Health Assessments of Koalas after Wildfire: A Temporal Comparison of Rehabilitated and Non-Rescued Resident Individuals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
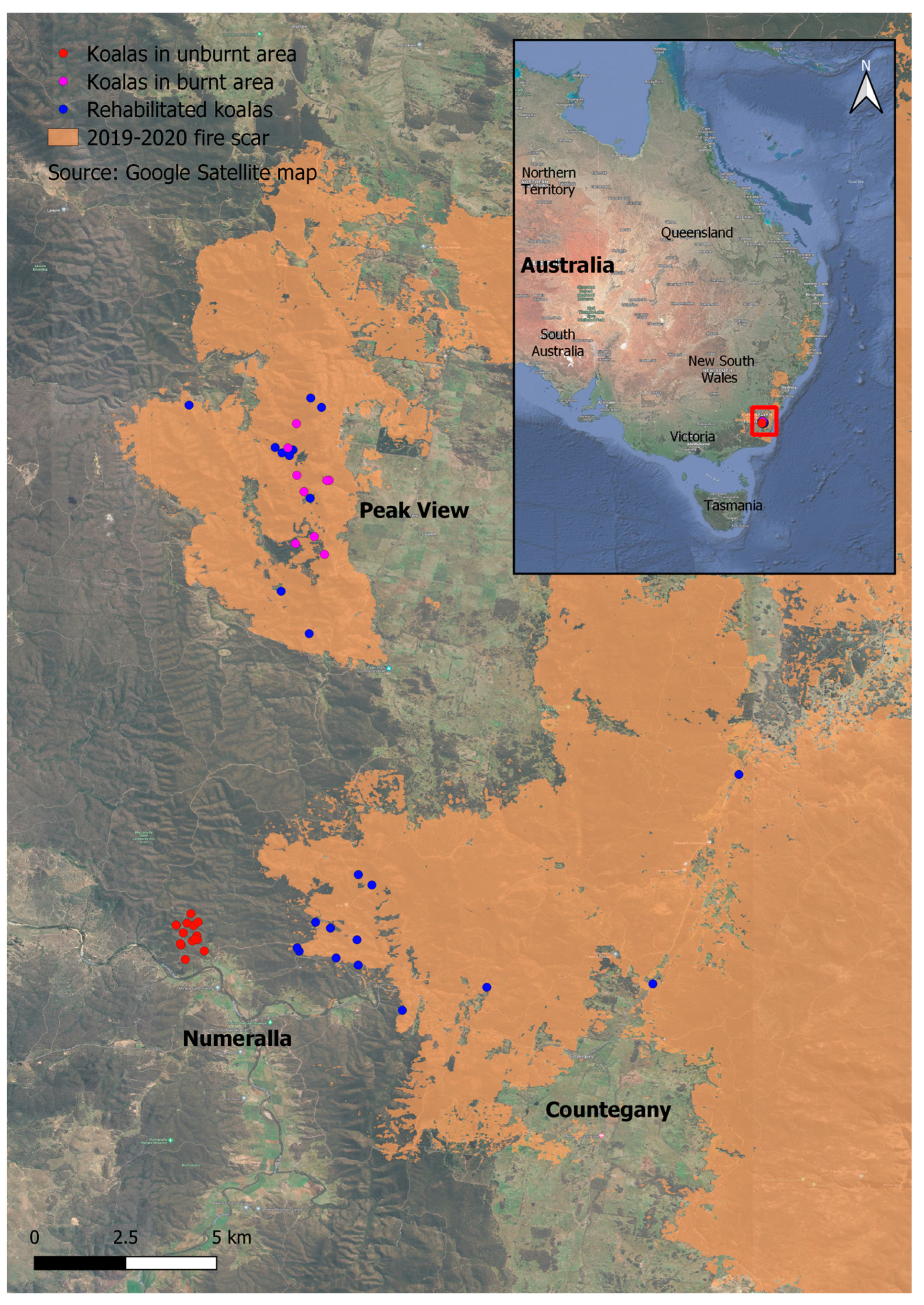
2.1. Study Area
2.2. Koala Groups
2.3. Data Collection
- Rescue: an initial health check within 24 h of rescue for the rehabilitated group only.
