Exploring the Toxic Effects of ZEA on IPEC-J2 Cells from the Inflammatory Response and Apoptosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Grouping
2.2. Measurement of Oxidative Stress and Antioxidant Indicators
2.3. ROS Activity Assay for IPEC-J2 Cells
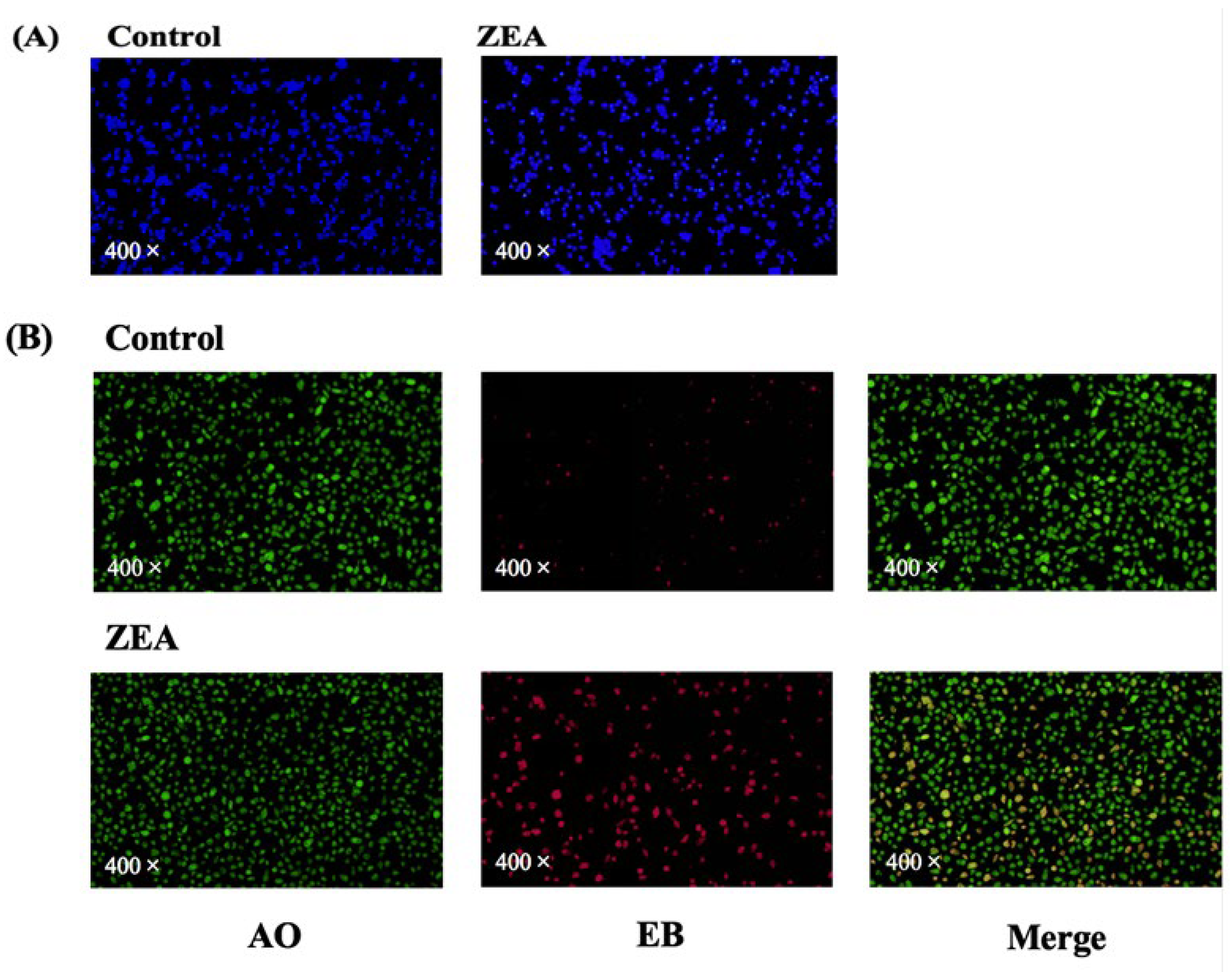
2.4. Hoechst Staining
2.5. Apoptotic Cell Death Assay
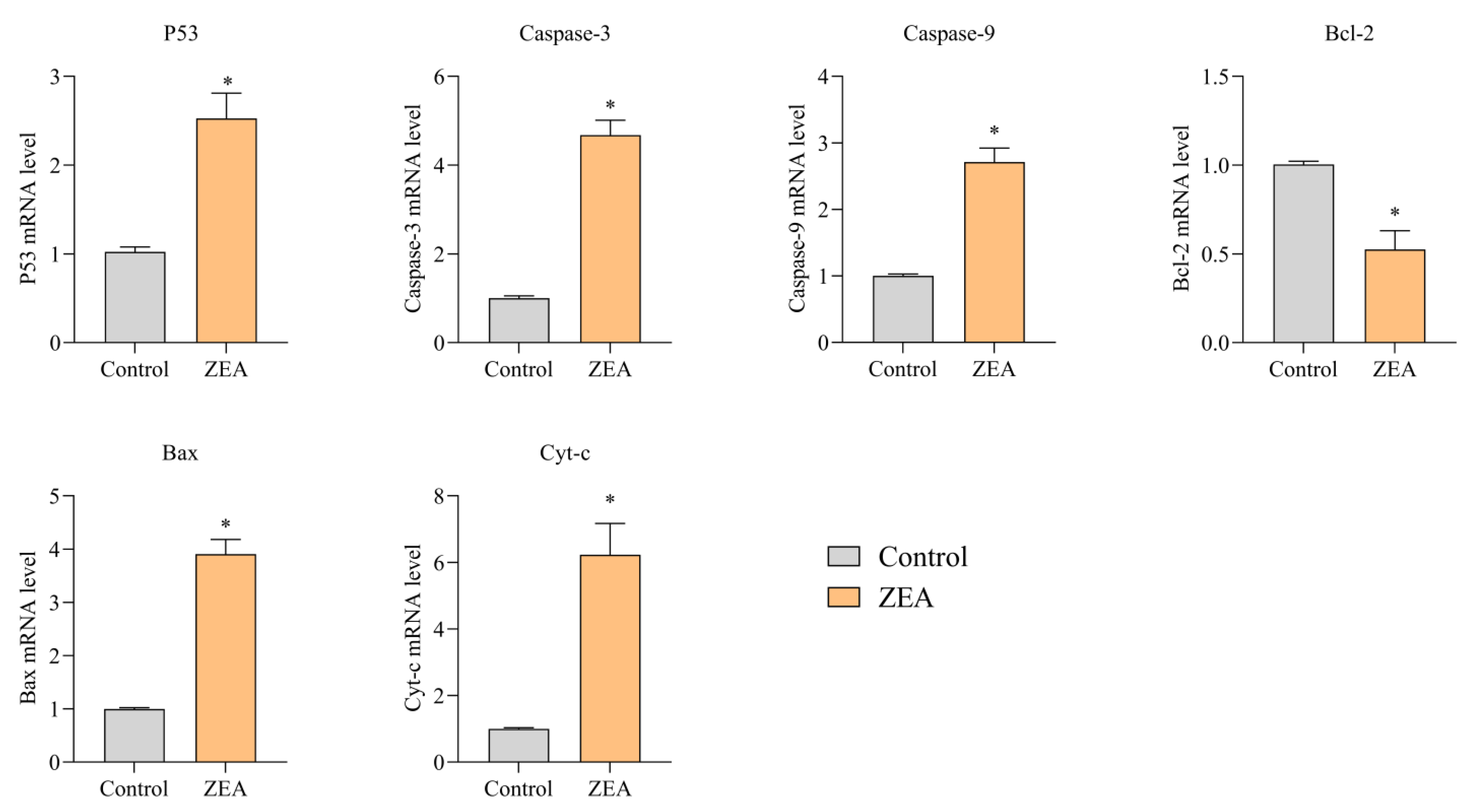
2.6. Detection of mRNA Expression of Apoptosis and Inflammatory Factor-Related Pathways
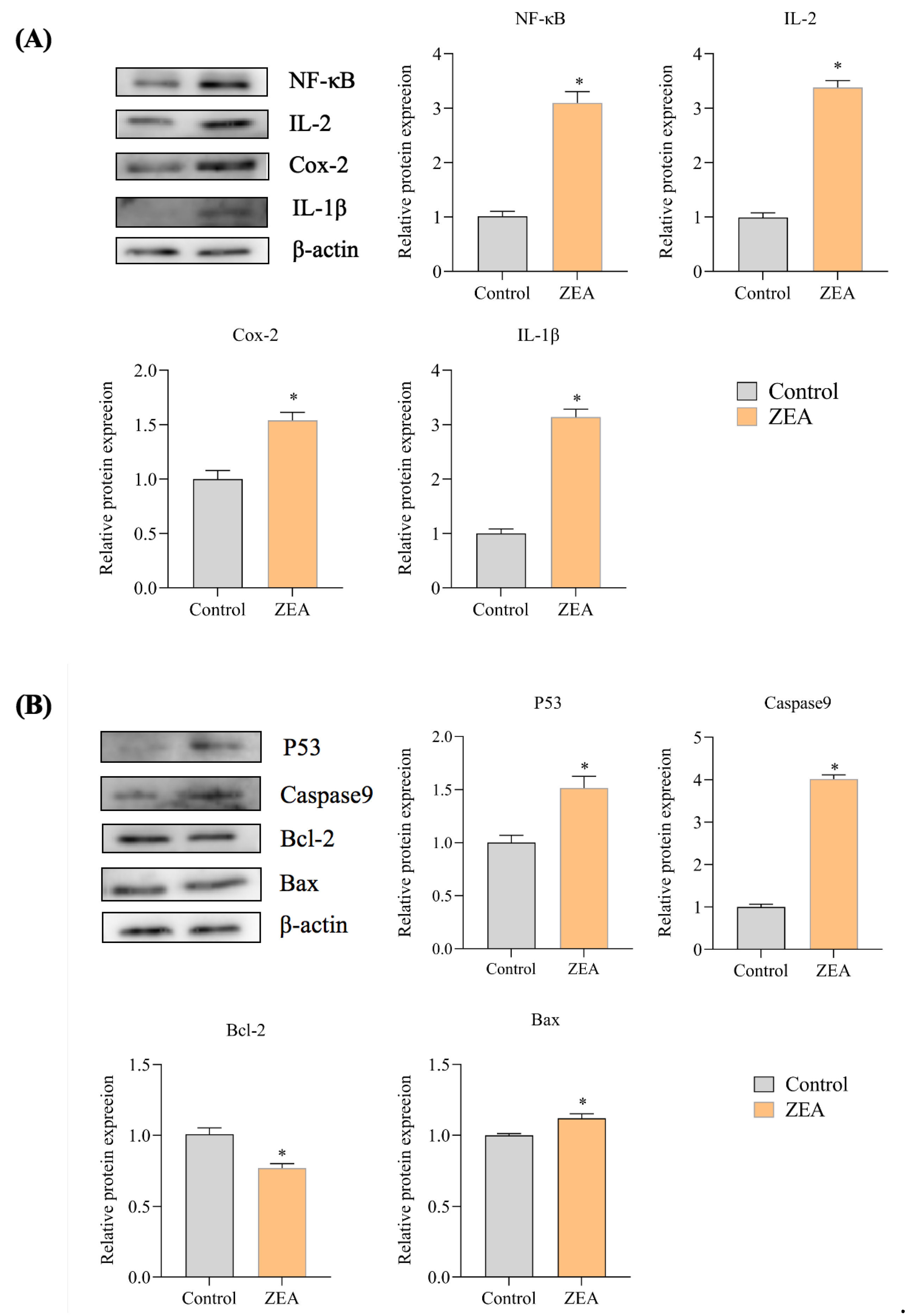
2.7. Protein Expression Detection of Apoptosis and Inflammatory Factor-Related Pathways
2.8. Statistical Analysis
3. Results
3.1. ZEA-Induced Changes in Oxidative Stress Parameters in IPEC-J2 Cells
3.2. Oxidative Stress Levels in IPEC-J2 Cells under the Influence of ZEA
3.3. Cell Death Assays of IPEC-J2 Cells
3.4. Effect of ZEA on Apoptosis and mRNA Expression of Inflammatory Response-Relb Gated Proteins in Porcine IPEC-J2 Cells
3.5. Effect of ZEA on the Expression of Proteins Associated with IPEC-J2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Richard, J.L. Some major mycotoxins and their mycotoxicosis—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Blaney, B.J.; Bloomfield, R.C.; Moore, C.J. Zearalenone intoxication of pigs. Aust. Vet. J. 1984, 61, 24–27. [Google Scholar] [CrossRef]
- Sundlof, S.F.; Strickland, C. Zearalenone and zeranol: Potential residue problems in livestock. Vet. Hum. Toxicol. 1986, 28, 242–250. [Google Scholar] [PubMed]
- Curtui, V.G.; Gareis, M.; Usleber, E.; Märtlbauer, E. Survey of Romanian slaughtered pigs for the occurrence of mycotoxins ochratoxins A and B, and zearalenone. Food Addit. Contam. 2001, 18, 730–738. [Google Scholar] [CrossRef]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C. Taranu.Zearalenone and the immune response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef]
