Population Status and Vulnerability of Mantidactylus pauliani from Ankaratra Protected Area, Madagascar
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Spatial Distribution and Elevational Range
3.2. Habitat Use
3.3. Vulnerability Analysis
4. Discussion
4.1. Spatial Distribution and Elevation Range
4.2. Habitat Use and Adaptation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreone, F.; Cox, N.A.; Glaw, F.; Köhler, J.; Rabibisoa, N.H.C.; Randriamahazo, H.; Randrianasolo, H.; Raxworthy, C.J.; Stuart, S.N.; Vallan, D.; et al. Update of the Global Amphibian Assessment for Madagascar in Light of Species Discoveries, Nomenclature Changes, and New Field Information. Monogr. Mus. Reg. Sci. Nat. Torino 2008, XLV, 419–438. [Google Scholar]
- Andreone, F.; Carpenter, A.I.; Cox, N.; du Preez, L.; Freeman, K.; Furrer, S.; Garcia, G.; Glaw, F.; Glos, J.; Knox, D.; et al. The Challenge of Conserving Amphibian Megadiversity in Madagascar. PLoS Biol. 2008, 6, e118. [Google Scholar] [CrossRef]
- Glaw, F.; Vences, M. A Field Guide to the Amphibians and Reptiles of Madagascar, 3rd ed.; Vences & Glaw: Cologne, Germany, 2007; ISBN 978-3-929449-03-7. [Google Scholar]
- Rakotonoely, S.A.X. Biologie et Écologie de Deux Espèces d’Amphibiens Boophis williamsi (GUIBE, 1974) et Mantidactylus pauliani (GUIBE, 1974) Critiquement en Danger du Massif de l’Ankaratra. Master’s Thesis, University of Antananarivo, Antananarivo, Madagascar, 2012. [Google Scholar]
- Rosa, S.C.F. Is There a Future for the Amphibians of the Ankaratra Massif Reserve? Understanding the Role of Landscape Change. Master’s Thesis, University of Porto, Porto, Portugal, 2017. [Google Scholar]
- Vences, M.; Andreone, F.; Glaw, F.; Raminosoa, N.; Randrianirina, J.E.; Vieites, D.R. Amphibians and Reptiles of the Ankaratra Massif: Reproductive Diversity, Biogeography and Conservation of a Montane Fauna in Madagascar. Ital. J. Zool. 2002, 69, 263–284. [Google Scholar] [CrossRef]
- Andreone, F.; Cadle, J.E.; Cox, N.; Glaw, F.; Nussbaum, R.A.; Raxworthy, C.J.; Stuart, S.N.; Vallan, D.; Vences, M. Species Review of Amphibian Extinction Risks in Madagascar: Conclusions from the Global Amphibian Assessment. Conserv. Biol. 2005, 19, 1790–1802. [Google Scholar] [CrossRef]
- Ndriantsoa, S.H.; Riemann, J.C.; Raminosoa, N.; Rödel, M.-O.; Glos, J.S. Amphibian Diversity in the Matrix of a Fragmented Landscape around Ranomafana in Madagascar Depends on Matrix Quality. Trop. Conserv. Sci. 2017, 10, 1940082916686065. [Google Scholar] [CrossRef]
- Suzzi-Simmons, A. Status of Deforestation of Madagascar. Glob. Ecol. Conserv. 2023, 42, e02389. [Google Scholar] [CrossRef]
