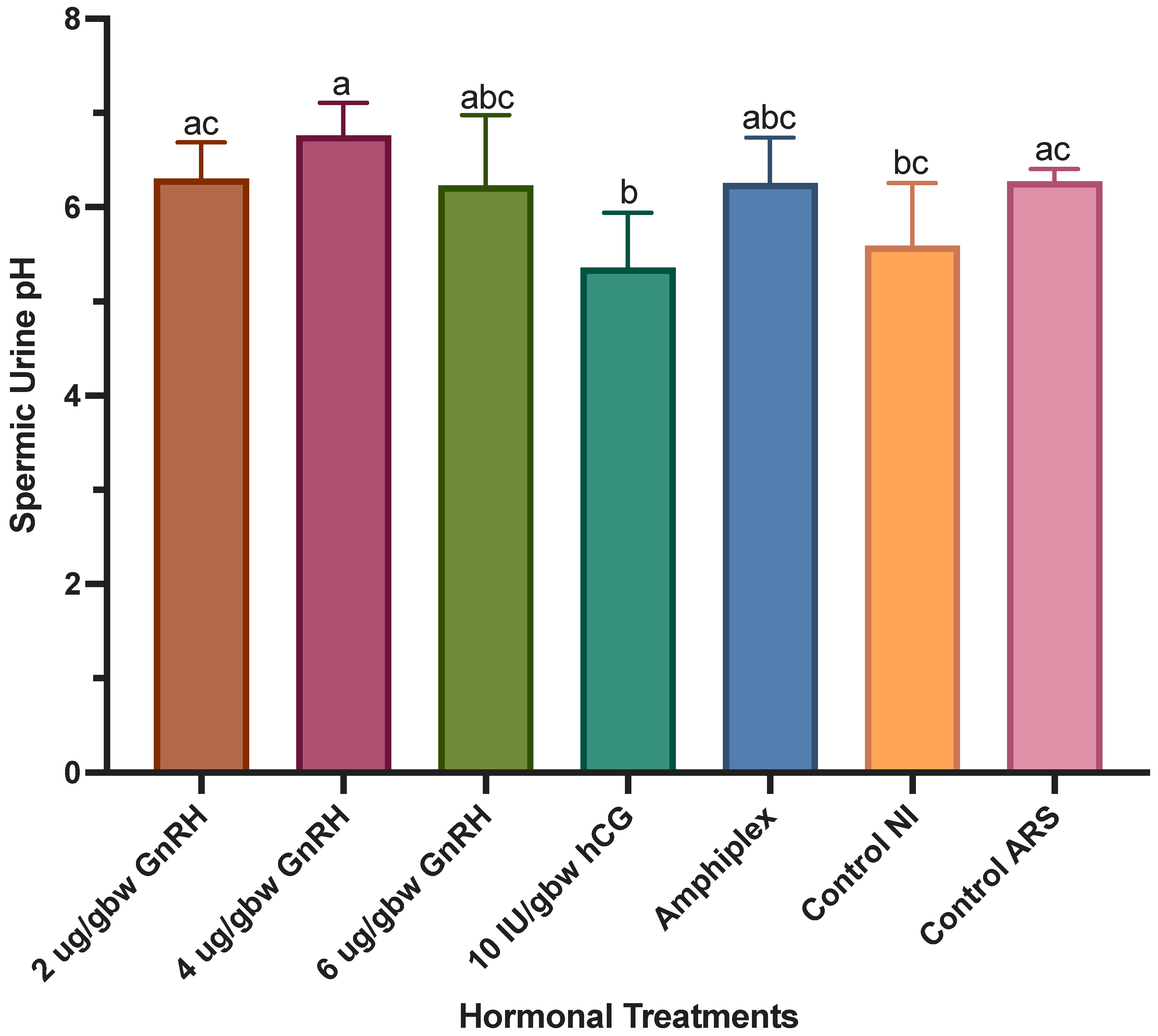
3.4. Effect of Hormonal Treatments on Sperm Quality Parameters
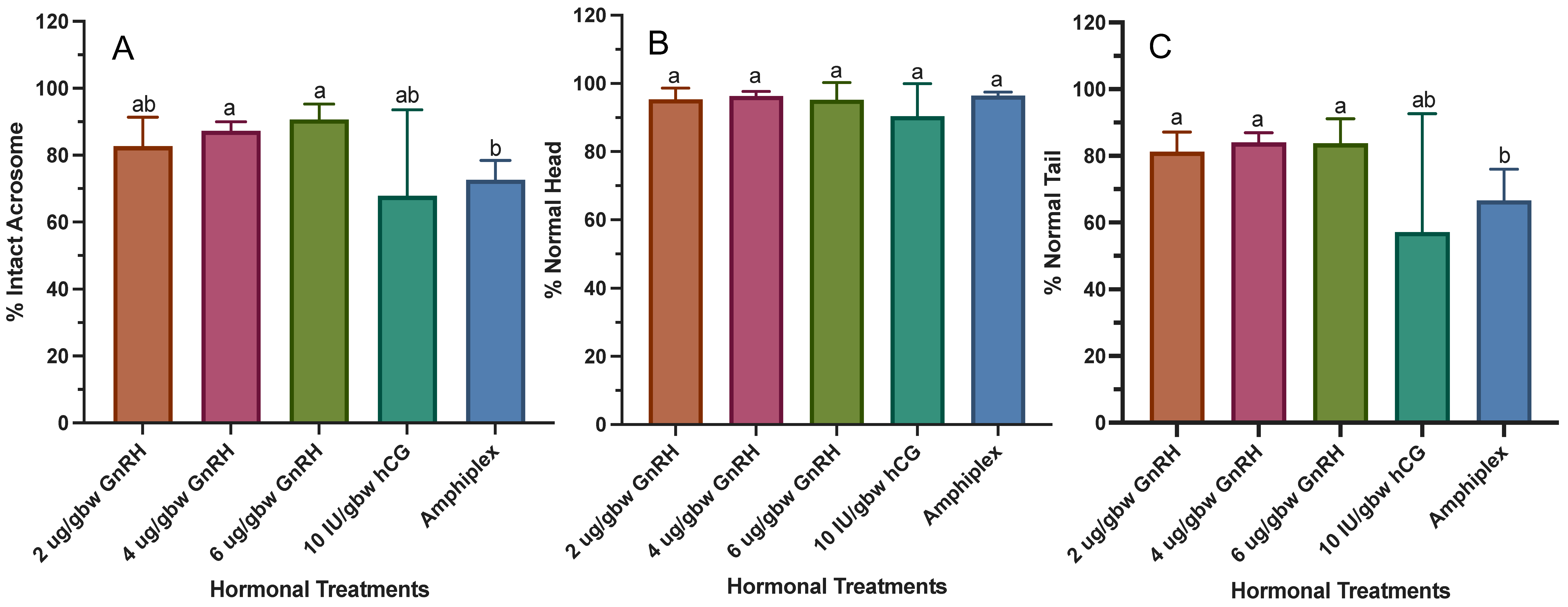
3.5. Morphological Assessment
4. Discussion
4.1. Sperm Concentration Post Hormone Induction
4.2. Hormonally Induced Sperm Concentration–Response Curves
4.3. Duration of Hormonal Stimulation Response
4.4. Effect of Hormonal Treatments on Sperm Quality Parameters
4.5. Morphological Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MiAmbiente. Plan de Acción para la Conservación de los Anfibios de Panamá; Ministry of Environment, Government of Panama: Panama City, Panama, 2011. [Google Scholar]
- Ibáñez, R. Status of Amphibians in Panama. 2021. Available online: https://phys.org/news/2020-09-superfungus-threatens-panamanian-golden-frogs.html (accessed on 22 March 2023).