- Pre-release: a pre-release check (5–9 months after rescue) for rehabilitated koalas who were selected for post-release monitoring, and a pre-collaring check for burnt resident and unburnt resident koalas captured for the monitoring study.
- Recapture: a final check up to nine months after the pre-release check for all monitored koalas who retained their tracking collars.
2.4. Sample Processing
2.5. Genetic Sample Extraction and Sequencing
2.6. Population Genetic Analysis
2.7. Data Analysis
3. Results
3.1. Survival Rates
3.2. Burn Injuries
3.3. Body Condition Score
3.4. Overall Health and Chlamydia Status
3.5. Blood Biochemistry
3.6. Haematology
3.7. Genetic Analysis
4. Discussion
4.1. Body Condition and Overall Health
4.2. Burn Injuries
4.3. Chlamydia Status
4.4. Genetics
4.5. Biochemical and Haematological Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Attiwill, P.M.; Adams, M.A. Mega-fires, inquiries and politics in the eucalypt forests of Victoria, south-eastern Australia. For. Ecol. Manag. 2013, 294, 45–53. [Google Scholar] [CrossRef]
- Penman, T.D.; Kavanagh, R.P.; Binns, D.L.; Melick, D.R. Patchiness of prescribed burns in dry sclerophyll eucalypt forests in south-eastern Australia. For. Ecol. Manag. 2007, 252, 24–32. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Yates, C.P.; Whitehead, P.J.; Smith, R.; Craig, R.; Allan, G.E.; Thackway, R.; Frakes, I.; Cridland, S.; Meyer, M.C.P.; et al. Bushfires ‘down under’: Patterns and implications of contemporary Australian landscape burning. Int. J. Wildland Fire 2007, 16, 361–377. [Google Scholar] [CrossRef]
- Wintle, B.A.; Legge, S.; Woinarski, J.C. After the megafires: What next for Australian wildlife? Trends Ecol. Evol. 2020, 35, 753–757. [Google Scholar] [CrossRef] [PubMed]
- NSW Department of Planning Industry and Environment. NSW Fire and the Environment 2019-20 Summary; NSW Department of Planning Industry and Environment: Parramatta, Australia, 2020.
- Van Eeden, L.M.; Nimmo, D.; Mahony, M.; Herman, K.; Ehmke, G.; Driessen, J.; O’Connor, J.; Bino, G.; Taylor, M.; Dickman, C. Impacts of the Unprecedented 2019–2020 Bushfires on Australian Animals; Report Prepared for WWF-Australia, Ultimo NSW; WWF-Australia: Sydney, Australia, 2020. [Google Scholar]
- Parrott, M.L.; Wicker, L.V.; Lamont, A.; Banks, C.; Lang, M.; Lynch, M.; McMeekin, B.; Miller, K.A.; Ryan, F.; Selwood, K.E. Emergency response to Australia’s black summer 2019–2020: The role of a zoo-based conservation organisation in wildlife triage, rescue, and resilience for the future. Animals 2021, 11, 1515. [Google Scholar] [CrossRef]
- Lunney, D.; Cope, H.; Sonawane, I.; Haering, R. A state-wide picture of koala rescue and rehabilitation in New South Wales during the 2019–2020 bushfires. Aust. Zool. 2022, 42, 243–255. [Google Scholar] [CrossRef]
- Dunstan, E.; Funnell, O.; McLelland, J.; Stoeckeler, F.; Nishimoto, E.; Mitchell, D.; Mitchell, S.; McLelland, D.J.; Kalvas, J.; Johnson, L. An analysis of demographic and triage assessment findings in bushfire-affected koalas (Phascolarctos cinereus) on Kangaroo Island, South Australia, 2019–2020. Animals 2021, 11, 3237. [Google Scholar] [CrossRef] [PubMed]
- Menkhorst, P.; Ramsey, D.; O’Brien, T.; Hynes, E.; Whisson, D. Survival and movements of koalas translocated from an over-abundant population. Wildl. Res. 2019, 46, 557–565. [Google Scholar] [CrossRef]
- Whisson, D.A.; Holland, G.J.; Carlyon, K. Translocation of overabundant species: Implications for translocated individuals. J. Wildl. Manag. 2012, 76, 1661–1669. [Google Scholar] [CrossRef]
- Lunney, D.; Gresser, S.; O’neill, L.E.; Matthews, A.; Rhodes, J. The impact of fire and dogs on koalas at Port Stephens, New South Wales, using population viability analysis. Pac. Conserv. Biol. 2007, 13, 189–201. [Google Scholar] [CrossRef]
- Zylstra, P. Fire Regimes for Risk Management of Koalas on the NSW Southern Tablelands; Centre for Environmental Risk Management of Bushfires, University of Wollongong: Wollongong, Australia, 2019. [Google Scholar]
