Integrated Metabolome and Transcriptome Analyses Reveal the Efficacy of Steroidal Saponins for Glucose and Lipid Metabolism in Hybrid Grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatu) Fed Higher-Lipid Diets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection and Chemical Analysis
2.3. Metabolome Analysis
2.4. Transcriptome Analysis
2.5. Quantitative RT-PCR Analysis of Gene
2.6. Statistical Analysis
3. Results
3.1. Activities of ALT, AST, and Levels of TP, ALB in Serum
3.2. Levels of LZM, ACP, and AKP in Serum
3.3. Liver Antioxidative Indexes
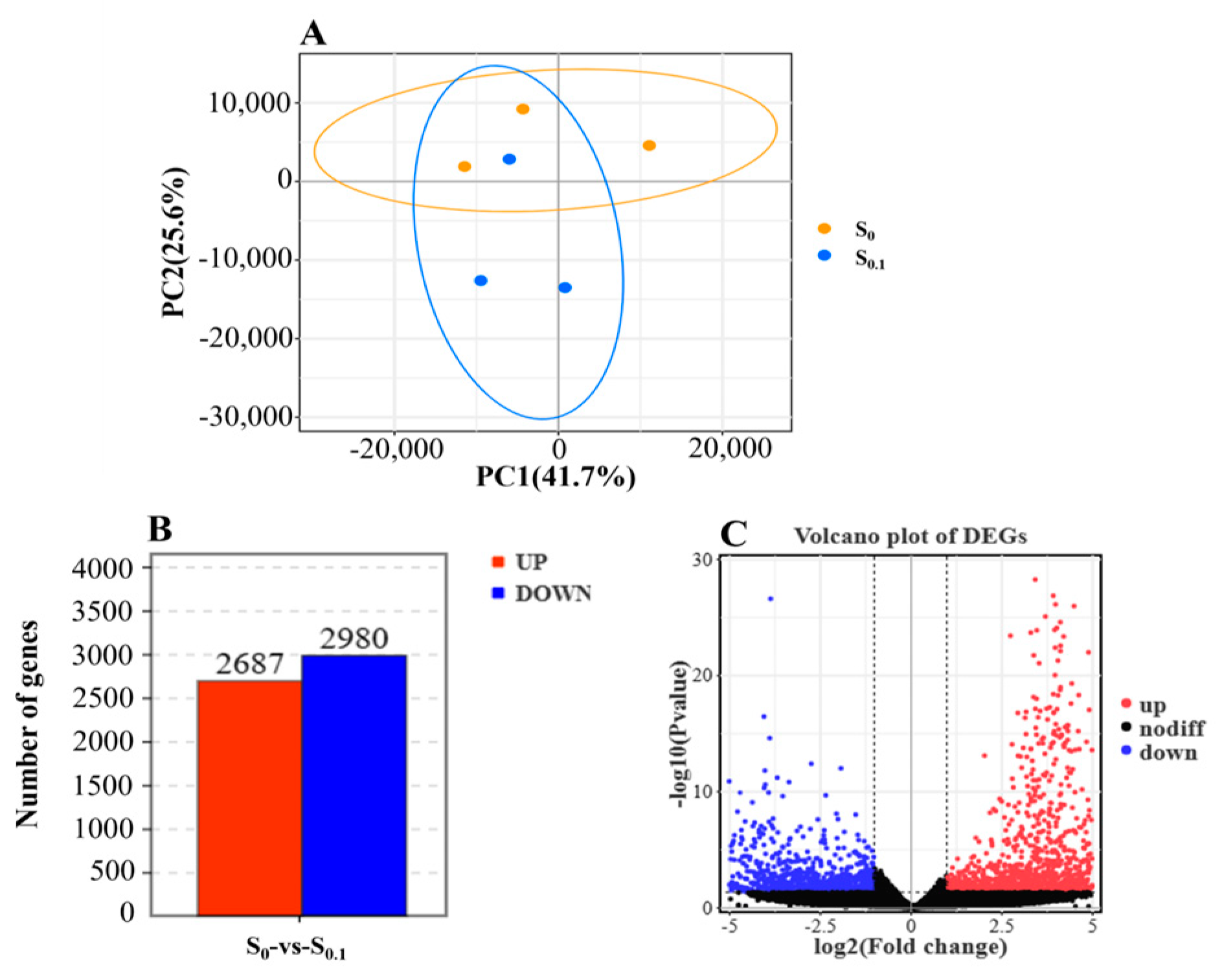
3.4. Transcriptome Analysis in S0 and S0.1 Group Fish Liver
3.5. Signaling Pathway Network and Key Genes of Glucose and Lipid Metabolism
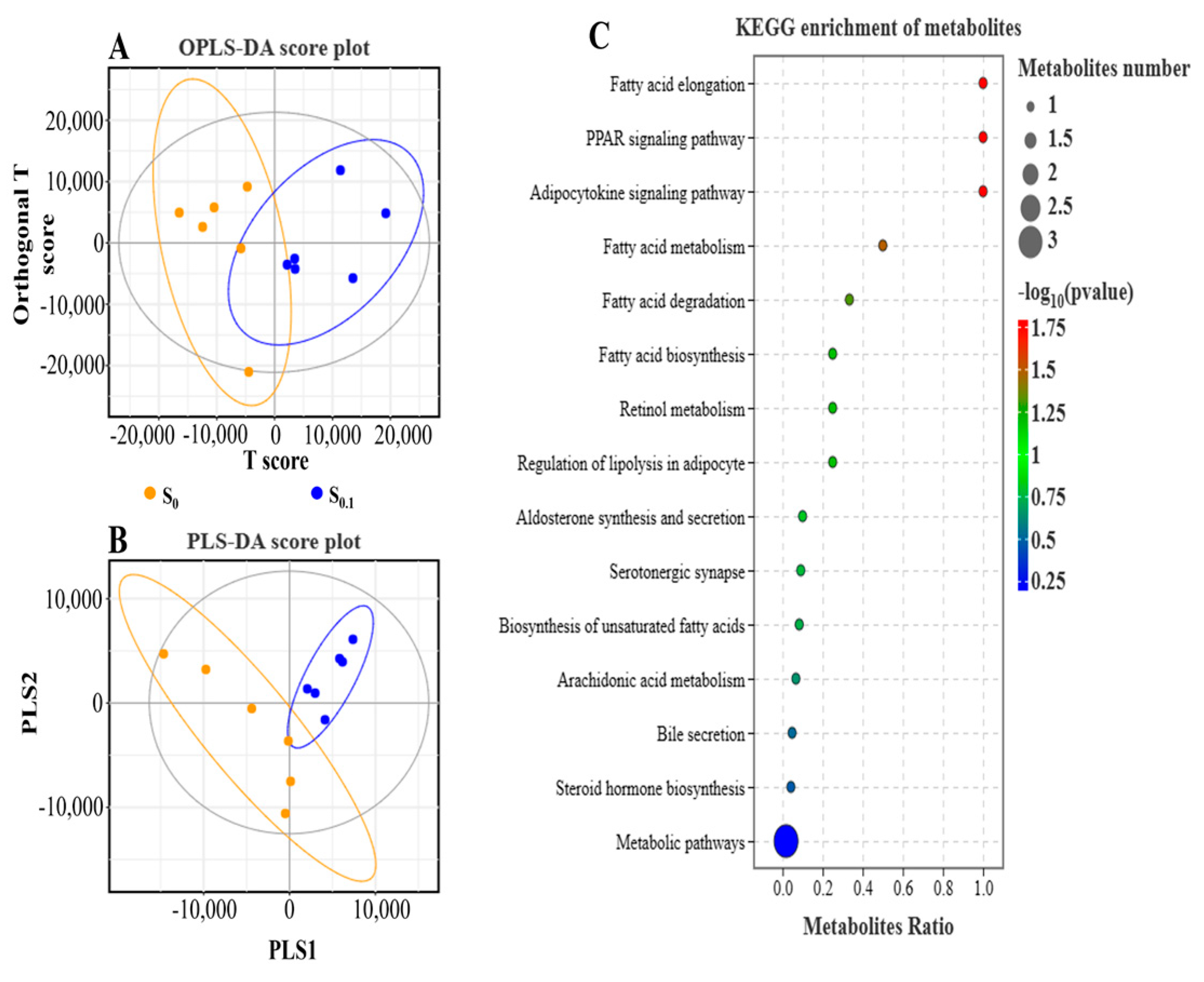
3.6. Metabolism Analysis in S0 and S0.1 Group Fish Liver
3.7. Combined Analysis of Transcriptomic and Metabolomic Data
3.8. Gene Expression of ptgs-1, ptges-1, and tbxas-2
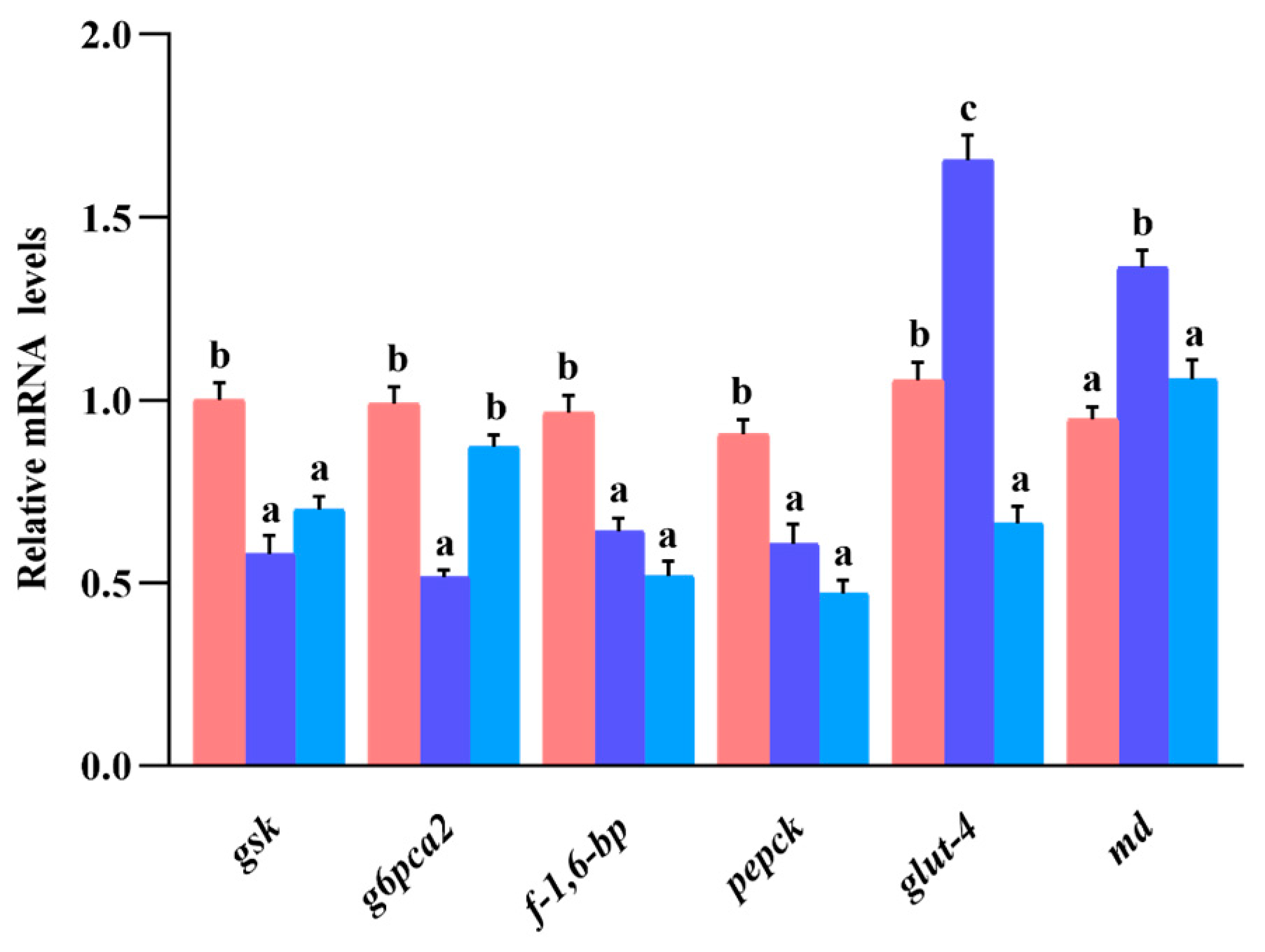
3.9. Gene Expression of Glucose Metabolism in Liver
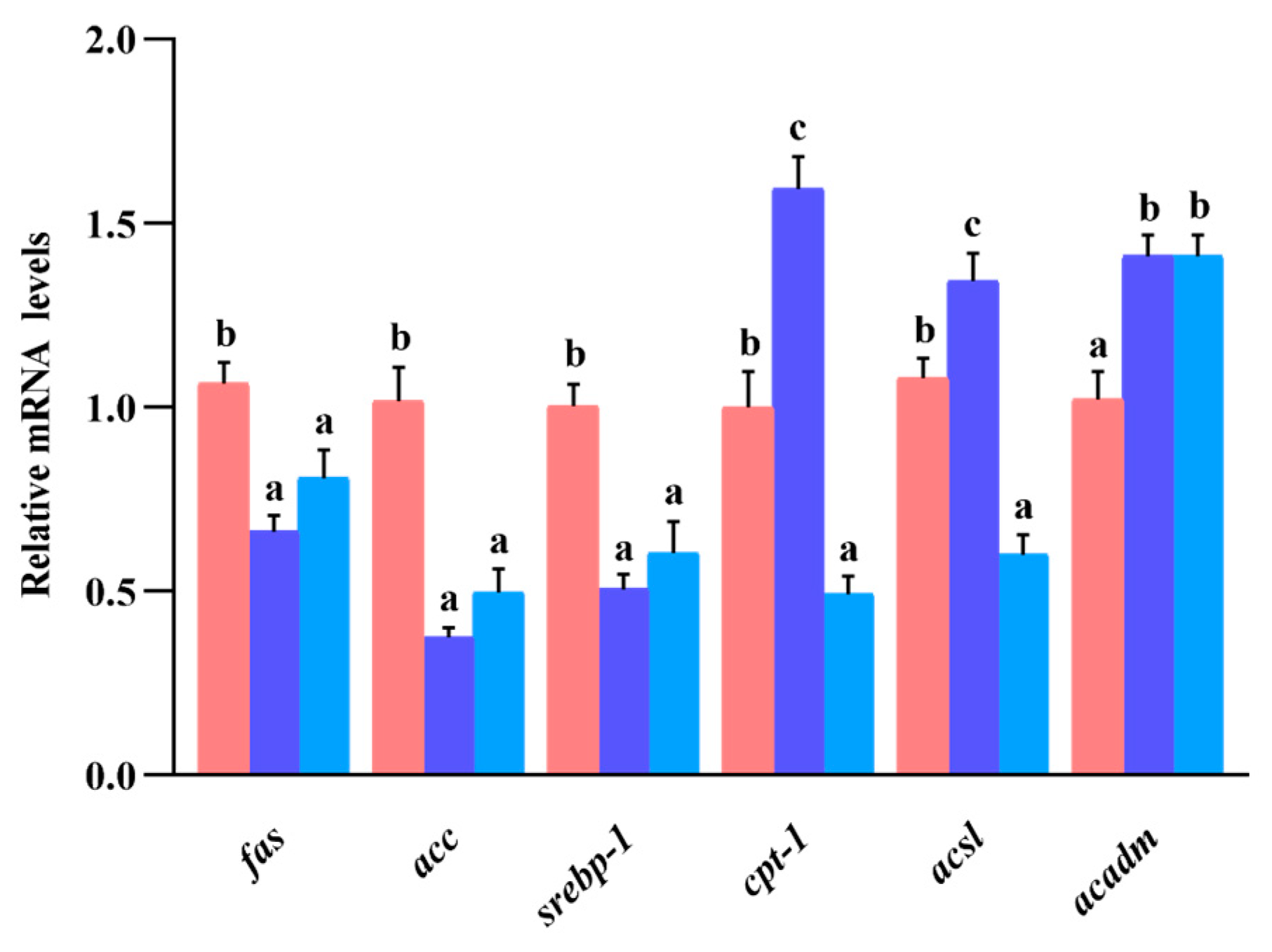
3.10. Gene Expression of Lipid Metabolism in Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisheries Administration of the Ministry of Agriculture and Rural Areas; National Fisheries Technology Extension Center; China Society of Fisheries. Fishery Statistical Yearbook 2018; Chinese Agricultural Press: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Fisheries Administration of the Ministry of Agriculture and Rural Areas; National Fisheries Technology Extension Center; China Society of Fisheries. Fishery Statistical Yearbook 2022; Chinese Agricultural Press: Beijing, China, 2022. (In Chinese) [Google Scholar]
- Siddaiah, G.M.; Kumar, R.; Kumari, R.; Chandan, N.K.; Debbarma, J.; Damle, D.K.; Giri, S.S. Dietary fishmeal replacement with Hermetia illucens (Black soldier fly, BSF) larvae meal affected production performance, whole body composition, antioxidant status, and health of snakehead (Channa striata) juveniles. Anim. Feed. Sci. Technol. 2023, 297, 115597. [Google Scholar] [CrossRef]
- Lin, S. Review of fish meal market in 2017 and prospect of situation in 2018. Guangdong Feed 2017, 27, 17–18. [Google Scholar]
- Lin, S. Review of fish meal market in 2021 and prospect of situation in 2022. Guangdong Feed 2021, 27, 20–22. [Google Scholar]
- Li, X.; Jiang, Y.; Liu, W.; Ge, X. Protein-sparing effect of dietary lipid in practical diets for blunt snout bream (Megalobrama amblycephala) fingerlings: Effects on digestive and metabolic responses. Fish Physiol. Biochem. 2012, 38, 529–541. [Google Scholar] [CrossRef]
