Molecular Characteristics of Chicken Infectious Anemia Virus in Central and Eastern China from 2020 to 2022
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Pathogen Detection
2.2. Detection, Polymerase Chain Reaction (PCR) Amplification, and Sequencing
2.3. Virus Isolation
2.4. Phylogenetic and Recombination Analysis
2.5. VP1 Structure Modeling
3. Results
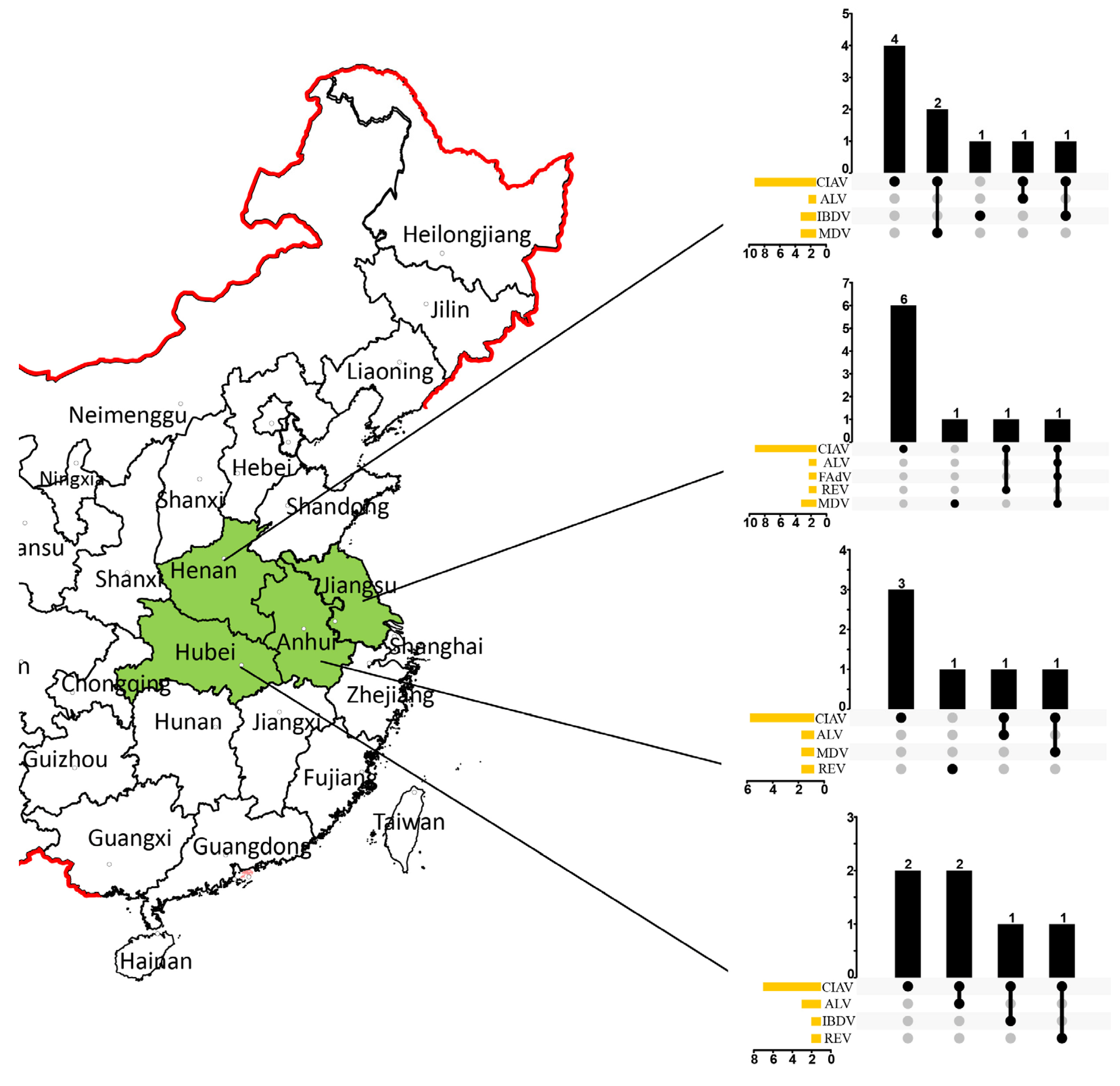
3.1. Sample Screening
3.2. DNA Alignment and Identity Analysis
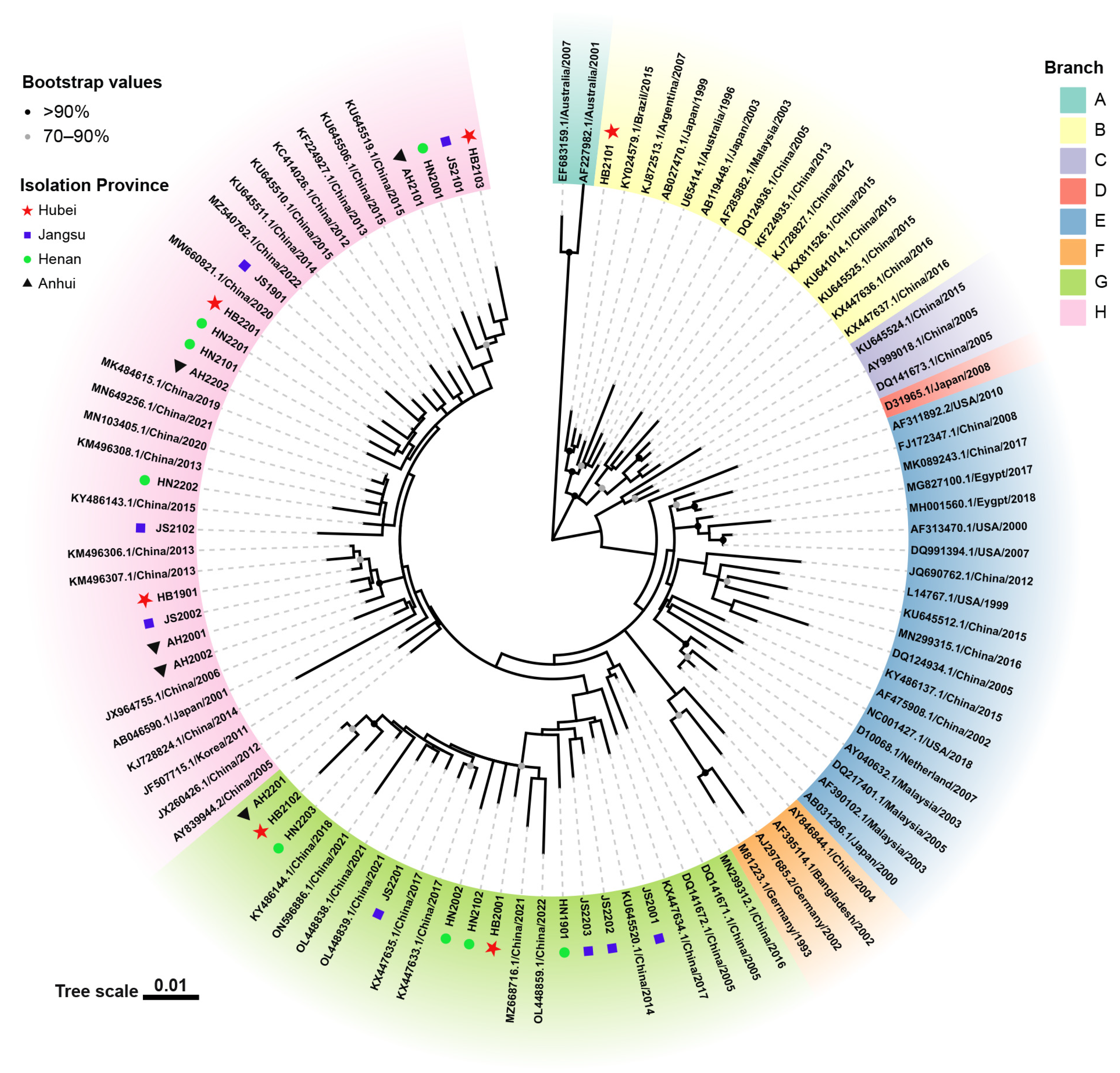
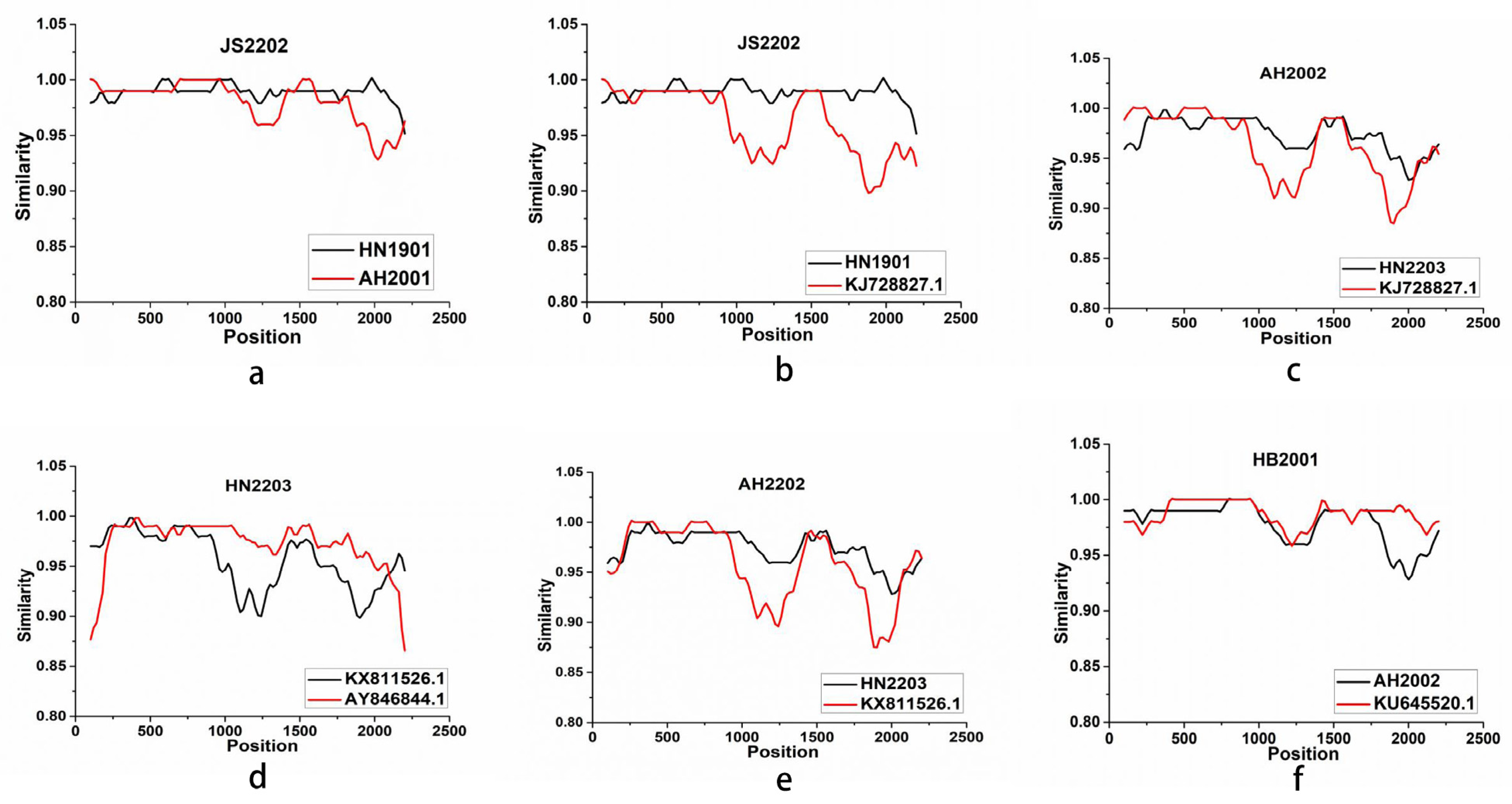
3.3. Phylogenetic and Recombination Analysis
3.4. Mutation Analysis of VP1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.R.; Kwon, Y.K.; Bae, Y.C.; Oem, J.K.; Lee, O.S. Molecular characterization of chicken infectious anemia viruses detected from breeder and broiler chickens in South Korea. Poult. Sci. 2010, 89, 2426–2431. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wu, B.; Sun, B.; Chen, F.; Ji, J.; Ma, J.; Xie, Q. Phylogenetic and molecular characterization of chicken anemia virus in southern China from 2011 to 2012. Sci. Rep. 2013, 3, 3519. [Google Scholar] [CrossRef]
- Hagood, L.T.; Kelly, T.F.; Wright, J.C.; Hoerr, F.J. Evaluation of chicken infectious anemia virus and associated risk factors with disease and production losses in broilers. Avian Dis. 2000, 44, 803–808. [Google Scholar] [CrossRef]
- Yuasa, N.; Taniguchi, T.; Yoshida, I. Isolation and Some Characteristics of an Agent Inducing Anemia in Chicks. Avian Dis. 1979, 23, 366–385. [Google Scholar] [CrossRef]
- Wang, X.W.; Feng, J.; Jin, J.X.; Zhu, X.J.; Sun, A.J.; Liu, H.Y.; Wang, J.J.; Wang, R.; Yang, X.; Chen, L.; et al. Molecular Epidemiology and Pathogenic Characterization of Novel Chicken Infectious Anemia Viruses in Henan Province of China. Front. Vet. Sci. 2022, 9, 871826. [Google Scholar] [CrossRef]
- Gimeno, I.M.; Schat, K.A. Virus-Induced Immunosuppression in Chickens. Avian Dis. 2018, 62, 272–285. [Google Scholar] [CrossRef]
- Rosario, K.; Breitbart, M.; Harrach, B.; Segalés, J.; Delwart, E.; Biagini, P.; Varsani, A. Revisiting the taxonomy of the family Circoviridae: Establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch. Virol. 2017, 162, 1447–1463. [Google Scholar] [CrossRef]