- IUCN. IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/resources/grid/summary-statistics (accessed on 16 March 2023).
- Ryan, M.J.; Savage, J.M.; Lips, K.R.; Giermakowski, J.T. A New Species of the Craugastor rugulosus Series (Anura: Craugastoridae) from West-Central Panama. Copeia 2010, 2010, 405–409. [Google Scholar] [CrossRef]
- Heinicke, M.P.; Duellman, W.E.; Trueb, L.; Means, D.B.; MacCulloch, R.D.; Hedges, S.B. A New Frog Family (Anura: Terrarana) from South America and an Expanded Direct-Developing Clade Revealed by Molecular Phylogeny. Zootaxa 2009, 2211, 1–35. [Google Scholar] [CrossRef]
- LaMarca, E.; Lips, K.R.; Lötters, S.; Puschendorf, R.; Ibáñez, R.; Rueda-Almonacid, J.V.; Schulte, R.; Marty, C.; Castro, F.; Manzanilla-Puppo, J.; et al. Catastrophic Population Declines and Extinctions in Neotropical Harlequin Frogs (Bufonidae: Atelopus) 1. Biotropica 2005, 37, 190–201. [Google Scholar] [CrossRef]
- PARC. Vanishing Robber Frog. Available online: http://amphibianrescue.org/meet-the-frogs/vanishing-robber-frog/ (accessed on 22 March 2023).
- Browne, R.K.; Wolfram, K.; García, G.; Bagaturov, M.F.; Pereboom, Z.J.J.M. Zoo-Based Amphibian Research and Conservation Breeding Programs. Amphib. Reptile Conserv. 2011, 3, 1–14. [Google Scholar]
- Lacy, R.C. Achieving True Sustainability of Zoo Populations. Zoo Biol. 2013, 32, 19–26. [Google Scholar] [CrossRef]
- Della-Togna, G.; Howell, L.G.; Clulow, J.; Langhorne, C.J.; Marcec-Greaves, R.; Calatayud, N.E. Evaluating Amphibian Biobanking and Reproduction for Captive Breeding Programs According to the Amphibian Conservation Action Plan Objectives. Theriogenology 2020, 150, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Della-Togna, G. Structural and Functional Characterization of the Panamanian Golden Frog (Atelopus zeteki) Spermatoza—Impact of Medium Osmolality and Cryopreservation on Motility and Cell Viability. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2015. [Google Scholar]
- Della-Togna, G.; Trudeau, V.L.; Gratwicke, B.; Evans, M.; Augustine, L.; Chia, H.; Bronikowski, E.J.; Murphy, J.B.; Comizzoli, P. Effects of Hormonal Stimulation on the Concentration and Quality of Excreted Spermatozoa in the Critically Endangered Panamanian Golden Frog (Atelopus zeteki). Theriogenology 2017, 91, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Clulow, J.; Mahony, M.; Browne, R.; Pomering, M.; Clarck, A. Applications of Assisted Reproductive Technologies (ART) to Endangered Anuran Amphibians. In Declines and Disappearances of Autralian Frogs; Campbell, A., Ed.; Environment Australia: Canberra, Australia, 1999; pp. 219–225. ISBN 0 642 54656 8. [Google Scholar]
- Browne, R.K.; Zippel, K. Reproduction and Larval Rearing of Amphibians. ILAR J. 2007, 48, 214–234. [Google Scholar] [CrossRef]
- Hopkins, B.K.; Herr, C. Cryopreservation of Frog (Rana pipiens) Sperm Cells Collected by Non-Lethal Methods. In Proceedings of the Annual Conference of the International Embryo Transfer Society, Denver, CO, USA, 5–9 January 2008; CSIRO: Denver, CO, USA, 2008; Volume 20, p. 120. [Google Scholar]