- Xie, R.T.; Amenyogbe, E.; Chen, G.; Huang, J.S. Effects of feed fat level on growth performance, body composition and serum biochemical indices of hybrid grouper (Epinephelus fuscoguttatus × Epinephelus polyphekadion). Aquaculture 2021, 530, 735813. [Google Scholar] [CrossRef]
- Meng, Y.; Tian, H.; Hu, X.; Han, B.; Li, X.; Cangzhong, L.; Ma, R. Effects of dietary lipid levels on the lipid deposition and metabolism of subadult triploid rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2022, 2022, 6924835. [Google Scholar] [CrossRef]
- Li, T.; Yan, X.; Dong, X.; Pan, S.; Tan, B.; Zhang, S.; Zhang, H. Choline Alleviates Disorders of Lipid Metabolism in Hybrid Grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus) Caused by High-Lipid Diet. Aquac. Nutr. 2022, 2022, 998849. [Google Scholar]
- Fan, Z.; Li, J.; Zhang, Y.; Wu, D.; Zheng, X.; Wang, C.A.; Wang, L. Excessive dietary lipid affecting growth performance, feed utilization, lipid deposition, and hepatopancreas lipometabolism of large-sized common carp (Cyprinus carpio). Front. Nutr. 2021, 8, 694426. [Google Scholar] [CrossRef]
- Fei, S.; Xia, Y.; Chen, Z.; Liu, C.; Liu, H.; Han, D.; Xie, S. A high-fat diet alters lipid accumulation and oxidative stress and reduces the disease resistance of overwintering hybrid yellow catfish (Pelteobagrus fulvidraco ♀ × P. vachelli ♂). Aquac. Rep. 2022, 23, 101043. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Ye, H.; Zou, C.; Ye, C.; Wang, A. Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology, and immune related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) fed high lipid diets. Fish Shellfish Immunol. 2018, 73, 234–244. [Google Scholar]
- Yang, Q.H.; Tan, B.P.; Dong, X.H.; Chi, S.Y.; Liu, H.Y. Effects of different levels of Yucca schidigera extract on the growth and nonspecific immunity of Pacific white shrimp (Litopenaeus vannamei) and on culture water quality. Aquaculture 2015, 439, 39–44. [Google Scholar] [CrossRef]
- Yun, B.; Ai, Q.; Qian, X.; Mai, K. Effects of soya saponins on feed intake, growth performance, and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.). Isr. J. Aquac. Bamidgeh 2015, 67, 20734. [Google Scholar] [CrossRef]
- Yu, C.H.; Xie, G.; He, R.R.; Zhai, Y.J.; Li, Y.F.; Tsoi, B.; Yang, D.P. Effects of a purified saponin mixture from alfalfa on plasma lipid metabolism in hyperlipidemic mice. J. Health Sci. 2011, 57, 401–405. [Google Scholar] [CrossRef]
- Upadhyay, S.; Jeena, G.S.; Shukla, R.K. Recent advances in steroidal saponins biosynthesis and in vitro production. Planta 2018, 248, 519–544. [Google Scholar] [CrossRef]