- Jeurissen, S.H.; Wagenaar, F.; Pol, J.M.; van der Eb, A.J.; Noteborn, M.H. Chicken anemia virus causes apoptosis of thymocytes after in vivo infection and of cell lines after in vitro infection. J. Virol. 1992, 66, 7383–7388. [Google Scholar] [CrossRef]
- Ducatez, M.F.; Owoade, A.A.; Abiola, J.O.; Muller, C.P. Molecular epidemiology of chicken anemia virus in Nigeria. Arch. Virol. 2006, 151, 97–111. [Google Scholar] [CrossRef]
- Peters, M.A.; Jackson, D.C.; Crabb, B.S.; Browning, G.F. Chicken anemia virus VP2 is a novel dual specificity protein phosphatase. J. Biol. Chem. 2002, 277, 39566–39573. [Google Scholar] [CrossRef]
- Koch, G.; van Roozelaar, D.J.; Verschueren, C.A.; van der Eb, A.J.; Noteborn, M.H. Immunogenic and protective properties of chicken anaemia virus proteins expressed by baculovirus. Vaccine 1995, 13, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Kaffashi, A.; Pagel, C.N.; Noormohammadi, A.H.; Browning, G.F. Evidence of apoptosis induced by viral protein 2 of chicken anaemia virus. Arch. Virol. 2015, 160, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.M.; Shvarts, A.; van Ormondt, H.; Jochemsen, A.G.; van der Eb, A.J.; Noteborn, M.H. Apoptin, a protein derived from chicken anemia virus, induces p53-independent apoptosis in human osteosarcoma cells. Cancer Res. 1995, 55, 486–489. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, X.; Cheng, A.; Wang, M.; Yin, Z.; Huang, J.; Jia, R. Apoptosis Triggered by ORF3 Proteins of the Circoviridae Family. Front. Cell Infect. Microbiol. 2020, 10, 609071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Fang, L.; Fu, J.; Cui, S.; Zhao, Y.; Cui, Z.; Chang, S.; Zhao, P. Genomic Analysis of the Chicken Infectious Anemia Virus in a Specific Pathogen-Free Chicken Population in China. BioMed Res. Int. 2016, 2016, 4275718. [Google Scholar] [CrossRef][Green Version]
- Meng, F.; Dong, G.; Zhang, Y.; Tian, S.; Cui, Z.; Chang, S.; Zhao, P. Co-infection of fowl adenovirus with different immunosuppressive viruses in a chicken flock. Poult. Sci. 2018, 97, 1699–1705. [Google Scholar] [CrossRef]
- Su, Q.; Wang, T.; Meng, F.; Cui, Z.; Chang, S.; Zhao, P. Synergetic pathogenicity of Newcastle disease vaccines LaSota strain and contaminated chicken infectious anemia virus. Poult. Sci. 2019, 98, 1985–1992. [Google Scholar] [CrossRef]
- Eltahir, Y.M.; Qian, K.; Jin, W.; Qin, A. Analysis of chicken anemia virus genome: Evidence of intersubtype recombination. Virol. J. 2011, 8, 512. [Google Scholar] [CrossRef]