- Bletz, M.C.; Rosa, G.M.; Andreone, F.; Courtois, E.A.; Schmeller, D.S.; Rabibisoa, N.H.C.; Rabemananjara, F.C.E.; Raharivololoniaina, L.; Vences, M.; Weldon, C.; et al. Widespread Presence of the Pathogenic Fungus Batrachochytrium Dendrobatidis in Wild Amphibian Communities in Madagascar. Sci. Rep. 2015, 5, 8633. [Google Scholar] [CrossRef]
- Rabemananjara, F.; Randriamahazo, H.; Rahantamalala, J.; Rahantalisoa, H.; Rakotoarisoa, J.M.; Rabibisoa, N.H.C.; Andreone, F. The Conservation Effort for Two Critically Endangered Amphibian Species of the Ankaratra Massif, Boophis Williamsi and Mantidactylus Pauliani. FrogLog 2012, 103, 29–31. [Google Scholar]
- Hazell, D. Frog Ecology in Modified Australian Landscapes: A Review. Wildl. Res. 2003, 30, 193–205. [Google Scholar] [CrossRef]
- Hernández-Ordóñez, O.; Martínez-Ramos, M.; Arroyo-Rodríguez, V.; González-Hernández, A.; González-Zamora, A.; Zárate, D.A.; Reynoso, V.H. Distribution and Conservation Status of Amphibian and Reptile Species in the Lacandona Rainforest, Mexico: An Update after 20 Years of Research. Trop. Conserv. Sci. 2014, 7, 1–25. [Google Scholar] [CrossRef]
- Fu, L.; Wang, X.; Yang, S.; Li, C.; Hu, J. Morphological Variation and Its Environmental Correlates in the Taihangshan Swelled-Vented Frog across the Qinling Mountains. Animals 2022, 12, 2328. [Google Scholar] [CrossRef]
- Raxworthy, C.J.; Nussbaum, R.A. A Rainforest Survey of Amphibians, Reptiles and Small Mammals at Montagne d’Ambre, Madagascar. Biol. Conserv. 1994, 69, 65–73. [Google Scholar] [CrossRef]
- Moisan, J.; Pelletier, L. Guide de Surveillance Biologique Basée sur les Macrosinvertébrées Benthiques d’Eau Douce du Québec: Cours d’Eaux Peu Profondes à Substrats Grossier; Direction du Suivi de l’Etat de l’Environnement, Ministère du Développement Durable, de l’Environnement et des Parcs: Québec, QC, Canada, 2008; ISBN 978-2-550-53590-4. [Google Scholar]
- ZICOMA. Evaluation de La Faune Aviaire dans les Zones Humides entre le Parc National de Ranomafana et Celui d’Andringitra; Projet d’Appui a la Gestion de l’Environnement, International Resources Group: Washington, DC, USA, 2000. [Google Scholar]
- Salafsky, N.; Margoluis, R. Threat Reduction Assessment: A Practical and Cost-effective Approach to Evaluating Conservation and Development Projects. Conserv. Biol. 1999, 13, 830–841. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species; Version 2022-2; Red list Authorities: Gland, Switzerland, 2022. [Google Scholar]
- Blommers-Schlösser, R.M.A. Biosystematics of the Malagasy Frogs. I. Mantellinae (Ranidae). Beaufortia 1979, 29, 1–77. [Google Scholar]