- Dawood, M.A.; Gewaily, M.S.; Monier, M.N.; Younis, E.M.; Van Doan, H.; Sewilam, H. The regulatory roles of yucca extract on the growth rate, hepato-renal function, histopathological alterations, and immune-related genes in common carp exposed with acute ammonia stress. Aquaculture 2021, 534, 736287. [Google Scholar] [CrossRef]
- Zheng, S.C.; Zhao, Q.T.; Sang, X.Y.; Jiang, J.J.; Xun, L.Y. Study progress on active ingredients of Chinese materia medica and their mechanism to improve insulin resistance. J. Tradit. Chin. Med. 2021, 40, 94–99. [Google Scholar]
- You, L.L.; Chen, Y.H.; Liu, J.F.; Jin, X.M.; Feng, C. Influence of bitter melon saponin on the glucose consumption of insulin resistance HepG2 cell in vitro. Sci. Technol. Food Ind. 2014, 35, 338–340+345. [Google Scholar]
- Zou, C.; Du, L.; Wu, J.; Gan, S.; Li, Q.; Babu, V.S.; Lin, L. Saikosaponin d alleviates high-fat-diet induced hepatic steatosis in hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) by targeting AMPK/PPARα pathway. Aquaculture 2022, 553, 738088. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, J.; Yang, Q.; Dong, X.; Zhang, S.; Liang, W.; Chi, S. Effects of Dietary Steroid Saponins on Growth Performance, Serum and Liver Glucose, Lipid Metabolism, and Immune Molecules of Hybrid Groupers (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatu) Fed High-Lipid Diets. Metabolites 2023, 13, 305. [Google Scholar] [CrossRef]
- Cui, W.X.; Ma, A.J. Transcriptome analysis provides insights into the effects of myoinositol on the turbot Scophthalmus maximus. Fish Shellfish Immunol. 2020, 106, 691–704. [Google Scholar] [CrossRef]
- Chen, M.; Bai, F.; Song, T.; Niu, X.; Wang, X.; Wang, K.; Ye, J. Hepatic transcriptome analysis provides new insight into the lipid-reducing effect of dietary taurine in high-fat fed groupers (Epinephelus coioides). Metabolites 2022, 12, 670. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, T.; Ma, A.; Huang, Z.; Liu, Z.; Cui, W.; Yuan, C. Metabolic responses in Scophthalmus maximus kidney subjected to thermal stress. Fish Shellfish Immunol. 2020, 103, 37–46. [Google Scholar] [CrossRef]
- Xiang, Q.; Yan, H.; Luo, X.; Kang, Y.; Hu, J.; Chen, L. Integration of transcriptomics and metabolomics reveals damage and recovery mechanisms of fish gills in response to nanosilver exposure. Aquat. Toxicol. 2021, 237, 105895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ma, A.; Yang, S.; Huang, Z. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 102937. [Google Scholar] [CrossRef]
- Pan, S.; Yan, X.; Dong, X.; Li, T.; Suo, X.; Tan, B.; Zhang, H. The positive effects of dietary inositol on juvenile hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatu) fed high-lipid diets: Growth performance, antioxidant capacity and immunity. Fish Shellfish Immunol. 2022, 26, 84–95. [Google Scholar] [CrossRef]