- Fang, L.; Li, Y.; Wang, Y.; Fu, J.; Cui, S.; Li, X.; Chang, S.; Zhao, P. Genetic Analysis of Two Chicken Infectious Anemia Virus Variants-Related Gyrovirus in Stray Mice and Dogs: The First Report in China, 2015. BioMed Res. Int. 2017, 2017, 6707868. [Google Scholar] [CrossRef]
- Gao, Q.Y.B.; Wang, Q.; Jiang, L.; Zhu, H.; Gao, Y.; Qin, L.; Wang, Y.; Qi, X.; Gao, H.; Wang, X.; et al. Development and application of a multiplex PCR method for rapid differential detection of subgroup A, B, and J avian leukosis viruses. J. Clin. Microbiol. 2014, 52, 37–44. [Google Scholar] [CrossRef]
- Fu, M.; Wang, B.; Chen, X.; He, Z.; Wang, Y.; Li, X.; Cao, H.; Zheng, S.J. MicroRNA gga-miR-130b Suppresses Infectious Bursal Disease Virus Replication via Targeting of the Viral Genome and Cellular Suppressors of Cytokine Signaling 5. J. Virol. 2017, 92, e01646-17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qi, X.; Gao, Y.; Hua, Y.; Li, K.; Deng, X.; Wang, Q.Z.L.; Chai, H.; Chen, Y.; Yin, C.; et al. Molecular characterization and phylogenetic analysis of the reticuloendotheliosis virus isolated from wild birds in Northeast China. Vet. Microbiol. 2013, 166, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef]
- Martin, D.; Rybicki, E. RDP: Detection of recombination amongst aligned sequences. Bioinformatics 2000, 16, 562–563. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Imada, T.; Kaji, N.; Mase, M.; Tsukamoto, K.; Tanimura, N.; Yuasa, N. Identification of a genetic determinant of pathogenicity in chicken anaemia virus. J. Gen. Virol. 2001, 82, 1233–1238. [Google Scholar] [CrossRef]
- Todd, D.; Scott, A.N.; Ball, N.W.; Borghmans, B.J.; Adair, B.M. Molecular basis of the attenuation exhibited by molecularly cloned highly passaged chicken anemia virus isolates. J. Virol. 2002, 76, 8472–8474. [Google Scholar] [CrossRef]
- Natesan, S.; Kataria, J.M.; Dhama, K.; Rahul, S.; Bhardwaj, N. Biological and molecular characterization of chicken anaemia virus isolates of Indian origin. Virus Res. 2006, 118, 78–86. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Cui, S.; Fu, J.; Li, X.; Zhang, H.; Cui, Z.; Chang, S.; Shi, W.; Zhao, P. Genomic Characterization of Recent Chicken Anemia Virus Isolates in China. Front. Microbiol. 2017, 8, 401. [Google Scholar] [CrossRef][Green Version]