- Lipke, C. Induced Spermiation and Sperm Morphology in the Green Poison Frog, Dendrobates auratus. Ph.D. Thesis, University of Veterinary Medicine Hannover, Hannover, Germany, 2008. [Google Scholar]
- Trudeau, V.L.; Somoza, G.M.; Natale, G.S.; Pauli, B.; Wignall, J.; Jackman, P.; Doe, K.; Schueler, F.W. Hormonal Induction of Spawning in 4 Species of Frogs by Coinjection with a Gonadotropin-Releasing Hormone Agonist and a Dopamine Antagonist. Reprod. Biol. Endocrinol. 2010, 8, 36. [Google Scholar] [CrossRef]
- Byrne, P.G.; Silla, A.J. Hormonal Induction of Gamete Release, and in-Vitro Fertilisation, in the Critically Endangered Southern Corroboree Frog, Pseudophryne corroboree. Reprod. Biol. Endocrinol. 2010, 8, 144. [Google Scholar] [CrossRef]
- Mann, R.M.; Hyne, R.V.; Choung, C.B. Hormonal Induction of Spermiation, Courting Behavior and Spawning in the Southern Bell Frog, Litoria raniformis. Zoo Biol. 2010, 29, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J. Effects of Luteinizing Hormone-Releasing Hormone and Arginine-Vasotocin on the Sperm-Release Response of Günther’s Toadlet, Pseudophryne guentheri. Reprod. Biol. Endocrinol. 2010, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Uteshev, V.K.; Shishova, N.V.; Kaurova, S.A.; Browne, R.K.; Gakhova, E.N. Hormonal Induction of Spermatozoa from Amphibians with Rana temporaria and Bufo bufo as Anuran Models. Reprod. Fertil. Dev. 2011, 24, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Della Togna, G.; Gratwicke, B.; Evans, M.; Augustine, L.; Chia, H.; Bronikowski, E.; Murphy, J.B.; Comizzoli, P. Influence of Extracellular Environment on the Motility and Structural Properties of Spermatozoa Collected from Hormonally Stimulated Panamanian Golden Frog (Atelopus zeteki). Theriogenology 2018, 108, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Mouttham, L.L.; Buhr, M.; Freeman, E.W.; Widowski, T.M.; Graham, L.H.; Brown, J.L. Interrelationship of Serum Testosterone, Dominance and Ovarian Cyclicity Status in Female African Elephants. Anim. Reprod. Sci. 2011, 126, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Clulow, J.; Pomering, M.; Herbert, D.; Upton, R.; Calatayud, N.; Clulow, S.; Mahony, M.J.; Trudeau, V.L. Differential Success in Obtaining Gametes between Male and Female Australian Temperate Frogs by Hormonal Induction—A Review. Gen. Comp. Endocrinol. 2018, 265, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Obringer, A.R.; O’Brien, J.K.; Saunders, R.L.; Yamamoto, K.; Kikuyama, S.; Roth, T.L. Characterization of the Spermiation Response, Luteinizing Hormone Release and Sperm Quality in the American Toad (Bufo americanus) and the Endangered Wyoming Toad (Bufo baxteri). Reprod. Fertil. Dev. 2000, 12, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C. GnRH: The Master Molecule of Reproduction; Springer Science+Business Media: New York, NY, USA, 2002; pp. 53–92. [Google Scholar] [CrossRef]
- Feder, M.E.; Burggren, W.W. Environmental Physiology of Amphibians; Feder, M.E., Burggren, W.W., Eds.; The University of Chicago Press: Chicago, IL, USA, 1992; ISBN 9780226239446. [Google Scholar]