- Su, B.; Chen, J.C. Effect of saponin immersion on enhancement of the immune response of white shrimp and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2008, 24, 74–81. [Google Scholar] [CrossRef]
- Ng’ambi, J.W.; Li, R.; Mu, C.; Song, W.; Liu, L.; Wang, C. Dietary administration of saponin stimulates growth of the swimming crab Portunus trituberculatus and enhances its resistance against Vibrio alginolyticus infection. Fish Shellfish Immunol. 2016, 59, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Wan, W.; Hu, J.; Chang, E.; Zhou, Z.; Zhou, Y.; Sun, L. Dietary arachidonic acid affects the innate immunity, antioxidant capacities, intestinal health, and microbiota in Chinese mitten crab (Eriocheir sinensis). Aquaculture 2022, 548, 737635. [Google Scholar] [CrossRef]
- Medagoda, N.; Lee, K.-J. Effects of dietary arachidonic acid supplementation in high plant protein diets on growth, feed utilization, and immunity of olive flounder, Paralichthys olivaceus. Aquaculture 2023, 571, 739431. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Ruan, Y.; Fan, Y.; Xie, Y.; Ma, C.; Mo, B.; Lai, Y.; Kuang, W. Modified Xiaoqing long decoction alleviates lipopolysaccharide-induced acute lung injury in mice by regulating arachidonic acid metabolism and exerting anti-apoptotic and anti-inflammatory effects. Anat. Rec. 2022, 305, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Sano, H.; Masuda, C.; Kawamura, M.; Tsubouchi, Y.; Chargui, J.; Wada, S. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer 2000, 89, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Shoieb, S.M.; El-Ghiaty, M.A.; Alqahtani, M.A.; El-Kadi, A.O. Cytochrome P450-derived eicosanoids and inflammation in liver diseases. Prostaglandins Other Lipid Mediat. 2020, 147, 106400. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Song, B.K.; Kim, J.H.; Siddiqi, M.Z.; Im, W.T. Production of Prosaikogenin F, Prosaikogenin G, Saikogenin F and Saikogenin G by the Recombinant Enzymatic Hydrolysis of Saikosaponin and their Anti-Cancer Effect. Molecules 2022, 27, 3255. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, S.G.; Nam, J.H.; Park, K.K.; Chung, W.Y.; Kim, W.B.; Park, H.J. Isolation of Saponins with the Inhibitory Effect on Nitric Oxide, Prostaglandin E2 and Tumor Necrosis Factor-α Production from Pleurospermum kamtschaticum. Biol. Pharm. Bull. 2005, 28, 1668–1671. [Google Scholar] [CrossRef]