- Crowther, M.S.; Lunney, D.; Lemon, J.; Stalenberg, E.; Wheeler, R.; Madani, G.; Ross, K.A.; Ellis, M. Climate-mediated habitat selection in an arboreal folivore. Ecography 2014, 37, 336–343. [Google Scholar] [CrossRef]
- Martin, R.; Handasyde, K.A. The Koala: Natural History, Conservation and Management; UNSW Press: Randwick, Australia, 1999. [Google Scholar]
- Matthews, A.; Lunney, D.; Gresser, S.; Maitz, W. Movement patterns of koalas in remnant forest after fire. Aust. Mammal. 2016, 38, 91–104. [Google Scholar] [CrossRef]
- Burrows, G. Epicormic strand structure in Angophora, Eucalyptus and Lophostemon (Myrtaceae): Implications for fire resistance and recovery. New Phytol. 2002, 153, 111–131. [Google Scholar] [CrossRef]
- Burrows, N. Linking fire ecology and fire management in south-west Australian forest landscapes. For. Ecol. Manag. 2008, 255, 2394–2406. [Google Scholar] [CrossRef]
- Clarke, J.; Warren, K.; Calver, M.; de Tores, P.; Mills, J.; Robertson, I. Hematologic and serum biochemical reference ranges and an assessment of exposure to infectious diseases prior to translocation of the threatened western ringtail possum (Pseudocheirus occidentalis). J. Wildl. Dis. 2013, 49, 831–840. [Google Scholar] [CrossRef]
- Waters, D.A.; Burrows, G.E.; Harper, J.D. Eucalyptus regnans (Myrtaceae): A fire-sensitive eucalypt with a resprouter epicormic structure. Am. J. Bot. 2010, 97, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.R.; Parsons, M.; Jensen, R.; Tran, C. Mechanisms of rainforest persistence and recruitment in frequently burnt wet tropical eucalypt forests. Austral Ecol. 2012, 37, 268–275. [Google Scholar] [CrossRef]
- McCallum, H.; Kerlin, D.H.; Ellis, W.; Carrick, F. Assessing the significance of endemic disease in conservation—Koalas, chlamydia, and koala retrovirus as a case study. Conserv. Lett. 2018, 11, e12425. [Google Scholar] [CrossRef]
- Polkinghorne, A.; Hanger, J.; Timms, P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Vet. Microbiol. 2013, 165, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Quigley, B.L.; Timms, P. Helping koalas battle disease–Recent advances in Chlamydia and koala retrovirus (KoRV) disease understanding and treatment in koalas. FEMS Microbiol. Rev. 2020, 44, 583–605. [Google Scholar] [CrossRef]
- Albery, G.F.; Turilli, I.; Joseph, M.B.; Foley, J.; Frere, C.H.; Bansal, S. From flames to inflammation: How wildfires affect patterns of wildlife disease. Fire Ecol. 2021, 17, 23. [Google Scholar] [CrossRef]
- Lucas, C.; Hennessy, K.; Mills, G.; Bathols, J. Bushfire Weather in Southeast Australia: Recent Trends and Projected Climate Change Impacts; Bushfire Cooperative Research Centre: Melbourne, Australia, 2007. [Google Scholar]
- Grogan, L.F.; Peel, A.J.; Kerlin, D.; Ellis, W.; Jones, D.; Hero, J.-M.; McCallum, H. Is disease a major causal factor in declines? An evidence framework and case study on koala chlamydiosis. Biol. Conserv. 2018, 221, 334–344. [Google Scholar] [CrossRef]
- Gonzalez-Astudillo, V.; Allavena, R.; McKinnon, A.; Larkin, R.; Henning, J. Decline causes of Koalas in South East Queensland, Australia: A 17-year retrospective study of mortality and morbidity. Sci. Rep. 2017, 7, 42587. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, C.; Lunney, D.; Melzer, A.; Menkhorst, P.; Phillips, S.; Phalen, D.; Ellis, W.; Foley, W.; Baxter, G.; De Villiers, D. Conserving koalas: A review of the contrasting regional trends, outlooks and policy challenges. Biol. Conserv. 2015, 192, 226–236. [Google Scholar] [CrossRef]
- Department of Agriculture, Water and the Environment. Conservation Advice for Phascolarctos cinereus (Koala) Combined Populations of Queensland, New South Wales and the Australian Capital Territory; Department of Agriculture, Water and the Environment: Canberra, Australia, 2022.