- Biehl, M.L.; Prelusky, D.B.; Koritz, G.D.; Hartin, K.E.; Buck, W.B.; Trenholm, H.L. Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicol. Appl. Pharmacol. 1993, 121, 152–159. [Google Scholar] [CrossRef]
- Przybylska-Gornowicz, B.; Tarasiuk, M.; Lewczuk, B.; Prusik, M.; Ziółkowska, N.; Zielonka, Ł.; Gajęcki, M.; Gajęcka, M. The effects of low doses of two Fusarium toxins, zearalenone and deoxynivalenol, on the pig jejunum. A light and electron microscopic study. Toxins 2015, 7, 4684–4705. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Zhao, A.H.; Wang, J.J.; Tian, Y.; Yan, Z.H.; Dri, M.; Shen, W.; De Felici, M.; Li, L. Oxidative stress as a plausible mechanism for zearalenone to induce genome toxicity. Gene 2022, 829, 146511. [Google Scholar] [CrossRef] [PubMed]
- AbuZahra, H.M.; Rajendran, P.; Ismail, M.B. Zerumbone exhibit protective effect against zearalenone induced toxicity via ameliorating inflammation and oxidative stress induced apoptosis. Antioxidants 2021, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Ouanes, Z.; Hassen, W.; Baudrimont, I.; Creppy, E.; Bacha, H. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol. Vitr. 2004, 18, 467–474. [Google Scholar] [CrossRef]
- Hassen, W.; Ayed-Boussema, I.; Oscoz, A.A.; Lopez, A.D.C.; Bacha, H. The role of oxidative stress in zearalenone-mediated toxicity in Hep G2 cells: Oxidative DNA damage, gluthatione depletion and stress proteins induction. Toxicology 2007, 232, 294–302. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, M.; Li, J.; Shan, A. DL-Selenomethionine Alleviates Oxidative Stress Induced by Zearalenone via Nrf2/Keap1 Signaling Pathway in IPEC-J2 Cells. Toxins 2021, 13, 557. [Google Scholar] [CrossRef] [PubMed]