- Gu, M.; Gu, J.N.; Penn, M.; Bakke, A.M.; Lein, I.; Krogdahl, A. Effects of diet supplementation of soya-saponins, isoflavones and phytosterols on Atlantic salmon (Salmo salar L.) fry fed from start-feeding. Aquac. Nutr. 2015, 21, 604–613. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Xue, J.; Chu, W.; Hu, Y. Effects of dietary soy isoflavone and soy saponin on growth performance, intestinal structure, intestinal immunity, and gut microbiota community on rice field eel (Monopterus albus). Aquaculture 2021, 537, 736506. [Google Scholar] [CrossRef]
- Chen, W.; Ai, Q.H.; Mai, K.M.; Xu, W.; Liufu, Z.G.; Zhang, W.B.; Cai, Y.H. Effects of dietary soybean saponins on feed intake, growth performance, digestibility, and intestinal structure in juvenile Japanese flounder (Paralichthys olivaceus). Aquaculture 2011, 318, 95–100. [Google Scholar] [CrossRef]
- Fan, Z.; Li, J.; Wu, D.; Li, C.; Cao, D.; Miao, L.; Wang, L. Preliminarily curative effectiveness of long-term bitter melon Momordica charantia saponins administration for the glucose homeostasis of juvenile common carp (Cyprinus carpio) fed a high-starch diet. Aquac. Rep. 2022, 25, 101232. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Liu, Q.; Ye, H.; Zou, C.; Ye, C.; Lin, H. Effects of dietary ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) fed high lipid diets. Fish Shellfish Immunol. 2018, 72, 399–409. [Google Scholar]
- Serrano, A.E., Jr. Effects of Quillaja saponins on growth, feed efficiency, digestive enzyme activities and metabolism of common carp (Cyprinus carpio L.). Aquac. Nutr. 2013, 19, 468–474. [Google Scholar] [CrossRef]
- Li, A.; Yuan, X.; Liang, X.F.; Liu, L.; Li, J.; Li, B.; Tao, Y.X. Adaptations of lipid metabolism and food intake in response to low and high fat diets in juvenile grass carp (Ctenopharyngodon idellus). Aquaculture 2016, 457, 43–49. [Google Scholar] [CrossRef]
- Tang, T.; Hu, Y.; Peng, M.; Chu, W.; Hu, Y.; Zhong, L. Effects of high-fat diet on growth performance, lipid accumulation and lipid metabolism related MicroRNA/gene expression in the liver of grass carp (Ctenopharyngodon idella). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 234, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.B.; Ma, Y.Y.; Hu, C.T.; Lin, Q.Y.; Chen, L.Q.; Zhang, M.L.; Qiao, F. The individual and combined effects of hypoxia and high-fat diet feeding on nutrient composition and flesh quality in Nile tilapia (Oreochromis niloticus). Food Chem. 2021, 343, 128479. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, A.P.; Inyang Etoh, A.P.; Inyang Etoh, P.C. Antibacterial efficacies of some plant extracts against aeromonas and pseudomonas diseases of farmed catfish (Heterobranchus longifilis). Vet. Sci. Dev. 2011, 1, 11. [Google Scholar] [CrossRef]
- Kwasek, K.; Wojno, M.; Patula, S.; Oliaro, F.; Cabay, C.; Pinnell, L.J. The effect of first feeding exposure of larval largemouth bass to a formulated soybean meal-based or soy saponin-supplemented diet on fish growth performance and gut microbiome. N. Am. J. Aquac. 2021, 83, 312–326. [Google Scholar] [CrossRef]