- Ou, S.C.; Lin, H.L.; Liu, P.C.; Huang, H.J.; Lee, M.S.; Lien, Y.Y.; Tsai, Y.L. Epidemiology and molecular characterization of chicken anaemia virus from commercial and native chickens in Taiwan. Transbound. Emerg. Dis. 2018, 65, 1493–1501. [Google Scholar] [CrossRef]
- Yao, S.; Tuo, T.; Gao, X.; Han, C.; Yan, N.; Liu, A.; Gao, H.; Gao, Y.; Cui, H.; Liu, C.; et al. Molecular epidemiology of chicken anaemia virus in sick chickens in China from 2014 to 2015. PLoS ONE 2019, 14, e0210696. [Google Scholar] [CrossRef] [PubMed]
- Techera, C.; Marandino, A.; Tomás, G.; Grecco, S.; Hernández, M.; Hernández, D.; Panzera, Y.; Pérez, R. Origin, spreading and genetic variability of chicken anaemia virus. Avian Pathol. 2021, 50, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Johne, R.; Raue, R.; Todd, D.; Müller, H. Sequence analysis of the full-length cloned DNA of a chicken anaemia virus (CAV) strain from Bangladesh: Evidence for genetic grouping of CAV strains based on the deduced VP1 amino acid sequences. J. Vet. Med. B Infect. Dis. Vet. Public Health 2002, 49, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, R.W.; Soiné, C.; Weinkle, T.; O’Connell, P.H.; Ohashi, K.; Watson, S.; Lucio, B.; Harrington, S.; Schat, K.A. A hypervariable region in VP1 of chicken infectious anemia virus mediates rate of spread and cell tropism in tissue culture. J. Virol. 1996, 70, 8872–8878. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgod, S.; Adel, A.; Arafa, A.S.; Hussein, H.A. Full genome sequences of chicken anemia virus demonstrate mutations associated with pathogenicity in two different field isolates in Egypt. Virusdisease 2018, 29, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, Z.; Lei, X.; Lu, J.; Yan, Z.; Qin, J.; Chen, F.; Xie, Q.; Lin, W. Epidemiology, molecular characterization, and recombination analysis of chicken anemia virus in Guangdong province, China. Arch. Virol. 2020, 165, 1409–1417. [Google Scholar] [CrossRef]
- Erfan, A.M.; Selim, A.A.; Naguib, M.M. Characterization of full genome sequences of chicken anemia viruses circulating in Egypt reveals distinct genetic diversity and evidence of recombination. Virus Res. 2018, 251, 78–85. [Google Scholar] [CrossRef]

| Accession No. | Strain Name | Species | Site of Isolation | Genome Length (bp) | Year |
|---|---|---|---|---|---|
| M81223 | M81223 | Chicken | Germany | 2298 | 1993 |