- Boeira, S.P.; Funck, V.R.; Borges Filho, C.; Del’Fabbro, L.; Gomes, M.G.d.; Donato, F.; Royes, L.F.F.; Oliveira, M.S.; Jesse, C.R.; Furian, A.F. Lycopene protects against acute zearalenone-induced oxidative, endocrine, inflammatory and reproductive damages in male mice. Chem. Biol. Interact. 2015, 230, 50–57. [Google Scholar] [PubMed]
- Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 2007, 137, 326–341. [Google Scholar] [CrossRef]
- MacDonald, S.J.; Anderson, S.; Brereton, P.; Wood, R.; Damant, A. Determination of zearalenone in barley, maize and wheat flour, polenta, and maize-based baby food by immunoaffinity column cleanup with liquid chromatography: Interlaboratory study. J. AOAC Int. 2005, 88, 1733–1740. [Google Scholar] [CrossRef]
- Engelhardt, G.; Barthel, J.; Sparrer, D. Fusarium mycotoxins and ochratoxin A in cereals and cereal products: Results from the Bavarian Health and Food Safety Authority in 2004. Mol. Nutr. Food Res. 2006, 50, 401–405. [Google Scholar] [CrossRef]
- Jo, H.; Kong, C.; Song, M.; Kim, B.G. Effects of dietary deoxynivalenol and zearalenone on apparent ileal digestibility of amino acids in growing pigs. Anim. Feed Sci. Technol. 2016, 219, 77–82. [Google Scholar] [CrossRef]
- Hartmann, N.; Erbs, M.; Wettstein, F.E.; Hoerger, C.C.; Schwarzenbach, R.P.; Bucheli, T.D. Quantification of zearalenone in various solid agroenvironmental samples using D6-zearalenone as the internal standard. J. Agric. Food Chem. 2008, 56, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, K.; Waśkiewicz, A.; Świetlik, J.; Bocianowski, J.; Goliński, P. Possible way of zearalenone migration in the agricultural environment. Plant Soil Environ. 2015, 61, 358–363. [Google Scholar] [CrossRef]