- Pozzi, A.G.; Rosemblit, C.; Ceballos, N.R. Effect of Human Gonadotropins on Spermiation and Androgen Biosynthesis in the Testis of the Toad Bufo arenarum (Amphibia, Anura). J. Exp. Zool. Part Comp. Exp. Biol. 2006, 305A, 96–102. [Google Scholar] [CrossRef]
- Conn, P.M. The Molecular Basis of Gonadotropin-Releasing Hormone Action. Endocr. Rev. 1986, 7, 3–10. [Google Scholar] [CrossRef]
- Xu, C.; Xu, X.-Z.; Nunemaker, C.S.; Moenter, S.M. Dose-Dependent Switch in Response of Gonadotropin-Releasing Hormone (GnRH) Neurons to GnRH Mediated through the Type I GnRH Receptor. Endocrinology 2004, 145, 728–735. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Jeong, K.-H.; Chin, W.W. Molecular Mechanisms of Gonadotropin-Releasing Hormone Receptor Gene Regulation. J. Soc. Gynecol. Investig. 1999, 6, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sandow, J.; Rechenberg, W.; Jerzabek, G.; Engelbart, K.; Kuhl, H.; Fraser, H. Hypothalamic-Pituitary-Testicular Function in Rats after Supraphysiological Doses of a Highly Active LRH Analogue (Buserelin). Eur. J. Endocrinol. 1980, 94, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; McFadden, M.S.; Byrne, P.G. Hormone-Induced Sperm-Release in the Critically Endangered Booroolong Frog (Litoria booroolongensis): Effects of Gonadotropin-Releasing Hormone and Human Chorionic Gonadotropin. Conserv. Physiol. 2019, 7, coy080. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.E.; Robertson, J.M.; Kaiser, K. Variation in Male Spermiation Response to Exogenous Hormones among Divergent Populations of Red-Eyed Treefrogs. Reprod. Biol. Endocrinol. 2016, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Bellisario, R.; Bahl, O. Human Chorionic Gonadotropin. V. Tissue Specificity of Binding and Partial Characterization of Soluble Human Chorionic Gonadotropin-Receptor Complexes. J. Biol. Chem. 1975, 250, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Arregui, L.; Diaz-Diaz, S.; Alonso-López, E.; Kouba, A.J. Hormonal Induction of Spermiation in a Eurasian Bufonid (Epidalea calamita). Reprod. Biol. Endocrinol. 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Iimori, E.; D’Occhio, M.J.; Lisle, A.T.; Johnston, S.D. Testosterone Secretion and Pharmacological Spermatozoal Recovery in the Cane Toad (Bufo marinus). Anim. Reprod. Sci. 2005, 90, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Roberts, J.D. Investigating Patterns in the Spermiation Response of Eight Australian Frogs Administered Human Chorionic Gonadotropin (HCG) and Luteinizing Hormone-Releasing Hormone (LHRHa). Gen. Comp. Endocrinol. 2012, 179, 128–136. [Google Scholar] [CrossRef]
- Pham, T.H.; Brannelly, L.A. Sperm Parameters Following Hormonal Induction of Spermiation in an Endangered Frog [the Alpine Tree Frog (Litoria verreauxii alpina). Reprod. Fertil. Dev. 2022, 34, 867–874. [Google Scholar] [CrossRef]
- Rastogi, R.K. Seasonal Cycle in Anuran (Amphibia) Testis: The Endocrine and Environmental Controls. Ital. J. Zool. 1976, 43, 151–172. [Google Scholar] [CrossRef]
- Clulow, J.; Upton, R.; Trudeau, V.L.; Clulow, S. Amphibian Assisted Reproductive Technologies: Moving from Technology to Application. In Reproductive Sciences in Animal Conservation Second Edition; Comizzoli, P., Brown, J.L., Holt, W.V., Eds.; Advances in Experimental Medicine and Biology; Springer Nature: Cham, Switzerland, 2019; Volume 1200, pp. 413–463. ISBN 9783030236328. [Google Scholar]
