An Outbreak of Aeromonas salmonicida in Juvenile Siberian Sturgeons (Acipenser baerii)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Tissue Sampling
2.2. Parasitological Study of Gills
2.3. Microbiological Study
2.4. Histological Study
2.5. Immunohistochemical Study
3. Results
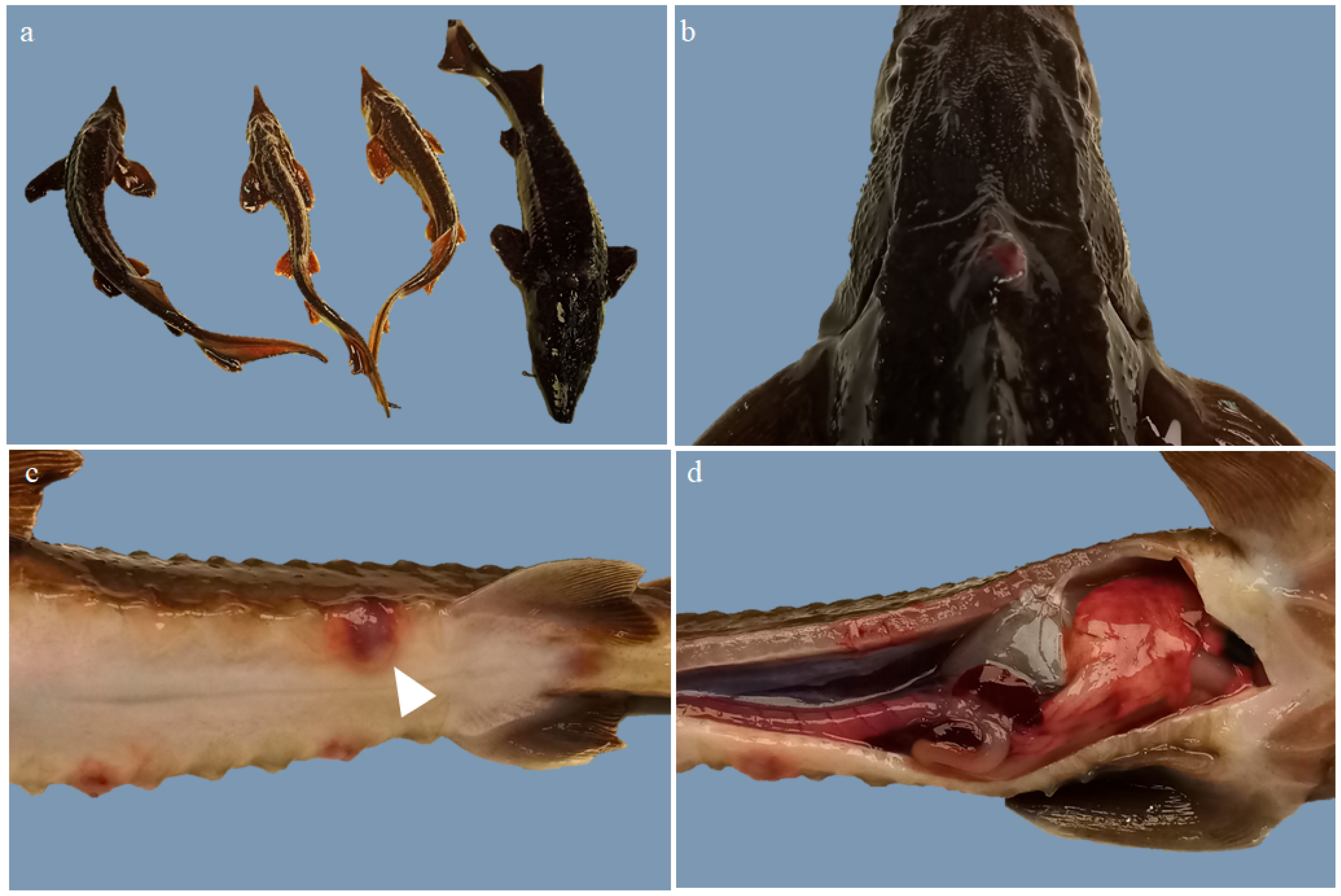
3.1. Macroscopic Study
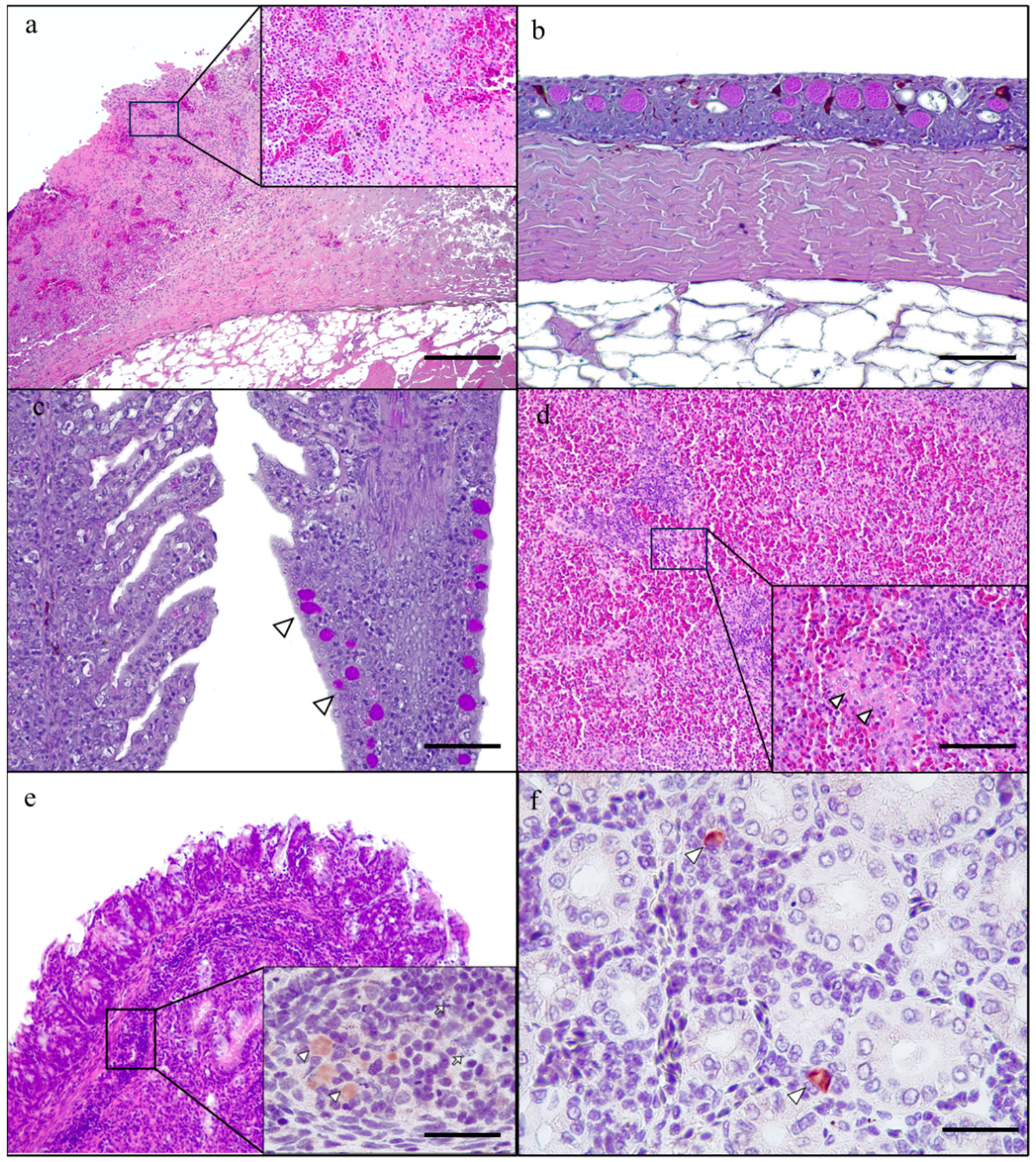
3.2. Histological Study
3.3. Microbiological Study
4. Discussion
5. Conclusions
- Juvenil sturgeons suffere from a hyperacute form of the disease;
- The A. salmonicida subsp. salmonicida strain isolated was susceptible to common antimicrobials used in aquaculture;
- Bacterial colonies were not seen histologically, but immunohistochemistry has been useful for detecting Aeromonas in tissues.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bronzi, P.; Rosenthal, H. Present and future sturgeon and caviar production and marketing: A global market overview. J. Appl. Ichthyol. 2014, 30, 1536–1546. [Google Scholar] [CrossRef]
- Radosavljević, V.; Milićević, V.; Maksimović-Zorić, J.; Veljović, L.; Nešić, K.; Pavlović, M.; Ljubojević Pelić, D.; Marković, Z. Sturgeon diseases in aquaculture. Arch. Vet. Sci. 2019, 12, 5–20. [Google Scholar] [CrossRef]
- Kayiş, Ş.; Er, A.; Kangel, P.; Kurtoğlu, I.Z. Bacterial pathogens and health problems of Acipenser gueldenstaedtii and Acipenser baerii sturgeons reared in the eastern Black Sea region of Turkey. Iran. J. Vet. Res. 2017, 18, 18–24. [Google Scholar]
- EUMOFA. The Caviar Market: Production, Trade and Consumption in and ouside the EU; Publications Office of the European Union: Luxembourg, 2021.