- Wen, M.; Fu, X.; Han, X.; Hu, X.; Dong, P.; Xu, J.; Wang, Y. Sea cucumber saponin echinoside A (EA) stimulates hepatic fatty acid β-oxidation and suppresses fatty acid biosynthesis coupling in a diurnal pattern. J. Nutr. Sci. Vitaminol. 2016, 62, 170–177. [Google Scholar] [CrossRef]



| Target Gene | Nucleotide Sequence (5′-3′) | Accession No. |
|---|---|---|
| Glycogen phosphorylase (gp) | F: TCTCCCGTGTTCTTTACCC R: GCCATTGCTGGATGAGTG | MT548584 |
| Glycogen synthase kinase (gsk) | F: TACACTGCCTGACCAAGACC R: TAATGTGGCTGGAGACGAAT | MT548583 |
| Hexokinase-1 (hk) | F: ACTTTGGGTGCAATCCTGAC R: AGACGACGCACTGTTTTGTG | BC067330.1 |
| Glucokinase (gk) | F: TGGGTTTTACCTTCTCCTT R: AGTCCCCTCGTCTCTTGAT | MH213270 |
| Phosphofructokinase (pfk) | F: AAACGCCCATGCAAACTAC R: CAACCTCTCTGACAGCCAC | MH213271 |
| Pyruvate kinase (pk) | F: TCCTGGAGCATCTGTGTCTG R: GTCTGGCGATGTTCATTCCT | BC152219.1 |
| Glucose-6-phosphatase (g-6-p) | F: GCCTGTGGATGCTAATGGG R: GGAGGTCAAGAAGAGAGTCGTG | MT548587 |
| Glucose-6-phosphatase catalytic subunit 2 (g6pca2) | F: CACAGTCCGTCCTCACAT R: GCAAAACAGCGTCCATAA | MH213269 |
| Fructose-1,6-bisphosphatase (f-1,6-bp) | F: ACAGTCTGAATGAAGGCTAC R: CTCATACAACAGCCTCAGCT | XM019099298.1 |
| Pyruvate carboxylase (pc) | F: GTTGCCCACAACTTCAGCAAG R: ATGCTGGGCTG TGAAGTCATC | NM_022172.3 |
| Phosphoenolpyruvate carboxykinase (pepck) | F: CGTGCTGGACTGGATGTTC R: CCAAGCCCTGGAGGTTCA | MT548586 |
| Glucose transporter 4 (glut-4) | F: ATTCCTCTACATCGTCCGC R: CCATCTTCCTCTTCTCCTC | XM_010737169.3 |
| Citrate synthase (cs) | F: GAAGGAACTCGGTGGTGAGG R: GACCATAGCCTGGCACAACT | BC165743.1 |
| Succinate dehydrogenase (sd) | F: ACCTTGGACCTGCTGTGTTGATG R: GCGGTAGAGGGAGAACGGAT | SRP152627 |
| Malate dehydrogenase (md) | F: ATGCCATGAATGGAAAGGAG R: TCCTCGCCCTTCTTGATAGA | XM_038711981.1 |
| Prostaglandin-endoperoxide synthase 1 (ptgs-1) | F: AAGGTCTGGGTCACGGAGTG R: CAAGAGAATCTCCATAAATGTGTCCA | GENE-020530-1 |
| Prostaglandin-endoperoxide synthase 2 (ptgs-2) | F: GAGTACTGGAAGCCGAGCAC R: GATATCACTGCCGCCTGAGT | AM296029 |
| Prostaglandin E synthase 1 (ptges-1) | F: TAATAACGGGACAAGTGAGGC R: GGCAAACAAGTAGGCAACG | XM_010750942 |
| Prostaglandin E synthase 2 (ptges-2) | F: TGGCTGTGTTTGGCGTCCTCA R: TCTTGGTGTTCTGCGGTGTCCT | XM_019272321 |
| Thromboxane-2 synthase (tbxas-2) | F: AGCTGCATGATGGGATCTGTCAATC R: AGGTGGATGATGCGATGTGTGAATC | AY398422 |
| Peroxisome proliferator-activated receptor γ (ppar-γ) | F: ACCGCAGCACGAAGAACAAC R: TGGACGCCATAGTGAAACCC | KM052849.1 |
| Peroxisome proliferator-activated receptor β (ppar-β) | F: GCTACAGAGCAGCACGACA R: CTCCTCATCTTCGCTTTCC | DQ232867.1 |