- Vu, M.; Trudeau, V.L. Neuroendocrine Control of Spawning in Amphibians and Its Practical Applications. Gen. Comp. Endocrinol. 2016, 234, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.; Weiler, B.; Trudeau, V.L. Time- and Dose-Related Effects of a Gonadotropin-Releasing Hormone Agonist and Dopamine Antagonist on Reproduction in the Northern Leopard Frog (Lithobates pipiens). Gen. Comp. Endocrinol. 2017, 254, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Daniels, E.; Licht, P. Effects of Gonadotropin-Releasing Hormone on the Levels of Plasma Gonadotrophins (FSH and LH) in the Bullfrog, Rana catesbeiana. Gen. Comp. Endocrinol. 1980, 42, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Matteo, L.D.; Minucci, S.; Fasano, S.; Pierantoni, R.; Varriale, B.; Chieffi, G. A Gonadotropin-Releasing Hormone (GnRH) Antagonist Decreases Androgen Production and Spermatogonial Multiplication in Frog (Rana esculenta): Indirect Evidence for the Existence of GnRH or GnRH-Like Material Receptors in the Hypophysis and Testis*. Endocrinology 1988, 122, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hinkson, K.M.; Baecher, J.A.; Poo, S. Cryopreservation and Hormonal Induction of Spermic Urine in a Novel Species: The Smooth-Sided Toad (Rhaebo guttatus). Cryobiology 2019, 89, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, C. Developing Assisted Reproductive Technologies for Endangered North American Amphibians; Mississippi State University: Starkville, MS, USA, 2016. [Google Scholar]
- Haczkiewicz, K.; Rozenblut-Kościsty, B.; Ogielska, M. Prespermatogenesis and Early Spermatogenesis in Frogs. Zoology 2017, 122, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Roco, Á.S.; Ruiz-García, A.; Bullejos, M. Testis Development and Differentiation in Amphibians. Genes 2021, 12, 578. [Google Scholar] [CrossRef]
- Brum, A.J.C.; Loebens, L.; Prado, C.P.A.; Cechin, S.Z. Reproductive Cycle, Sexual Maturity and Longevity of Odontophrynus americanus (Anura: Odontophrynidae) in South Brazil. Acta Zool. 2022, 103, 99–111. [Google Scholar] [CrossRef]
- Burgos, M.H.; Vitale-Calpe, R. The Mechanism of Spermiation in the Toad. Am. J. Anat. 1967, 120, 227–251. [Google Scholar] [CrossRef]
- Silla, A.J.; Byrne, P.G. The Role of Reproductive Technologies in Amphibian Conservation Breeding Programs. Annu. Rev. Anim. Biosci. 2018, 7, 499–519. [Google Scholar] [CrossRef]
- Méndez-Tepepa, M.; Morales-Cruz, C.; García-Nieto, E.; Anaya-Hernández, A. A Review of the Reproductive System in Anuran Amphibians. Zool. Lett. 2023, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.K.; Seratt, J.; Vance, C.; Kouba, A. Hormonal Priming, Induction of Ovulation and in-Vitro Fertilization of the Endangered Wyoming Toad (Bufo baxteri). Reprod. Biol. Endocrinol. 2006, 4, 34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silla, A.J.; McFadden, M.; Byrne, P.G. Hormone-Induced Spawning of the Critically Endangered Northern Corroboree Frog Pseudophryne pengilleyi. Reprod. Fertil. Dev. 2018, 30, 1352. [Google Scholar] [CrossRef]