- Williot, P.; Brun, R. Ovarian development and cycles in cultured Siberian sturgeon, Acipenser baeri. Aquat. Living Resour. 1998, 11, 111–118. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens, Diseases of Farmed and Wild Fish, 4th ed.; Praxis Publishing Ltd.: Chichester, UK, 2007. [Google Scholar]
- Chakraborty, S.; Hossain, A.; Cao, T.; Gnanagobal, H.; Segovia, C.; Hill, S.; Monk, J.; Porter, J.; Boyce, D.; Hall, J.R.; et al. Multi-Organ Transcriptome Response of Lumpfish (Cyclopterus lumpus) to Aeromonas salmonicida Subspecies salmonicida Systemic Infection. Microorganisms 2022, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Diamanka, A.; Loch, T.P.; Cipriano, R.C.; Faisal, M. Polyphasic characterization of Aeromonas salmonicida isolates recovered from salmonid and non-salmonid fish. J. Fish Dis. 2013, 36, 949–963. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Zhao, J.; Li, T.; Tafalla, C.; Zhang, Q.; Wang, X.; Gong, X.; Shen, Z.; Li, A. Transcriptomic and proteomic analyses of splenic immune mechanisms of rainbow trout (Oncorhynchus mykiss) infected by Aeromonas salmonicida subsp. salmonicida. J. Proteom. 2015, 122, 41–54. [Google Scholar] [CrossRef]
- Orozova, P.; Barker, M.; Austin, D.A.; Austin, B. Identification and pathogenicity to rainbow trout, Oncorhynchus mykiss (Walbaum), of some aeromonads. J. Fish Dis. 2009, 32, 865–871. [Google Scholar] [CrossRef]
- Menanteau-Ledouble, S.; Kumar, G.; Saleh, M.; El-Matbouli, M. Aeromonas salmonicida: Updates on an old acquaintance. Dis. Aquat. Org. 2016, 120, 49–68. [Google Scholar] [CrossRef]
- Faisal, M.; Eissa, A.E.; Elsayed, E.E. Isolation of Aeromonas salmonicida from sea lamprey (Petromyzon marinus) with furuncle-like lesions in Lake Ontario. J. Wildl. Dis. 2007, 43, 618–622. [Google Scholar] [CrossRef][Green Version]
- Lian, Z.; Bai, J.; Hu, X.; Lü, A.; Sun, J.; Guo, Y.; Song, Y. Detection and characterization of Aeromonas salmonicida subsp. salmonicida infection in crucian carp Carassius auratus. Vet. Res. Commun. 2020, 44, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Polinski, M.P.; Fehringer, T.R.; Johnson, K.A.; Snekvik, K.R.; Lapatra, S.E.; Lafrentz, B.R.; Ireland, S.C.; Cain, K.D. Characterization of susceptibility and carrier status of burbot, Lota lota (L.), to IHNV, IPNV, Flavobacterium psychrophilum, Aeromonas salmonicida and Renibacterium salmoninarum. J. Fish Dis. 2010, 33, 559–570. [Google Scholar] [CrossRef]
- Zepeda-Velázquez, A.P.; Vega-Sánchez, V.; Salgado-Miranda, C.; Soriano-Vargas, E. Histopathological findings in farmed rainbow trout (Oncorhynchus mykiss) naturally infected with 3 different Aeromonas species. Can. J. Vet. Res. 2015, 79, 250–254. [Google Scholar] [PubMed]
- Salehi, M.R.; Shadvar, S.; Sadeghian, M.; Doomanlou, M.; Abdollahi, A.; Manshadi, S.A.D.; Sardari, A.; Rahdar, H.A.; Feizabadi, M.M. Endocarditis with Aeromonas salmonicida. IDCases 2019, 18, e00625. [Google Scholar] [CrossRef]
- Varshney, A.; Das, M.; Chaudhary, P.; Kumari, R.; Yadav, K. Aeromonas salmonicida as a Causative Agent for Postoperative Endophthalmitis. Middle East Afr. J. Ophthalmol. 2017, 24, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.T.; Charette, S.J. To Be or Not to Be Mesophilic, That Is the Question for Aeromonas salmonicida. Microorganisms 2022, 10, 240. [Google Scholar] [CrossRef]