- Snowy Monaro Regional Council. Snowy Monaro Draft Rural Land Use Strategy; Snowy Monaro Regional Council: Cooma, Australia, 2020.
- State Government of NSW and Department of Planning and Environment. Native Vegetation of the Cooma-Monaro Shire; VIS_ID 4064 (Dataset); State Government of NSW and Department of Planning and Environment: Parramatta, Australia, 2014.
- Allen, C. OEH Koala Habitat Study: Towards a Comprehensive Koala Plan of Management for North East Monaro; Regional Operations Group, NSW Office of Environment and Heritage: Albury, Australia, 2015.
- Leavesley, A.; Rhind, S. Vertebrate Fauna of Monaro Plains Conservation Reserves; Department of Environment and Climate Change: Queanbeyan, Australia, 2008.
- Office of Environment and Heritage NSW. Plan of Management: Northern Monaro Reserves; Office of Environment and Heritage: Parramatta, Australia, 2012.
- Martin, S.; Youngentob, K.N.; Clark, R.G.; Foley, W.J.; Marsh, K.J. The distribution and abundance of an unusual resource for koalas (Phascolarctos cinereus) in a sodium-poor environment. PLoS ONE 2020, 15, e0234515. [Google Scholar] [CrossRef]
- Witt, R.R.; Beranek, C.T.; Howell, L.G.; Ryan, S.A.; Clulow, J.; Jordan, N.R.; Denholm, B.; Roff, A. Real-time drone derived thermal imagery outperforms traditional survey methods for an arboreal forest mammal. PLoS ONE 2020, 15, e0242204. [Google Scholar] [CrossRef]
- Madani, G.; Ashman, K.; Mella, V.; Whisson, D. A review of the ‘noose and flag’method to capture free-ranging koalas. Aust. Mammal. 2020, 42, 341–348. [Google Scholar] [CrossRef]
- State Government of NSW and Department of Planning and Environment. Fire Extent and Severity Mapping; State Government of NSW and Department of Planning and Environment: Parramatta, Australia, 2020.
- Martin, R. Age-specific fertility in three populations of the koala, Phascolarctos cinereus Goldfuss, in Victoria. Wildl. Res. 1981, 8, 275–283. [Google Scholar] [CrossRef]
- Legione, A.R.; Patterson, J.L.S.; Whiteley, P.; Firestone, S.M.; Curnick, M.; Bodley, K.; Lynch, M.; Gilkerson, J.R.; Sansom, F.M.; Devlin, J.M. Koala retrovirus genotyping analyses reveal a low prevalence of KoRV-A in Victorian koalas and an association with clinical disease. J. Med. Microbiol. 2017, 66, 236–244. [Google Scholar] [CrossRef]
- Patterson, J.L.; Lynch, M.; Anderson, G.A.; Noormohammadi, A.H.; Legione, A.; Gilkerson, J.R.; Devlin, J.M. The prevalence and clinical significance of Chlamydia infection in island and mainland populations of Victorian koalas (Phascolarctos cinereus). J. Wildl. Dis. 2015, 51, 309–317. [Google Scholar] [CrossRef]
- Sarker, N.; Fabijan, J.; Owen, H.; Seddon, J.; Simmons, G.; Speight, N.; Kaler, J.; Woolford, L.; Emes, R.D.; Hemmatzadeh, F. Koala retrovirus viral load and disease burden in distinct northern and southern koala populations. Sci. Rep. 2020, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Hulse, L.S.; Hickey, D.; Mitchell, J.M.; Beagley, K.W.; Ellis, W.; Johnston, S.D. Development and application of two multiplex real-time PCR assays for detection and speciation of bacterial pathogens in the koala. J. Vet. Diagn. Investig. 2018, 30, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Hogg, C.J.; Silver, L.; McLennan, E.A.; Belov, K. Koala Genome Survey: An Open Data Resource to Improve Conservation Planning. Genes 2023, 14, 546. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.A.; Farrow, E.G.; Gibson, M.; Willig, L.K.; Twist, G.; Yoo, B.; Marrs, T.; Corder, S.; Krivohlavek, L.; Walter, A. A 26-hour system of highly sensitive whole genome sequencing for emergency management of genetic diseases. Genome Med. 2015, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.; Daly, M.J. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Published Online 2020; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An analysis tool set for population genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Square Means; R Package 687 Version 1.6.3; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Koala Health Hub The University of Sydney. Koala Examination Form. Available online: https://drive.google.com/file/d/1rtyYUkgTcDxFZS7UH8q1-VLzHyRZE4k6/view (accessed on 16 June 2023).