| U65414.1 | 704 | Chicken | Australia | 2298 | 1996 |
| L14767.1 | L14767.1 | Chicken | USA | 2298 | 1999 |
| AB027470.1 | TR20 | Chicken | Japan | 2298 | 1999 |
| AF313470.1 | Del-Rosa a | Chicken | USA | 2294 | 2000 |
| AB031296.1 | A2 | Chicken | Japan | 2298 | 2000 |
| AB046590.1 | C369 | Chicken | Japan | 2298 | 2001 |
| AF227982.1 | AF227982 | Chicken | Australia | 2286 | 2001 |
| AJ297685.2 | clone34 | Chicken | Germany | 2297 | 2002 |
| AF475908.1 | AF475908 | Chicken | China | 2298 | 2002 |
| AB119448.1 | G6 | Chicken | Japan | 2298 | 2003 |
| AY040632.1 | 3-IP60 | Chicken | Malaysia | 2298 | 2003 |
| AF285882.1 | SMSC-1 | Chicken | Malaysia | 2298 | 2003 |
| AF390102.1 | SMSC-IP60 | Chicken | Malaysia | 2298 | 2003 |
| AY846844.1 | TJBD40 | Chicken | China | 2298 | 2004 |
| AF395114 | BD-3 | Chicken | Bangladesh | 2298 | 2004 |
| AY999018 | SD24 | Chicken | China | 2298 | 2005 |
| DQ124936.1 | AH4 | Chicken | China | 2298 | 2005 |
| AY839944.2 | LF4 | Chicken | China | 2298 | 2005 |
| DQ141673.1 | SD22 | Chicken | China | 2298 | 2005 |
| DQ124934.1 | HA4 | Chicken | China | 2298 | 2005 |
| DQ217401 | SMSC-1P123WT | Chicken | Malaysia | 2298 | 2005 |
| DQ141671.1 | SH16 | Chicken | China | 2298 | 2005 |
| DQ141672 | HN9 | Chicken | China | 2298 | 2005 |
| JX964755 | GXC060821 | Chicken | China | 2292 | 2006 |
| EF683159 | 3711 | Chicken | Australia | 2279 | 2007 |
| DQ991394 | 01-4201 | Chicken | USA | 2298 | 2007 |
| KJ872513 | CIAV-10 | Chicken | Argentina | 2298 | 2007 |
| D10068.1 | D10068.1 | Chicken | Netherland | 2298 | 2007 |
| FJ172347.1 | SDLY08 | Chicken | China | 2298 | 2008 |
| D31965.1 | 82-2 | Chicken | Japan | 2319 | 2008 |
| AF311892.2 | 98D02512 | Chicken | USA | 2298 | 2010 |
| JF507715.1 | CIAVV89-69 | Chicken | Korea | 2298 | 2011 |
| KJ728827.1 | 18 | Chicken | China | 2298 | 2012 |
| JX260426.1 | GD-1-12 | Chicken | China | 2298 | 2012 |
| KC414026 | Cat-Gyv | Cat | China | 2295 | 2012 |
| JQ690762.1 | JQ690762.1 | Human | China | 2316 | 2012 |
| KM496308.1 | SC-NC1 | Chicken | China | 2298 | 2013 |
| KF224935.1 | GD-K-12 | Chicken | China | 2298 | 2013 |
| KM496307 | SC-MZ42A | Chicken | China | 2298 | 2013 |
| KF224927.1 | GD-C-12 | Chicken | China | 2298 | 2013 |
| KM496306.1 | SC-MZ | Chicken | China | 2298 | 2013 |
| KJ728824 | 14 | Chicken | China | 2298 | 2014 |