- Rabibisoa, N.H.C.; Raxworthy, C.J.; Andreone, F. Changement Climatique et Amphibien. In Sahonagasy Action Plan; Andreone, F., Randriamahazo, H., Rabibisoa, N., Eds.; MRSN, CI, IUCN/SSC-ASG: Turin, Italy, 2008; pp. 43–48. ISBN 978–88-860-41-83-6. [Google Scholar]
- Haramura, T. Microhabitat Selection by Tadpoles of Buergeria Japonica Inhabiting the Coastal Area. J. Ethol. 2007, 25, 3–7. [Google Scholar] [CrossRef]
- Dittrich, C.; Drakulić, S.; Schellenberg, M.; Thein, J.; Rödel, M.-O. Some like It Hot? Developmental Differences in Yellow-Bellied Toad (Bombina variegata) Tadpoles from Geographically Close but Different Habitats. Can. J. Zool. 2016, 94, 69–77. [Google Scholar] [CrossRef]
- Brattstrom, B.H. Thermal Control of Aggregation Behavior in Tadpoles. Herpetologica 1962, 18, 38–46. [Google Scholar]
- Edmonds, D.; Rakotoarisoa, J.C.; Rasoanantenaina, S.; Sam, S.S.; Soamiarimampionona, J.; Tsimialomanana, E.; Rainer Dolch, Y.; Rabemananjara, F.; Rabibisoa, N.; Robsomanitrandrasana, E. Captive Husbandry, Reproduction, and Fecundity of the Golden Mantella (Mantella aurantiaca) at the Mitsinjo Breeding Facility in Madagascar. Salamandra 2015, 51, 315–325. [Google Scholar]
- Eterovick, P.C.; Ferreira, A.D.M. Breeding Habitat and Microhabitat Choices by Male and Female Frogs: Are There Differences Between Sexes and Seasons? Herpetologica 2008, 64, 397–405. [Google Scholar] [CrossRef]
- Porcel, X.; Dubos, N.; Nöel, J.; Lava, H.; Velo, J.H.; Melo, M.; Rosa, G.M.; Andreone, F.; Crottini, A. Male Parental Care in Malagasy Stream-Dwelling Frogs of the Mantidactylus Femoralis Group (Anura: Mantellidae: Ochthomantis): Egg Guarding in Ochthomantis. Herpetol. Notes 2022, 15, 55–61. [Google Scholar]
- Raxworthy, C.J.; Nussbaum, R.A. Montane Amphibian and Reptile Communities in Madagascar. Conserv. Biol. 1996, 10, 750–756. [Google Scholar] [CrossRef]
- Cocca, W.; Andreone, F.; Belluardo, F.; Rosa, G.M.; Randrianirina, J.E.; Glaw, F.; Crottini, A. Resolving a Taxonomic and Nomenclatural Puzzle in Mantellid Frogs: Synonymization of Gephyromantis azzurrae with G. Corvus, and Description of Gephyromantis kintana sp. Nov. from the Isalo Massif, Western Madagascar. ZooKeys 2020, 951, 133–157. [Google Scholar] [CrossRef]
- Welsh, H.H.; Ollivier, L.M. Stream Amphibians as indicators of ecosystem stress:a case study from California’s redwoods. Ecol. Appl. 1998, 8, 1118–1132. [Google Scholar] [CrossRef]
- Konopik, O.; Steffan-Dewenter, I.; Grafe, T.U. Effects of Logging and Oil Palm Expansion on Stream Frog Communities on Borneo, Southeast Asia. Biotropica 2015, 47, 636–643. [Google Scholar] [CrossRef]
- Tabarelli, M.; Gascon, C. Lessons from Fragmentation Research: Improving Management and Policy Guidelines for Biodiversity Conservation. Conserv. Biol. 2005, 19, 734–739. [Google Scholar] [CrossRef]