- Marin, D.E.; Taranu, I. Overview on aflatoxins and oxidative stress. Toxin Rev. 2012, 31, 32–43. [Google Scholar] [CrossRef]
- Yu, J.Y.; Zheng, Z.H.; Son, Y.O.; Shi, X.; Jang, Y.O.; Lee, J.C. Mycotoxin zearalenone induces AIF- and ROS-mediated cell death through p53- and MAPK-dependent signaling pathways in RAW264.7 macrophages. Toxicol. Vitr. 2011, 25, 1654–1663. [Google Scholar] [CrossRef]
- Abid-Essefi, S.; Bouaziz, C.; Golli-Bennour, E.E.; Ouanes, Z.; Bacha, H. Comparative study of toxic effects of zearalenone and its two major metabolites α-zearalenol and β-zearalenol on cultured human Caco-2 cells. J. Biochem. Mol. Toxicol. 2009, 23, 233–243. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307, 135662. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Cai, J.; Liu, P.; Zhang, X.; Shi, B.; Jiang, Y.; Qiao, S.; Liu, Q.; Fang, C.; Zhang, Z. Micro-algal astaxanthin improves lambda-cyhalothrin-induced necroptosis and inflammatory responses via the ROS-mediated NF-κB signaling in lymphocytes of carp (Cyprinus carpio L.). Fish Shellfish. Immunol. 2023, 139, 108929. [Google Scholar] [CrossRef]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21944. [Google Scholar] [CrossRef]
- Ruh, M.F.; Bi, Y.; Cox, L.; Berk, D.; Howlett, A.C.; Bellone, C.J. Effect of environmental estrogens on IL-1beta promoter activity in a macrophage cell line. Endocrine 1998, 9, 207–211. [Google Scholar] [CrossRef]
- Ben Salah-Abbès, J.; Abbès, S.; Houas, Z.; Abdel-Wahhab, M.A.; Oueslati, R. Zearalenone induces immunotoxicity in mice: Possible protective effects of radish extract (Raphanus sativus). J. Pharm. Pharmacol. 