- Canfield, P.; O’Neill, M.; Smith, E. Haematological and biochemical reference values for the koala (Phascolarctos cinereus). Aust. Vet. J. 1989, 66, 324–326. [Google Scholar] [CrossRef]
- Leigh, K.A.; Hofweber, L.N.; Sloggett, B.K.; Inman, V.L.; Pettit, L.J.; Sriram, A.; Haering, R. Outcomes for an arboreal folivore after rehabilitation and implications for management. Sci. Rep. 2023, 13, 6542. [Google Scholar] [CrossRef] [PubMed]
- Melzer, A.; Carrick, F.; Menkhorst, P.; Lunney, D.; John, B.S. Overview, critical assessment, and conservation implications of koala distribution and abundance. Conserv. Biol. 2000, 14, 619–628. [Google Scholar] [CrossRef]
- Seabrook, L.; McAlpine, C.; Baxter, G.; Rhodes, J.; Bradley, A.; Lunney, D. Drought-driven change in wildlife distribution and numbers: A case study of koalas in south west Queensland. Wildl. Res. 2011, 38, 509–524. [Google Scholar] [CrossRef]
- Degabriele, R. A relative shortage of nitrogenous food in the ecology of the koala (Phascolarctos cinereus). Aust. J. Ecol. 1981, 6, 139–141. [Google Scholar] [CrossRef]
- Gordon, G.; Brown, A.; Pulsford, T. A koala (Phascolarctos cinereus Goldfuss) population crash during drought and heatwave conditions in south-western Queensland. Aust. J. Ecol. 1988, 13, 451–461. [Google Scholar] [CrossRef]
- Cristescu, R.H.; Gardiner, R.; Terraube, J.; McDonald, K.; Powell, D.; Levengood, A.L.; Frère, C.H. Difficulties of assessing the impacts of the 2019–2020 bushfires on koalas. Austral Ecol. 2023, 48, 12–18. [Google Scholar] [CrossRef]
- Lunney, D.; Stalenberg, E.; Santika, T.; Rhodes, J.R. Extinction in Eden: Identifying the role of climate change in the decline of the koala in south-eastern NSW. Wildl. Res. 2014, 41, 22–34. [Google Scholar] [CrossRef]
- Hulse, L.S.; Beagley, K.; Ellis, W.; Fitzgibbon, S.; Gillett, A.; Barth, B.; Robbins, A.; Pyne, M.; Larkin, R.; Johnston, S.D. Epidemiology of Chlamydia-induced reproductive disease in male koalas (Phascolarctos cinereus) from southeast Queensland, Australia as assessed from penile urethral swabs and semen. J. Wildl. Dis. 2020, 56, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Cockram, F.; Jackson, A. Keratoconjunctivitis of the koala, Phascolarctos cinereus, caused by Chlamydia psittaci. J. Wildl. Dis. 1981, 17, 497–504. [Google Scholar] [CrossRef]
- Wan, C.; Loader, J.; Hanger, J.; Beagley, K.; Timms, P.; Polkinghorne, A. Using quantitative polymerase chain reaction to correlate Chlamydia pecorum infectious load with ocular, urinary and reproductive tract disease in the koala (Phascolarctos cinereus). Aust. Vet. J. 2011, 89, 409–412. [Google Scholar] [CrossRef]
- Nyari, S.; Waugh, C.A.; Dong, J.; Quigley, B.L.; Hanger, J.; Loader, J.; Polkinghorne, A.; Timms, P. Epidemiology of chlamydial infection and disease in a free-ranging koala (Phascolarctos cinereus) population. PLoS ONE 2017, 12, e0190114. [Google Scholar] [CrossRef] [PubMed]