- McDonough, C.E.; Martin, M.W.; Vance, C.K.; Cole, J.A.; Kouba, A.J. Frequency of Exogenous Hormone Therapy Impacts Spermiation in Male Fowler’s Toad (Bufo fowleri). Reprod. Fertil. Dev. 2015, 28, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Zadmajid, V. Comparative Effects of Human Chorionic Gonadotropin (HCG) and OvaprimTM (SGnRHa+domperidone) on the Reproductive Characteristics of Wild-Caught Male Longspine Scraper, Capoeta trutta (Heckel, 1843). Aquaculture 2016, 463, 7–15. [Google Scholar] [CrossRef]
- Zadmajid, V.; Bashiri, S.; Sharafi, N.; Butts, I.A.E. Effect of HCG and OvaprimTM on Reproductive Characteristics of Male Levantine Scraper, Capoeta damascina (Valenciennes, 1842). Theriogenology 2018, 115, 45–56. [Google Scholar] [CrossRef]
- Seifi, T.; Imanpoor, M.R.; Golpour, A. The Effect of Different Hormonal Treatments on Semen Quality Parameters in Cultured and Wild Carp. Turk. J. Fish. Aquat. Sci. 2011, 11, 595–602. [Google Scholar]
- Michael, S.F.; Jones, C. Cryopreservation of Spermatozoa of the Terrestrial Puerto Rican Frog, Eleutherodactylus coqui. Cryobiology 2004, 48, 90–94. [Google Scholar] [CrossRef]
- Christensen, J.R.; Pauli, B.D.; Richardson, J.S.; Bishop, C.A.; Elliott, J. Effects of PH and Dilution on African Clawed Frog (Xenopus laevis) Sperm Motility. Can. J. Zool. 2004, 82, 555–563. [Google Scholar] [CrossRef]
- Browne, R.K.; Silla, A.J.; Upton, R.; Della-Togna, G.; Marcec-Greaves, R.; Shishova, N.V.; Uteshev, V.K.; Proaño, B.; Pérez, O.D.; Mansour, N.; et al. Sperm Collection and Storage for the Sustainable Management of Amphibian Biodiversity. Theriogenology 2019, 133, 187–200. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Cosson, J. Sperm Motility in Fishes. I. Effects of Temperature and PH: A Review. Cell Biol. Int. 2005, 29, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Inoda, T.; Morisawa, M. Effect of Osmolality on the Initiation of Sperm Motility in Xenopus laevis. Comp. Biochem. Physiol. Part Physiol. 1987, 88, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.M.H.; Cosson, J. Sperm Motility in Fishes. (II) Effects of Ions and Osmolality: A Review. Cell Biol. Int. 2006, 30, 1–14. [Google Scholar] [CrossRef]
- Brischoux, F.; Cheron, M. Osmotic ‘Cost’ of Reproduction in Breeding Male Toads. Biol. Lett. 2019, 15, 20190689. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, J.P.; Mugnano, J.A.; Wehrheim, H.M.; Lee, R.E. Osmotic and Freezing Tolerance in Spermatozoa of Freeze-Tolerant and -Intolerant Frogs. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 275, R713–R719. [Google Scholar] [CrossRef]
- Raut, S.; Deshpande, S.; Balasinor, N. Unveiling the Role of Prolactin and Its Receptor in Male Reproduction. Horm. Metab. Res. 2019, 51, 215–219. [Google Scholar] [CrossRef]
- Panidis, D.; Rousso, D.; Skiadopoulos, S.; Panidou, E.; Mamopoulos, M. Evaluation of Semen Parameters in Man with Hyperprolactinemia Induced by Metoclopramide. Arch. Androl. 1997, 39, 237–242. [Google Scholar] [CrossRef][Green Version]



| Hormones | Concentrations |
|---|---|