- Vincent, A.T.; Fernández-Bravo, A.; Sanchis, M.; Mayayo, E.; Figueras, M.J.; Charette, S.J. Investigation of the virulence and genomics of Aeromonas salmonicida strains isolated from human patients. Infect. Genet. Evol. 2019, 68, 1–9. [Google Scholar] [CrossRef]
- Fernandez-Ávarez, C.; Gijón, D.; Álvarez, M.; Santos, Y. First isolation of Aeromonas salmonicida subspecie salmonicida from diseased sea bass, Dicentrarchus labrax (L.), cultured in Spain. Aquac. Rep. 2016, 4, 36–41. [Google Scholar] [CrossRef]
- Novak, C.W.; Lewis, D.L.; Collicutt, B.; Verkaik, K.; Barker, D.E. Investigations on the role of the salmon louse, Lepeophtheirus salmonis (Caligidae), as a vector in the transmission of Aeromonas salmonicida subsp. salmonicida. J. Fish Dis. 2016, 39, 1165–1178. [Google Scholar] [CrossRef]
- Viršek, M.K.; Lovšin, M.N.; Koren, Š.; Kržan, A.; Peterlin, M. Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar. Pollut. Bull. 2017, 125, 301–309. [Google Scholar] [CrossRef]
- Bartkova, S.; Kokotovic, B.; Dalsgaard, I. Infection routes of Aeromonas salmonicida in rainbow trout monitored in vivo by real-time bioluminescence imaging. J. Fish Dis. 2017, 40, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Jutfelt, F.; Olsen, R.E.; Glette, J.; Ringø, E.; Sundell, K. Translocation of viable Aeromonas salmonicida across the intestine of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2006, 29, 255–262. [Google Scholar] [CrossRef]
- Ringø, E.; Jutfelt, F.; Kanapathippillai, P.; Bakken, Y.; Sundell, K.; Glette, J.; Mayhew, T.M.; Myklebust, R.; Olsen, R.E. Damaging effect of the fish pathogen Aeromonas salmonicida ssp. salmonicida on intestinal enterocytes of Atlantic salmon (Salmo salar L.). Cell Tissue Res. 2004, 318, 305–311. [Google Scholar] [CrossRef]
- Svendsen, Y.S.; Dalmo, R.A.; Bøgwald, J. Tissue localization of Aeromonas salmonicida in Atlantic salmon, Salmo salar L., following experimental challenge. J. Fish Dis. 2001, 22, 125–131. [Google Scholar] [CrossRef]
- Coscelli, G.A.; Bermúdez, R.; Losada, A.; Faílde, L.D.; Santos, Y.; Quiroga, M.I. Acute Aeromonas salmonicida infection in turbot (Scophthalmus maximus L.). Histopathological and immunohistochemical studies. Aquaculture 2014, 430, 79–85. [Google Scholar] [CrossRef]
- Padra, J.T.; Sundh, H.; Jin, C.; Karlsson, N.G.; Sundell, K.; Lindén, S.K. Aeromonas salmonicida binds differentially to mucins isolated from skin and intestinal regions of Atlantic salmon in an N-acetylneuraminic acid-dependent manner. Infect. Immun. 2014, 82, 5235–5245. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, C.; Xu, W.; Li, D.; Feng, Y.; Wu, J.; Luo, W.; Du, X.; Du, Z.; Huang, X. Heat Stress Decreases Intestinal Physiological Function and Facilitates the Proliferation of Harmful Intestinal Microbiota in Sturgeons. Front. Microbiol. 2022, 13, 755369. [Google Scholar] [CrossRef]
- Vasquez, I.; Hossain, A.; Gnanagobal, H.; Valderrama, K.; Campbell, B.; Ness, M.; Charette, S.J.; Gamperl, A.K.; Cipriano, R.; Segovia, C.; et al. Comparative Genomics of Typical and Atypical Aeromonas salmonicida Complete Genomes Revealed New Insights into Pathogenesis Evolution. Microorganisms 2022, 10, 189. [Google Scholar] [CrossRef]