| Fatty acid synthase (fas) | F: GGCGGCATTGTAGGCATTA R: CAATCAAAGTGTAGCCTCGGTAG | FJ196231.1 |
| Acetyl-CoA carboxylase (acc) | F: GAGAAGGCACCAGAAGATCATAG R: CACAGTACCTGCACTCACATAG | KX066238.1 |
| Sterol regulatory element-binding protein 1 (srebp-1) | F: TGGAGTTTGGAGGACTGTTTG R: AAGATGAGGGTGGAGTTGGA | KU179485.1 |
| Acyl-CoA synthetase (acsl) | F: CCATACACATCCACACCGAGT R: ACAGCTCTTCTATCTGGGGTC | GLEAN_10004723 |
| Carnitine acyltransferase 1 (cpt-1) | F: TGCTCCACGGAAAGTGCTAC R: GCAGTGACCCTCCTCAGTGTAT | HM037343.1 |
| Carnitine acyltransferase 2 (cpt-2) | F: CTATCTGGAGTGACATCATG R: CTCACTCACAGGTAGAGATG | XM019122007.1 |
| Acyl-CoA dehydrogenase (acadm) | F: ACAAGGTTTTGAGGGCAGGT R: TTGGCACTAGCTTGAGCACT | NM_213010.2 |
| β-actin | F: GGCTACTCCTTCACCACCACA R: TCTGGGCAACGGAACCTCT | AY510710.2 |
| Indexes | S0 | S0.1 | S0.2 |
|---|---|---|---|
| ALT/(U/L) | 66.92 ± 2.29 b | 45.32 ± 1.05 a | 45.34 ± 1.71 a |
| AST/(U/L) | 8.02 ± 0.28 b | 6.32 ± 0.22 a | 7.55 ± 0.18 b |
| TP/(mg/mL) | 3.24 ± 0.14 | 3.84 ± 0.18 | 3.26 ± 0.23 |
| ALB/(g/L) | 52.05 ± 0.68 c | 29.72 ± 0.31 a | 35.89 ± 0.29 b |
| Indexes | S0 | S0.1 | S0.2 |
|---|---|---|---|
| LZM/(U/mL) | 48.97 ± 4.48 a | 77.81 ± 4.38 b | 52.69 ± 1.25 a |
| ACP/(King’s unit/L) | 3.60 ± 0.05 a | 5.96 ± 0.10 b | 4.36 ± 0.18 a |
| AKP/(King’s unit/L) | 3.04 ± 0.11 a | 4.66 ± 0.11 b | 3.22 ± 0.12 a |
| Indexes | S0 | S0.1 | S0.2 |
|---|---|---|---|
| MDA/(nmol/mgprot) | 3.95 ± 0.27 b | 3.24 ± 0.11 a | 4.24 ± 0.21 a |
| SOD/(U/mgprot) | 137.35 ± 2.65 a | 164.22 ± 1.73 b | 133.57 ± 1.98 a |
| CAT/(U/mgprot) | 1.68 ± 0.06 a | 3.43 ± 0.15 b | 2.11 ± 0.04 a |
| GSH-PX/(U) | 4.73 ± 0.09 a | 7.61 ± 0.04 c | 6.54 ± 0.18 b |
| Gene Symbol | Related Description | Gene ID | Log2FC | p Value |
|---|---|---|---|---|
| pepck | phosphoenolpyruvate carboxykinase | Unigene0066979 | −11.53 | 0.00 |
| tbxas-2 | thromboxane synthase | Unigene0016223 | −11.32 | 0.00 |
| scs | succinyl-CoA synthetase | Unigene0027404 | −11.21 | 0.00 |
| scs | succinyl-CoA synthetase | Unigene0027402 | −10.94 | 0.00 |
| scs | succinyl-CoA synthetase | Unigene0027400 | −10.49 | 0.00 |
| tbxas-2 | thromboxane synthase | Unigene0025629 | −10.16 | 0.01 |
| ptges | prostaglandin E synthase | Unigene0066035 | −10.01 | 0.00 |
| scs | succinyl-CoA synthetase | Unigene0052415 | −9.90 | 0.00 |
| gsk-1 | glycogen synthase kinase | Unigene0014315 | −9.72 | 0.03 |
| scs | succinyl-CoA synthetase | Unigene0027399 | −9.67 | 0.00 |
| scs | succinyl-CoA synthetase | Unigene0027397 | −9.58 | 0.00 |
| f-1,6-bp | fructose-1,6-bisphosphatase | Unigene0026457 | −9.22 | 0.04 |
| gsk-1 | glycogen synthase kinase | Unigene0014320 | −9.04 | 0.02 |