2008, 60, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Huang, J.; Yang, J.; Chen, X.; Zhang, H.; Zhu, Y.; Liu, Q.; Zhang, Z. The protective effect of selenoprotein M on non-alcoholic fatty liver disease: The role of the AMPKα1-MFN2 pathway and Parkin mitophagy. Cell. Mol. Life Sci. 2022, 79, 354. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Burlacu, R.; Tudor, D.S. Effects of zearalenone and its derivatives on the innate immune response of swine. Toxicon 2010, 56, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Burlacu, R.; Manda, G.; Motiu, M.; Neagoe, I.; Dragomir, C.; Stancu, M.; Calin, L. Effects of zearalenone and its derivatives on porcine immune response. Toxicol. Vitr. 2011, 25, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Pistol, G.; Stancu, M. Effects of zearalenone and its metabolites on the swine epithelial intestinal cell line: IPEC 1. Proc. Nutr. Soc. 2013, 72, E40. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Neagoe, I.V.; Calin, L.; Taranu, I. Effects of zearalenone on oxidative stress and inflammation in weanling piglets. Food Chem. Toxicol. 2013, 58, 408–415. [Google Scholar] [CrossRef]
- Fan, W.; Lv, Y.; Ren, S.; Shao, M.; Shen, T.; Huang, K.; Zhou, J.; Yan, L.; Song, S. Zearalenone (ZEA)-induced intestinal inflammation is mediated by the NLRP3 inflammasome. Chemosphere 2018, 190, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, G.S. Fusarium Toxins in Cereals: A Risk Assessment; Nordic Council of Ministers: Lohusalu, Estonia, 1998. [Google Scholar]
- McGinty, A.; Chang, Y.W.; Sorokin, A.; Bokemeyer, D.; Dunn, M.J. Cyclooxygenase-2 expression inhibits trophic withdrawal apoptosis in nerve growth factor-differentiated PC12 cells. J. Biol. Chem. 2000, 275, 12095–12101. [Google Scholar] [CrossRef]
- Winn, R.K.; Harlan, J.M. The role of endothelial cell apoptosis in inflammatory and immune diseases. J. Thromb. Haemost. 2005, 3, 1815–1824. [Google Scholar] [CrossRef]