- White, N.; Timms, P. Chlamydia psittaci in a Koala (Phascolarctos cinereus) population in south-east Queensland. Wildl. Res. 1994, 21, 41–47. [Google Scholar] [CrossRef]
- Santamaria, F.; Schlagloth, R. The effect of Chlamydia on translocated Chlamydia-naïve koalas: A case study. Aust. Zool. 2016, 38, 192–202. [Google Scholar] [CrossRef]
- Weigler, B.J.; Girjes, A.A.; White, N.A.; Kunst, N.D.; Carrick, F.N.; Lavin, M.F. Aspects of the epidemiology of Chlamydia psittaci infection in a population of koalas (Phascolarctos cinereus) in southeastern Queensland, Australia. J. Wildl. Dis. 1988, 24, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, S.R.; Raadsma, H.W.; Leigh, K.A.; Tobey, J.R.; Phalen, D.; Krockenberger, A.; Ellis, W.A.; Hynes, E.; Higgins, D.P.; Zenger, K.R. Genomic comparisons reveal biogeographic and anthropogenic impacts in the koala (Phascolarctos cinereus): A dietary-specialist species distributed across heterogeneous environments. Heredity 2019, 122, 525–544. [Google Scholar] [CrossRef]
- Kjeldsen, S.R.; Zenger, K.R.; Leigh, K.; Ellis, W.; Tobey, J.; Phalen, D.; Melzer, A.; FitzGibbon, S.; Raadsma, H.W. Genome-wide SNP loci reveal novel insights into koala (Phascolarctos cinereus) population variability across its range. Conserv. Genet. 2016, 17, 337–353. [Google Scholar] [CrossRef]
- Curik, I.; Ferenčaković, M.; Sölkner, J. Inbreeding and runs of homozygosity: A possible solution to an old problem. Livest. Sci. 2014, 166, 26–34. [Google Scholar] [CrossRef]
- Foote, A.D.; Hooper, R.; Alexander, A.; Baird, R.W.; Baker, C.S.; Ballance, L.; Barlow, J.; Brownlow, A.; Collins, T.; Constantine, R. Runs of homozygosity in killer whale genomes provide a global record of demographic histories. Mol. Ecol. 2021, 30, 6162–6177. [Google Scholar] [CrossRef]
- Liu, S.; Westbury, M.V.; Dussex, N.; Mitchell, K.J.; Sinding, M.-H.S.; Heintzman, P.D.; Duchêne, D.A.; Kapp, J.D.; Von Seth, J.; Heiniger, H. Ancient and modern genomes unravel the evolutionary history of the rhinoceros family. Cell 2021, 184, 4874–4885.e16. [Google Scholar] [CrossRef]
- Braun, J.-P.; Lefebvre, H.; Watson, A. Creatinine in the dog: A review. Vet. Clin. Pathol. 2003, 32, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Finco, D.R. Kidney function. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 441–484. [Google Scholar]
- Yakubu, M.T.; Musa, I.F. Liver and kidney functional indices of pregnant rats following the administration of the crude alkaloids from Senna alata (Linn. Roxb) leaves. Iran. J. Toxicol. 2012, 6, 615–625. [Google Scholar]
- Natural Resources Commission, NSW Government. Summary Paper: Koala and Habitat Response after the 2019-20 Wildfires in North East NSW; Natural Resources Commission: Sydney, Australia, 2022.