- Funk, W.C.; Greene, A.E.; Corn, P.S.; Allendorf, F.W. High Dispersal in a Frog Species Suggests That It Is Vulnerable to Habitat Fragmentation. Biol. Lett. 2005, 1, 13–16. [Google Scholar] [CrossRef]
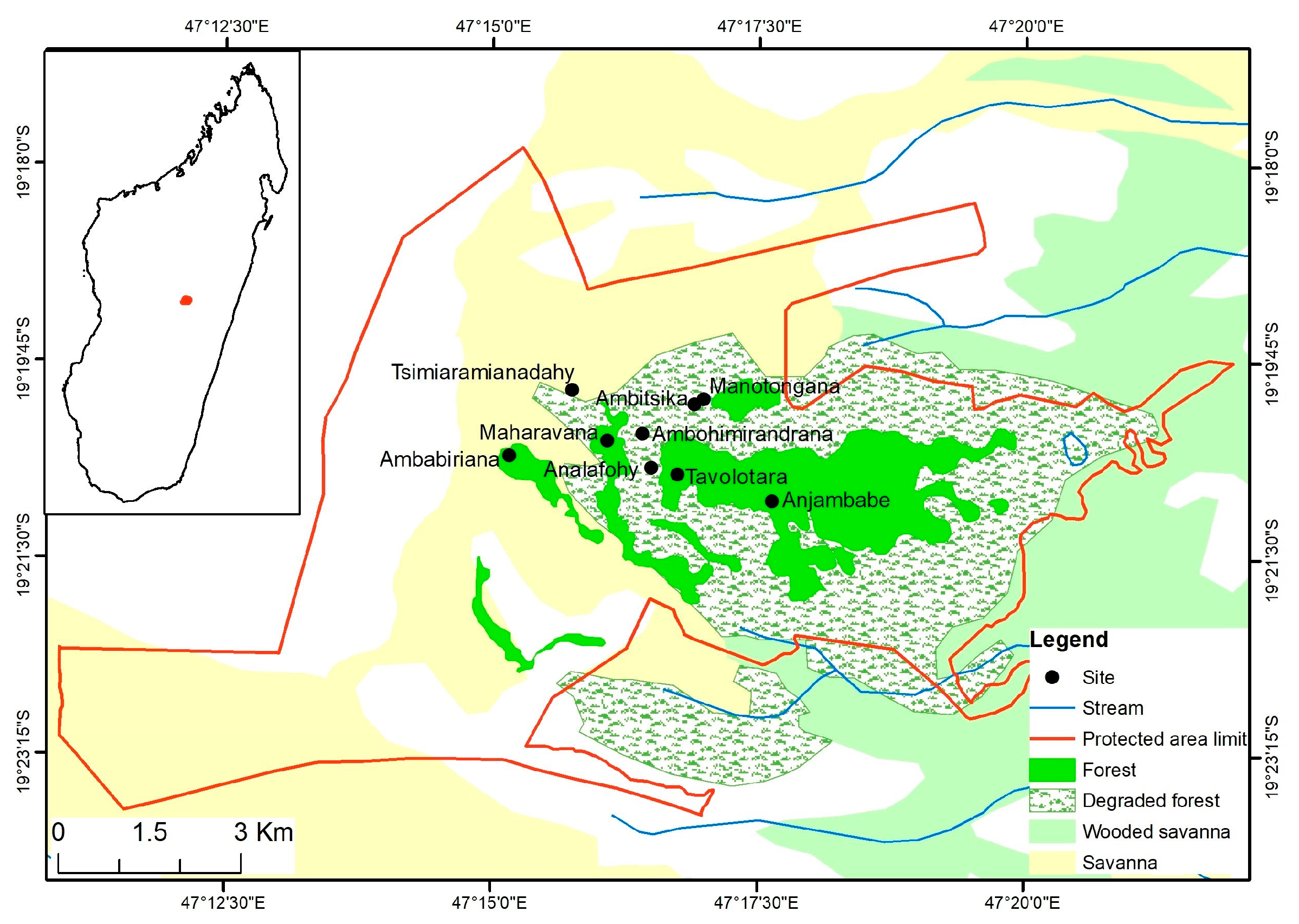



| Transect | Elevation (m) | Description |
|---|---|---|
| Anjambabe | 1900 | Natural forest |
| Tavolotara | 1993 | Natural forest |
| Maharavana | 2100 | Natural forest |
| Analafohy | 2126 | Degraded forest |
| Manotongana | 2167 | Edge 1 |
| Ambaniriana | 2166 | Galery forest |
| Ambitsika | 2202 | Savanna |
| Ambohimirandrana | 2244 | Savanna |
| Tsimiaramianadahy | 2378 | Savanna |
| Alt | Dry Season (2018) | Total | Humid Season (2019) | Total | Humid Season (2020) | Total | Dry Season (2021) | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | J | T | A | J | T | A | J | T | A | J | T | |||||
| 1900 m | 36 | 16 | 24 | 76 | 2 | 7 | 34 | 43 | 0 | 0 | 12 | 12 | 3 | 0 | 12 | 15 |
| 1993 m | 155 | 40 | 50 | 245 | 28 | 13 | 15 | 56 | 10 | 3 | 11 | 24 | 31 | 11 | 5 | 47 |
| 2100 m | - | - | - | - | - | - | - | - | 9 | 8 | 48 | 65 | 26 | 14 | 0 | 40 |
| 2126 m | - | - | - | - | - | - | - | - | 5 | 5 | 30 | 40 | 31 | 28 | 8 | 67 |
| 2166 m | 38 | 39 | 11 | 88 | 17 | 6 | 13 | 36 | 3 | 2 | 34 | 39 | 15 | 39 | 4 | 58 |
| 2167 m | 1 | 2 | 22 | 25 | 0 | 0 | 26 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2202 m | 36 | 29 | 9 | 74 | 2 | 2 | 44 | 48 | 2 | 5 | 61 | 68 | 11 | 8 | 5 | 24 |
| 2244 m | - | - | - | - | - | - | - | - | 0 | 1 | 69 | 70 | 1 | 0 | 0 | 1 |
| 2378 m | 23 | 6 | 11 | 40 | 1 | 1 | 15 | 17 | 2 | 0 | 32 | 34 | 3 | 1 | 14 | 18 |
| Total | 289 | 132 | 127 | 548 | 50 | 29 | 147 | 226 | 31 | 24 | 297 | 352 | 121 | 101 | 48 | 270 |
| Season | Forest | Savannah | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Dry season (2018) | 87 | 158 | 24 | 20 |
| Humid season (2019) | 13 | 34 | 2 | 1 |
| Humid season (2020) | 6 | 21 | 3 | 1 |
| Dry season (2021) | 35 | 71 | 6 | 9 |
| Environment Types | Sites | Threat Types | Score | Evaluation | ||
|---|---|---|---|---|---|---|
| Length | Intensity | Importance | ||||
| Forest environment | Anjambabe (1900 m) | Bushfires | 1 | 1 | 1 | Minor (3) |
| Charcoal production | 1 | 2 | 1 | Minor (4) | ||
| Logging | 2 | 1 | 1 | Minor (4) | ||
| Livestock grazing | 1 | 3 | 1 | Medium (5) | ||
| Evaluation total [Index of pressure PI] | 16 [0.44] | |||||
| Tavolotara (1993 m) | Bushfires | 2 | 1 | 1 | Minor (4) | |
| Charcoal production | 2 | 1 | 1 | Minor (4) | ||
| Logging | 1 | 2 | 1 | Minor (4) | ||
| Livestock grazing | 1 | 2 | 1 | Minor (4) | ||
| Evaluation total [Index of pressure PI] | 16 [0.44] | |||||