| KU645520.1 | HN1405 | Chicken | China | 2298 | 2014 |
| KU645511.1 | LN1402 | Chicken | China | 2298 | 2014 |
| KY024579.1 | RS-BR-15 | Chicken | Brazil | 2298 | 2015 |
| KU645512.1 | HN1504 | Chicken | China | 2298 | 2015 |
| KU641014.1 | JN1503 | Chicken | China | 2298 | 2015 |
| KX811526.1 | SD15 | Chicken | China | 2298 | 2015 |
| KU645506.1 | SD1512 | Chicken | China | 2298 | 2015 |
| KY486137.1 | HLJ15108 | Chicken | China | 2298 | 2015 |
| KU645519 | SD1508 | Chicken | China | 2298 | 2015 |
| KU645510.1 | SD1509 | Chicken | China | 2298 | 2015 |
| KY486143.1 | HLJ15169 | Chicken | China | 2298 | 2015 |
| KU645524 | CIAV-Dog | Dog | China | 2298 | 2015 |
| KU645525 | CIAV-Mouse | Mouse | China | 2298 | 2015 |
| KX447636.1 | LY-1 | Chicken | China | 2298 | 2016 |
| KX447637.1 | LY-2 | Chicken | China | 2298 | 2016 |
| MN299312.1 | 1716TW | Chicken | China | 2298 | 2016 |
| MN299315.1 | 1535TW | Chicken | China | 2298 | 2016 |
| KX447634.1 | BS-C2 | Chicken | China | 2298 | 2017 |
| MG827100.1 | CAV-SK4-2017 | Chicken | Egypt | 2298 | 2017 |
| KX447633.1 | BS-C1 | Chicken | China | 2298 | 2017 |
| MK089243.1 | 17SY0902 | Chicken | China | 2298 | 2017 |
| KX447635 | HB160430 | Chicken | China | 2298 | 2017 |
| NC001427 | Cux-1 a | Chicken | USA | 2319 | 2018 |
| MH001560.1 | CAV-EG-13 | Chicken | Egypt | 2298 | 2018 |
| KY486144.1 | HLJ15170 | Chicken | China | 2298 | 2018 |
| MK484615.1 | GX1804 | Chicken | China | 2298 | 2019 |
| MN103405.1 | GX1805 | Chicken | China | 2298 | 2020 |
| MW660821.1 | SDSPF2020 | Chicken | China | 2298 | 2020 |
| MN649256.1 | GX1908L2 | Chicken | China | 2298 | 2021 |
| MZ668716.1 | HN2021-1414 | Chicken | China | 2298 | 2021 |
| ON596886.1 | Guangxi/2298/2021 | Chicken | China | 2298 | 2021 |
| OL448839.1 | SD2008 | Chicken | China | 2298 | 2021 |
| OL448838.1 | SD2007 | Chicken | China | 2298 | 2021 |
| MZ540762.1 | YN04 | Chicken | China | 2298 | 2022 |
| Accession No. | Strain Name | Species | Site of Isolation | Genome Length (bp) | Year |
|---|---|---|---|---|---|
| OQ869186 | HN1901 | Chicken | China | 2298 | 2019 |
| OQ869187 | HB1901 | Chicken | China | 2298 | 2019 |
| OQ869188 | JS1901 | Chicken | China | 2298 | 2019 |
| OQ869189 | HN2001 | Chicken | China | 2298 | 2020 |
| OQ869190 | HB2001 | Chicken | China | 2298 | 2020 |
| OQ869191 | JS2001 | Chicken | China | 2298 | 2020 |