- Robert, K.A.; Schwanz, L.E. Monitoring the health status of free-ranging tammar wallabies using hematology, serum biochemistry, and parasite loads. J. Wildl. Manag. 2013, 77, 1232–1243. [Google Scholar] [CrossRef]
- Dellow, D.; Hume, I. Studies on the Nutrition of Macropodine Marsupials. 2. Urea and Water Metabolism in Thylogale Thetis and Macropus Eugenii; Two Wallabies from Divergent Habitats. Aust. J. Zool. 1982, 30, 399–406. [Google Scholar] [CrossRef]
- Kaplan, M.M. Alkaline phosphatase. Gastroenterology 1972, 62, 452–468. [Google Scholar] [CrossRef]
- Sherman, K.E. Alanine aminotransferase in clinical practice: A review. Arch. Intern. Med. 1991, 151, 260–265. [Google Scholar] [CrossRef]
- Stojević, Z.; Piršljin, J.; Milinković-Tur, S.; Zdelar-Tuk, M.; Beer Ljubić, B. Activities of AST, ALT and GGT in clinically healthy dairy cows during lactation and in the dry period. Vet. Arh. 2005, 75, 67–73. [Google Scholar]
- Whitehead, M.; Hawkes, N.; Hainsworth, I.; Kingham, J. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut 1999, 45, 129–133. [Google Scholar] [CrossRef]
- Whitfield, J. Gamma glutamyl transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef]
- Bennett, M.D.; Woolford, L.; O’Hara, A.J.; Nicholls, P.K.; Warren, K.S. Clinical chemistry values and tissue enzyme activities in western barred bandicoots (Perameles bougainville). Vet. Clin. Pathol. 2008, 37, 221–224. [Google Scholar] [CrossRef]
- Center, S.A. Interpretation of liver enzymes. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 297–333. [Google Scholar] [CrossRef]
- Spencer, A.J.; Canfield, P.J. Lymphoid neoplasia in the koala (Phascolarctos cinereus): A review and classification of 31 cases. J. Zoo Wildl. Med. 1996, 27, 303–314. [Google Scholar]
- Hemsley, S.; Govendir, M.; Canfield, P.; Connolly, J. Diabetes mellitus in a koala (Phascolarctos cinereus). Aust. Vet. J. 1998, 76, 203–208. [Google Scholar] [CrossRef]
- Speight, K.N.; Haynes, J.I.; Boardman, W.; Breed, W.G.; Taggart, D.A.; Rich, B.; Woolford, L. Plasma biochemistry and urinalysis variables of koalas (Phascolarctos cinereus) with and without oxalate nephrosis. Vet. Clin. Pathol. 2014, 43, 244–254. [Google Scholar] [CrossRef]
- Griffith, J.E. Studies into the Diagnosis, Treatment and Management of Chlamydiosis in Koalas. Ph.D. Thesis, The University of Sydney, Sydney, Australia, 2010. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Letendre, C.; Sawyer, E.; Young, L.J.; Old, J.M. Immunosenescence in a captive semelparous marsupial, the red-tailed phascogale (Phascogale calura). BMC Zool. 2018, 3, 10. [Google Scholar] [CrossRef]
- Gaughwin, M.; Judson, G. Haematology and clinical chemistry of hairy-nosed wombats (Lasiorhinus latifrons). J. Wildl. Dis. 1980, 16, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Bosma, P.J. Inherited disorders of bilirubin metabolism. J. Hepatol. 2003, 38, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Wolf, P.L. Biochemical diagnosis of liver disease. Indian J. Clin. Biochem. 1999, 14, 59–90. [Google Scholar] [CrossRef]
- Gronwall, R.; Mia, A.S. Fasting hyperbilirubinemia in horses. Am. J. Dig. Dis. 1972, 17, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. The components of the immune system. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York City, NY, USA, 2001. [Google Scholar]
- Faria, S.S.; Fernandes, P.C., Jr.; Silva, M.J.B.; Lima, V.C.; Fontes, W.; Freitas-Junior, R.; Eterovic, A.K.; Forget, P. The neutrophil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience 2016, 10, 702. [Google Scholar] [PubMed]