- Horna, G.; Ruiz, J. Type 3 secretion system of Pseudomonas aeruginosa. Microbiol. Res. 2021, 246, 126719. [Google Scholar] [CrossRef]
- Miletic, S.; Fahrenkamp, D.; Goessweiner-Mohr, N.; Wald, J.; Pantel, M.; Vesper, O.; Kotov, V.; Marlovits, T.C. Substrate-engaged type III secretion system structures reveal gating mechanism for unfolded protein translocation. Nat. Commun. 2021, 12, 1546. [Google Scholar] [CrossRef] [PubMed]
- Radics, J.; Königsmaier, L.; Marlovits, T.C. Structure of a pathogenic type 3 secretion system in action. Nat. Struct. Mol. Biol. 2014, 21, 82–87. [Google Scholar] [CrossRef]
- Vanden Bergh, P.; Frey, J. Aeromonas salmonicida subsp. salmonicida in the light of its type-three secretion system. Microb. Biotechnol. 2014, 7, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Dallaire-Dufresne, S.; Tanaka, K.H.; Trudel, M.V.; Lafaille, A.; Charette, S.J. Virulence, genomic features, and plasticity of Aeromonas salmonicida subsp. salmonicida, the causative agent of fish furunculosis. Vet. Microbiol. 2014, 169, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Starliper, C.E. The Effect of Depuration on Transmission of Aeromonas salmonicida between the Freshwater Bivalve Amblema plicata and Arctic Char. J. Aquat. Anim. Health 2011, 1, 56–62. [Google Scholar] [CrossRef]
- Mugetti, D.; Pastorino, P.; Menconi, V.; Pedron, C.; Prearo, M. The Old and the New on Viral Diseases in Sturgeon. Pathogens 2020, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Beaz-Hidalgo, R.; Magi, G.E.; Balboa, S.; Barja, J.L.; Romalde, J.L. Development of a PCR protocol for the detection of Aeromonas salmonicida in fish by amplification of the fstA (ferric siderophore receptor) gene. Vet. Microbiol. 2008, 128, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Bigarré, L.; Lesne, M.; Lautraite, A.; Chesneau, V.; Leroux, A.; Jamin, M.; Boitard, P.M.; Toffan, A.; Prearo, M.; Labrut, S.; et al. Molecular identification of iridoviruses infecting various sturgeon species in Europe. J. Fish Dis. 2017, 40, 105–118. [Google Scholar] [CrossRef]
- Zepeda-Velázquez, A.P. Aeromonas spp.: La infección en la trucha arcoíris (Oncorhynchus mykiss) y su aislamiento en México. AquaTIC 2015, 42, 1–16. [Google Scholar]
- Jin, S.; Fu, S.; Li, R.; Dang, H.; Gao, D.; Ye, S.; Jiang, Z. Identification and histopathological and pathogenicity analysis of Aeromonas salmonicida salmonicida from goldfish (Carassius auratus) in North China. Fish. Aquac. J. 2020, 5, 36–41. [Google Scholar] [CrossRef]
- Colussi, S.; Gasparri, F.; Brunetti, R.; Ferrari, A.; Marturano, S.; Prearo, M. Infezione da Aeromonas hydrophila in storioni siberiani (Acipenser baeri) d’allevamento. Ittiopatologia 2005, 2, 105–110. [Google Scholar]
- Mohler, J.W. Culture Manual for the Atlantic sturgeon, Acipenser oxyrinchus oxyrinchus; U.S. Fish and Wildlife Service Publication: Hadley, MA, USA, 2004. [Google Scholar]
- Santi, M.; Pastorino, P.; Foglini, C.; Righetti, M.; Pedron, C.; Prearo, M.A. survey of bacterial infections in sturgeon farming in Italy. J. Appl. Ichthyol. 2019, 35, 275–282. [Google Scholar] [CrossRef]