| OQ869192 | AH2001 | Chicken | China | 2298 | 2020 |
| OQ869193 | JS2002 | Chicken | China | 2298 | 2020 |
| OQ869194 | HN2002 | Chicken | China | 2298 | 2020 |
| OQ869195 | AH2002 | Chicken | China | 2298 | 2020 |
| OQ869196 | HB2101 | Chicken | China | 2298 | 2021 |
| OQ869197 | HB2102 | Chicken | China | 2298 | 2021 |
| OQ869198 | HN2101 | Chicken | China | 2298 | 2021 |
| OQ869199 | JS2101 | Chicken | China | 2298 | 2021 |
| OQ869200 | JS2102 | Chicken | China | 2298 | 2021 |
| OQ869201 | HN2102 | Chicken | China | 2298 | 2021 |
| OQ869202 | HB2103 | Chicken | China | 2298 | 2021 |
| OQ869203 | AH2101 | Chicken | China | 2298 | 2021 |
| OQ869204 | HB2201 | Chicken | China | 2298 | 2022 |
| OQ869205 | JS2201 | Chicken | China | 2298 | 2022 |
| OQ869206 | HN2201 | Chicken | China | 2298 | 2022 |
| OQ869207 | HN2202 | Chicken | China | 2298 | 2022 |
| OQ869208 | AH2201 | Chicken | China | 2298 | 2022 |
| OQ869209 | HN2203 | Chicken | China | 2298 | 2022 |
| OQ869210 | AH2202 | Chicken | China | 2298 | 2022 |
| OQ869211 | JS2202 | Chicken | China | 2298 | 2022 |
| OQ869212 | JS2203 | Chicken | China | 2298 | 2022 |
| Methods | RDP | GENECONV | Bootscan | Maxchi | Chimaera | SiSscan | 3Seq |
|---|---|---|---|---|---|---|---|
| p-Value | 8.26 × 10−7 | 7.35 × 10−6 | 9.20 × 10−6 | 2.27 × 10−9 | 4.03 × 10−10 | 1.46 × 10−15 | 7.56 × 10−17 |
| Strain | Substitution of the Amino Acid Residues in VP1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 22 | 75 | 89 | 97 | 125 | 139 | 141 | 144 | 157 | 287 | 290 | 370 | 376 | 394 | 413 | 446 | |
| SD24 | H | V | T | M | L | K | Q | E | V | S | A | S | L | Q | A | G |
| HN1901 | - | - | - | - | - | - | - | - | - | N | P | A | - | - | - | - |
| HB1901 | - | - | - | - | - | - | - | - | M | - | - | G | I | - | S | - |
| JS1901 | - | - | - | - | - | - | - | - | M | - | - | G | I | - | S | - |
| HN2001 | - | I | - | L | I | Q | - | Q | - | - | - | G | I | - | S | - |
| HB2001 | - | - | - | L | - | - | - | - | M | - | P | - | - | - | - | - |
| JS2001 | - | - | - | - | - | - | - | - | - | - | - | - | M | - | - | - |
| AH2001 | - | - | - | - | - | - | - | - | - | - | - | G | I | - | S | - |
| JS2002 | - | - | - | - | - | - | - | - | - | - | - | G | I | - | S | - |
| HN2002 | Q | - | - | L | - | - | - | - | M | T | P | - | - | - | - | - |
| AH2002 | - | - | - | - | - | - | - | - | - | - | - | G | I | - | S | - |