| GnRH-A | 2 μg/gbw |
| 4 μg/gbw | |
| 6 μg/gbw | |
| hCG | 5 IU/gbw |
| 10 IU/gbw | |
| Amphiplex | 0.4 μg/gbw GnRH-A + 10 μg/gbw MET |
| Hormones | Statistical Parameters | ||
|---|---|---|---|
| Optimum Concentration | Sub-Optimum Concentration | CI-95% | p |
| 4 μg/gbw GnRH-A | 2 μg/gbw GnRH-A | −4.30 × 106, −9.24 × 105 | 0.0003 |
| 4 μg/gbw GnRH-A | 6 μg/gbw GnRH-A | 1.24 × 106, 4.97 × 106 | <0.0001 |
| 4 μg/gbw GnRH-A | 5 IU/gbw hCG | 2.63 × 106, 6.10 × 106 | <0.0001 |
| 4 μg/gbw GnRH-A | 10 IU/gbw hCG | 2.61 × 106, 6.07 × 106 | <0.0001 |
| 4 μg/gbw GnRH-A | Control ARS | 2.57 × 106, 6.16 × 106 | <0.0001 |
| 4 μg/gbw GnRH-A | Control No Injection | 2.57 × 106, 6.16 × 106 | <0.0001 |
| Hormones | Statistical Parameters | ||
|---|---|---|---|
| Sub-Optimum Concentration | Non-Optimum Concentration | CI-95% | p |
| 2 μg/gbw GnRH-A | 5 IU/gbw hCG | 1.28 × 105, 3.36 × 106 | 0.0258 |
| Amphiplex | 5 IU/gbw hCG | −4.64 × 106, −1.17 × 106 | <0.0001 |
| 2 μg/gbw GnRH-A | 10 IU/gbw hCG | 1.04 × 105, 3.35 × 106 | 0.0295 |
| Amphiplex | 10 IU/gbw hCG | −4.61 × 106, −1.15 × 106 | <0.0001 |
| 2 μg/gbw GnRH-A | Controls | 6.93 × 104, 3.45 × 106 | 0.0358 |
| Amphiplex | Controls | 1.12 × 106, 4.70 × 106 | 0.0001 |
| Hormone | Sperm Concentration Peaks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Collection Time Points (Hours Post Injection) | ||||||||||
| 0.5 | 1.5 | 2.5 | 3.5 | 4.5 | 5.5 | 6.5 | 24 | 28 | 48 | |
| 2 μg/gpc GnRH | ||||||||||
| 4 μg/gpc GnRH | ||||||||||
| 6 μg/gpc GnRH | ||||||||||
| 5 IU/gpc hCG | ||||||||||
| 10 IU/gpc hCG | ||||||||||
| Amphiplex | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, Y.; Calatayud, N.E.; Arcia, I.D.; Mariscal, D.; Samaniego, D.; Rodríguez, D.; Rodríguez, K.; Guerrel, J.; Ibáñez, R.; Della Togna, G. Recovery and Characterization of Spermatozoa in a Neotropical, Terrestrial, Direct-Developing Riparian Frog (Craugastor evanesco) through Hormonal Stimulation. Animals 2023, 13, 2689. https://doi.org/10.3390/ani13172689
Otero Y, Calatayud NE, Arcia ID, Mariscal D, Samaniego D, Rodríguez D, Rodríguez K, Guerrel J, Ibáñez R, Della Togna G. Recovery and Characterization of Spermatozoa in a Neotropical, Terrestrial, Direct-Developing Riparian Frog (Craugastor evanesco) through Hormonal Stimulation. Animals. 2023; 13(17):2689. https://doi.org/10.3390/ani13172689
Chicago/Turabian StyleOtero, Yineska, Natalie E. Calatayud, Igli D. Arcia, Denise Mariscal, Diego Samaniego, Dionel Rodríguez, Karina Rodríguez, Jorge Guerrel, Roberto Ibáñez, and Gina Della Togna. 2023. "Recovery and Characterization of Spermatozoa in a Neotropical, Terrestrial, Direct-Developing Riparian Frog (Craugastor evanesco) through Hormonal Stimulation" Animals 13, no. 17: 2689. https://doi.org/10.3390/ani13172689
APA StyleOtero, Y., Calatayud, N. E., Arcia, I. D., Mariscal, D., Samaniego, D., Rodríguez, D., Rodríguez, K., Guerrel, J., Ibáñez, R., & Della Togna, G. (2023). Recovery and Characterization of Spermatozoa in a Neotropical, Terrestrial, Direct-Developing Riparian Frog (Craugastor evanesco) through Hormonal Stimulation. Animals, 13(17), 2689. https://doi.org/10.3390/ani13172689