- Haanen, C.; Vermes, I. Apoptosis and inflammation. Mediat. Inflamm. 1995, 4, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Apoptotic pathways: Ten minutes to dead. Cell 2005, 121, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. The cell’s dilemma, or the story of cell death: An entertainment in three acts. FEBS J. 2016, 283, 2568–2576. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, H.; Guo, C.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Wen, L.; He, Z. Zearalenone induces apoptosis and necrosis in porcine granulosa cells via a caspase-3- and caspase-9-dependent mitochondrial signaling pathway. J. Cell. Physiol. 2012, 227, 1814–1820. [Google Scholar] [CrossRef]
- Shu, K.X.; Li, B.; Wu, L.X. The p53 network: p53 and its downstream genes. Colloids Surf. B Biointerfaces 2007, 55, 10–18. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, H.; Ma, W.; Wu, Q.; Cai, J.; Zhang, Z. Exploring the Toxic Effects of ZEA on IPEC-J2 Cells from the Inflammatory Response and Apoptosis. Animals 2023, 13, 2731. https://doi.org/10.3390/ani13172731
Guan H, Ma W, Wu Q, Cai J, Zhang Z. Exploring the Toxic Effects of ZEA on IPEC-J2 Cells from the Inflammatory Response and Apoptosis. Animals. 2023; 13(17):2731. https://doi.org/10.3390/ani13172731
Chicago/Turabian StyleGuan, Haoyue, Wenxue Ma, Qiong Wu, Jingzeng Cai, and Ziwei Zhang. 2023. "Exploring the Toxic Effects of ZEA on IPEC-J2 Cells from the Inflammatory Response and Apoptosis" Animals 13, no. 17: 2731. https://doi.org/10.3390/ani13172731
APA StyleGuan, H., Ma, W., Wu, Q., Cai, J., & Zhang, Z. (2023). Exploring the Toxic Effects of ZEA on IPEC-J2 Cells from the Inflammatory Response and Apoptosis. Animals, 13(17), 2731. https://doi.org/10.3390/ani13172731