| HB2101 | - | I | - | L | I | Q | - | Q | - | T | P | T | - | - | - | - |
| HB2102 | - | - | - | L | - | - | - | - | M | T | P | - | - | - | - | - |
| HN2101 | - | - | - | - | - | - | - | - | M | - | - | G | I | - | S | - |
| JS2101 | N | I | - | L | I | Q | - | Q | - | - | - | G | I | - | S | - |
| JS2102 | - | - | - | - | - | - | - | - | - | - | - | G | I | - | S | - |
| HN2102 | - | - | - | - | P | - | - | - | M | T | P | - | - | - | - | - |
| HB2103 | N | I | - | L | I | Q | - | Q | - | A | P | G | I | - | S | - |
| AH2101 | N | I | - | L | I | Q | - | Q | - | - | - | G | I | - | S | - |
| HB2201 | - | - | - | - | - | - | - | - | M | - | - | G | I | - | S | - |
| JS2201 | - | - | - | L | - | - | - | - | M | - | P | - | - | - | - | - |
| HN2201 | - | - | - | - | - | - | - | - | M | - | - | G | I | - | S | - |
| HN2202 | - | - | - | - | - | - | - | - | - | - | - | G | I | - | S | - |
| AH2201 | - | - | - | L | - | - | - | - | M | T | P | - | - | - | - | - |
| HN2203 | - | - | - | L | - | - | - | - | M | T | P | - | - | - | - | - |
| AH2202 | - | - | - | - | - | - | - | - | - | - | - | G | I | - | S | - |
| JS2202 | - | - | - | - | - | - | - | - | M | T | P | - | - | - | - | - |
| JS2203 | - | - | - | - | - | - | - | - | M | T | P | R | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Zhang, Z.; Xu, X.; Ji, J.; Yao, L.; Kan, Y.; Xie, Q.; Bi, Y. Molecular Characteristics of Chicken Infectious Anemia Virus in Central and Eastern China from 2020 to 2022. Animals 2023, 13, 2709. https://doi.org/10.3390/ani13172709
Xu S, Zhang Z, Xu X, Ji J, Yao L, Kan Y, Xie Q, Bi Y. Molecular Characteristics of Chicken Infectious Anemia Virus in Central and Eastern China from 2020 to 2022. Animals. 2023; 13(17):2709. https://doi.org/10.3390/ani13172709
Chicago/Turabian StyleXu, Shuqi, Zhibin Zhang, Xin Xu, Jun Ji, Lunguang Yao, Yunchao Kan, Qingmei Xie, and Yingzuo Bi. 2023. "Molecular Characteristics of Chicken Infectious Anemia Virus in Central and Eastern China from 2020 to 2022" Animals 13, no. 17: 2709. https://doi.org/10.3390/ani13172709
APA StyleXu, S., Zhang, Z., Xu, X., Ji, J., Yao, L., Kan, Y., Xie, Q., & Bi, Y. (2023). Molecular Characteristics of Chicken Infectious Anemia Virus in Central and Eastern China from 2020 to 2022. Animals, 13(17), 2709. https://doi.org/10.3390/ani13172709