| Maharavana (2100 m) | Bushfires | 1 | 2 | 1 | Minor (4) | |
| Charcoal production | 1 | 2 | 1 | Minor (4) | ||
| Logging | 1 | 1 | 1 | Minor (3) | ||
| Evaluation total [Index of pressure PI] | 11 [0.41] | |||||
| Analafohy (2126 m) | Charcoal production | 2 | 2 | 1 | Medium (5) | |
| Logging | 2 | 2 | 1 | Medium (5) | ||
| Livestock grazing | 1 | 2 | 1 | Minor (4) | ||
| Expansion of agricultural land | 1 | 1 | 1 | Minor (3) | ||
| Evaluation total [Index of pressure PI] | 17 [0.47] | |||||
| Ambaniriana (2166 m) | Charcoal production | 1 | 2 | 1 | Minor (4) | |
| Logging | 1 | 1 | 1 | Minor (3) | ||
| Livestock grazing | 2 | 2 | 1 | Medium (5) | ||
| Evaluation total [Index of pressure PI] | 12 [0.44] | |||||
| Edge | Manotongana (2167 m) | Bushfires | 2 | 3 | 1 | Medium (6) |
| Charcoal production | 2 | 3 | 1 | Medium (6) | ||
| Logging | 2 | 2 | 1 | Medium (5) | ||
| Evaluation total [Index of pressure PI] | 17 [0.63] | |||||
| Savannah | Ambitsika (2202 m) | Bushfires | 1 | 2 | 1 | Minor (4) |
| Charcoal production | 2 | 1 | 1 | Minor (4) | ||
| Feux de pâturage | 1 | 2 | 1 | Minor (4) | ||
| Livestock grazing | 2 | 3 | 1 | Medium (6) | ||
| Evaluation total [Index of pressure PI] | 18 [0.5] | |||||
| Ambohimirandrana (2244 m) | Charcoal production | 1 | 2 | 1 | Minor (4) | |
| Livestock grazing | 2 | 3 | 1 | Medium (6) | ||
| Evaluation total [Index of pressure PI] | 10 [0.55] | |||||
| Tsimiaramianadahy (2378 m) | Bushfires | 2 | 3 | 1 | Medium (6) | |
| Livestock grazing | 2 | 3 | 1 | Medium (6) | ||
| Charcoal production | 1 | 2 | 1 | Minor (4) | ||
| Evaluation total [Index of pressure PI] | 16 [0.59] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oninjatovo Radonirina, H.; Randriamahatantsoa, B.; Rabibisoa, N.H.C. Population Status and Vulnerability of Mantidactylus pauliani from Ankaratra Protected Area, Madagascar. Animals 2023, 13, 2706. https://doi.org/10.3390/ani13172706
Oninjatovo Radonirina H, Randriamahatantsoa B, Rabibisoa NHC. Population Status and Vulnerability of Mantidactylus pauliani from Ankaratra Protected Area, Madagascar. Animals. 2023; 13(17):2706. https://doi.org/10.3390/ani13172706
Chicago/Turabian StyleOninjatovo Radonirina, Herizo, Bernard Randriamahatantsoa, and Nirhy H. C. Rabibisoa. 2023. "Population Status and Vulnerability of Mantidactylus pauliani from Ankaratra Protected Area, Madagascar" Animals 13, no. 17: 2706. https://doi.org/10.3390/ani13172706
APA StyleOninjatovo Radonirina, H., Randriamahatantsoa, B., & Rabibisoa, N. H. C. (2023). Population Status and Vulnerability of Mantidactylus pauliani from Ankaratra Protected Area, Madagascar. Animals, 13(17), 2706. https://doi.org/10.3390/ani13172706