- Forget, P.; Khalifa, C.; Defour, J.-P.; Latinne, D.; Van Pel, M.-C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Hickman, D.L. Evaluation of the neutrophil: Lymphocyte ratio as an indicator of chronic distress in the laboratory mouse. Lab Anim. 2017, 46, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Swan, M.P.; Hickman, D.L. Evaluation of the neutrophil-lymphocyte ratio as a measure of distress in rats. Lab Anim. 2014, 43, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in dogs and cats with acute pancreatitis. Vet. Clin. Pathol. 2021, 50, 45–51. [Google Scholar] [CrossRef]
- Constantino, B.T.; Cogionis, B. Nucleated RBCs—Significance in the peripheral blood film. Lab. Med. 2000, 31, 223–229. [Google Scholar] [CrossRef]
- Schaefer, M.; Rowan, R. The clinical relevance of nucleated red blood cell counts. Sysmex J. Int. 2000, 10, 59–63. [Google Scholar]
- Hajduk, P.; Copland, M.D.; Schultz, D.A. Effects of capture on hematological values and plasma cortisol levels of free-range koalas (Phascolarctos cinereus). J. Wildl. Dis. 1992, 28, 502–506. [Google Scholar] [CrossRef]
- Bolliger, A.; Backhouse, T. The blood of the koala (Phascolarctos cinereus). Aust. J. Zool. 1960, 8, 363–370. [Google Scholar] [CrossRef]
- Spencer, A.; Canfield, P. Bone marrow examination in the Koala (Phascolarctos cinereus). Comp. Haematol. Int. 1995, 5, 31–37. [Google Scholar] [CrossRef]
- Spencer, A.; Canfield, P. Enhanced Heinz body formation, cell lysis and anaemia in a koala (Phascolarctos cinereus). Comp. Haematol. Int. 1994, 4, 114–117. [Google Scholar] [CrossRef]
- Fabijan, J.; Sarker, N.; Speight, N.; Owen, H.; Meers, J.; Simmons, G.; Seddon, J.; Emes, R.; Tarlinton, R.; Hemmatzadeh, F. Pathological findings in koala retrovirus-positive koalas (Phascolarctos cinereus) from Northern and Southern Australia. J. Comp. Pathol. 2020, 176, 50–66. [Google Scholar] [CrossRef] [PubMed]





| Health Check (Timeframe) | Rehabilitated | Non-Rescued Residents in the Burnt Habitat | Non-Rescued Residents in the Unburnt Habitat |
|---|---|---|---|
| Rescue (23 January 2020–13 March 2020) | 31 | NA | NA |
| Pre-release (25 May 2020–18 December 2020) | 12 | 9 | 16 |
| Recapture (5 February 2021–16 May 2021) | 4 | 8 | 6 |
| Number of Individuals with Ocular Chlamydia Only | Number of Individuals with Urogenital Chlamydia Only | Number of Individuals with both Ocular and Urogenital Chlamydia | |
|---|---|---|---|
| Rehabilitated koalas (n = 31) | 2 | 17 | 1 |
| Residents in the burnt habitat (n = 9) | 0 | 4 | 0 |
| Residents in the unburnt habitat (n = 16) | 0 | 10 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lane, M.R.; Lowe, A.; Vukcevic, J.; Clark, R.G.; Madani, G.; Higgins, D.P.; Silver, L.; Belov, K.; Hogg, C.J.; Marsh, K.J. Health Assessments of Koalas after Wildfire: A Temporal Comparison of Rehabilitated and Non-Rescued Resident Individuals. Animals 2023, 13, 2863. https://doi.org/10.3390/ani13182863
Lane MR, Lowe A, Vukcevic J, Clark RG, Madani G, Higgins DP, Silver L, Belov K, Hogg CJ, Marsh KJ. Health Assessments of Koalas after Wildfire: A Temporal Comparison of Rehabilitated and Non-Rescued Resident Individuals. Animals. 2023; 13(18):2863. https://doi.org/10.3390/ani13182863
Chicago/Turabian StyleLane, Murraya R., Arianne Lowe, Jelena Vukcevic, Robert G. Clark, George Madani, Damien P. Higgins, Luke Silver, Katherine Belov, Carolyn J. Hogg, and Karen J. Marsh. 2023. "Health Assessments of Koalas after Wildfire: A Temporal Comparison of Rehabilitated and Non-Rescued Resident Individuals" Animals 13, no. 18: 2863. https://doi.org/10.3390/ani13182863
APA StyleLane, M. R., Lowe, A., Vukcevic, J., Clark, R. G., Madani, G., Higgins, D. P., Silver, L., Belov, K., Hogg, C. J., & Marsh, K. J. (2023). Health Assessments of Koalas after Wildfire: A Temporal Comparison of Rehabilitated and Non-Rescued Resident Individuals. Animals, 13(18), 2863. https://doi.org/10.3390/ani13182863