- Vercauteren, M.; De Swaef, E.; Declercq, A.M.; Aerts, J.; Ampe, B.; Gulla, S.; Haesebrouck, F.; Devriese, L.; Decostere, A.; Chiers, K. Pinpointing the role of Aeromonas salmonicida in the development of skin ulcerations in common dab (Limanda limanda). J. Fish Dis. 2020, 43, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Chebanov, M.; Rosenthal, H.; Gessner, J.; Anrooy, R.; Doukakis, P.; Pourkazemi, M.; Williot, P. Sturgeon Hatchery Practices and Management for Release; Guidelines. Fisheries and Aquaculture Technical Paper; FAO: Quebec, QC, Canada, 2011. [Google Scholar]
- Kent, M.L.; Benda, S.; St-Hilaire, S.; Schreck, C.B. Sensitivity and specificity of histology for diagnoses of four common pathogens and detection of nontarget pathogens in adult Chinook salmon (Oncorhynchus tshawytscha) in fresh water. J. Vet. Diagn. Invest. 2013, 25, 341–351. [Google Scholar] [CrossRef]
- Kolman, H. Primary humoral response in Siberian sturgeon after exposure to anti-furunculosis bacterin. Czech J. Anim. Sci. 2002, 47, 183–188. [Google Scholar]
- Kolman, H.; Siwicki, A.K.; Kolman, R. Influence of O-antigen Aeromonas salmonicida on non-specific and specific immune responses in siberian sturgeon, Acipenser baeri Brandt. Fish. Aquat. Sci. 1999, 7, 93–102. [Google Scholar]
- Wang, X.; Chen, J.; Zhang, R.; Liu, L.; Ma, G.; Zhu, H. Interleukin-6 in Siberian sturgeon (Acipenser baeri): Molecular characterization and immune functional activity. Fish Shellfish Immunol. 2020, 102, 296–306. [Google Scholar] [CrossRef]
- Wang, X.; Ma, G.; Zhang, R.; Liu, L.; Zhu, J.; Zhu, H. Molecular characterization and biological functioning of interleukin-8 in Siberian sturgeon (Acipenser baeri). Fish Shellfish Immunol. 2019, 90, 91–101. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, R.; Liu, L.; Ma, G.; Zhu, H. An IL-1β homologue induced inflammation and antibacterial immune defense in Siberian sturgeon (Acipenser baeri). Fish Shellfish Immunol. 2021, 118, 283–293. [Google Scholar] [CrossRef]
- Khajanchi, B.K.; Fadl, A.A.; Borchardt, M.A.; Berg, R.L.; Horneman, A.J.; Stemper, M.E.; Joseph, S.W.; Moyer, N.P.; Sha, J.; Chopra, A.K. Distribution of virulence factors and molecular fingerprinting of Aeromonas species isolates from water and clinical samples: Suggestive evidence of water-to-human transmission. Appl. Environ. Microbiol. 2010, 76, 2313–2325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Fernández, E.; Chinchilla, B.; Rebollada-Merino, A.; Domínguez, L.; Rodríguez-Bertos, A. An Outbreak of Aeromonas salmonicida in Juvenile Siberian Sturgeons (Acipenser baerii). Animals 2023, 13, 2697. https://doi.org/10.3390/ani13172697
Vázquez-Fernández E, Chinchilla B, Rebollada-Merino A, Domínguez L, Rodríguez-Bertos A. An Outbreak of Aeromonas salmonicida in Juvenile Siberian Sturgeons (Acipenser baerii). Animals. 2023; 13(17):2697. https://doi.org/10.3390/ani13172697
Chicago/Turabian StyleVázquez-Fernández, Esther, Blanca Chinchilla, Agustín Rebollada-Merino, Lucas Domínguez, and Antonio Rodríguez-Bertos. 2023. "An Outbreak of Aeromonas salmonicida in Juvenile Siberian Sturgeons (Acipenser baerii)" Animals 13, no. 17: 2697. https://doi.org/10.3390/ani13172697
APA StyleVázquez-Fernández, E., Chinchilla, B., Rebollada-Merino, A., Domínguez, L., & Rodríguez-Bertos, A. (2023). An Outbreak of Aeromonas salmonicida in Juvenile Siberian Sturgeons (Acipenser baerii). Animals, 13(17), 2697. https://doi.org/10.3390/ani13172697