| fas | fatty acid synthase | Unigene0053299 | −8.94 | 0.11 |
| ppar-γ | peroxisome proliferator-activated receptor gamma | Unigene0038259 | −1.20 | 0.00 |
| f-1,6-bp | fructose-1,6-bisphosphatase | Unigene0086471 | −0.75 | 0.01 |
| pc | pyruvate carboxylase | Unigene0012223 | −0.55 | 0.13 |
| g-6-p | glucose-6-phosphatase | Unigene0029240 | −0.38 | 0.28 |
| cpt-1 | carnitine palmitoyltransferase 1 | Unigene0055448 | 0.77 | 0.08 |
| gk | glucokinase | Unigene0045717 | 1.52 | 0.00 |
| cpt-2 | carnitine palmitoyltransferase 2 | Unigene0047890 | 2.06 | 0.21 |
| cs | citrate synthase | Unigene0018103 | 2.14 | 0.00 |
| sd | succinate dehydrogenase | Unigene0069170 | 2.16 | 0.03 |
| acadm | acyl-CoA dehydrogenase | Unigene0074561 | 2.16 | 0.03 |
| sod-2 | superoxide dismutase 2 | Unigene0077359 | 2.34 | 0.00 |
| sd | succinate dehydrogenase | Unigene0044006 | 2.90 | 0.03 |
| md | malate dehydrogenase | Unigene0052666 | 2.90 | 0.00 |
| cort | carnitine | Unigene0065047 | 3.08 | 0.03 |
| id | isocitrate dehydrogenase | Unigene0080007 | 3.72 | 0.00 |
| pfk | phosphofructokinase | Unigene0004488 | 3.79 | 0.03 |
| pk | pyruvate kinase | Unigene0059944 | 3.88 | 0.00 |
| sd | succinate dehydrogenase | Unigene0002948 | 4.39 | 0.01 |
| gp | glycogen phosphorylase | Unigene0043745 | 4.46 | 0.00 |
| hk | hexokinase | Unigene0071637 | 4.88 | 0.00 |
| id | isocitrate dehydrogenase | Unigene0007929 | 5.21 | 0.00 |
| gsh | glutathione peroxidase | Unigene0004325 | 9.52 | 0.37 |
| md | malate dehydrogenase | Unigene0060423 | 9.57 | 0.01 |
| ptgs-2 | prostaglandin G/H synthase | Unigene0043998 | 9.60 | 0.03 |
| md | malate dehydrogenase | Unigene0017911 | 10.03 | 0.03 |
| id | isocitrate dehydrogenase | Unigene0022151 | 10.11 | 0.04 |
| acadm | acyl-CoA dehydrogenase | Unigene0028577 | 10.21 | 0.01 |
| cat | catalase | Unigene0028584 | 10.35 | 0.08 |
| acsl | very long-chain-specific acyl-CoA dehydrogenase | Unigene0071297 | 10.38 | 0.03 |
| acadm | acyl-CoA dehydrogenase | Unigene0027315 | 10.41 | 0.02 |
| ptgs-1 | prostaglandin G/H synthase | Unigene0043999 | 10.48 | 0.00 |
| sod-1 | superoxide dismutase 1 | Unigene0052377 | 10.67 | 0.00 |
| Metabolite Name | Metabolite Index | Log2FC | p Value |
|---|---|---|---|
| Lipid metabolism | |||
| Methyl palmitate | Com_5883_pos | −2.18 | 0.00 |
| Pentadecanoic acid | Com_12401_pos | −1.95 | 0.00 |
| 11(Z),14(Z),17(Zz)-Eicosatrienoic acid | Com_7643_pos | −1.87 | 0.00 |
| 11-Deoxy prostaglandin F1β | Com_422_pos | −0.93 | 0.01 |
| Cytidine 5′-diphosphocholine | Com_1078_pos | −0.40 | 0.04 |
| Lipoamide | Com_4536_pos | −0.39 | 0.02 |
| Prostaglandin H1 | Com_5241_neg | −0.36 | 0.53 |
| 15(R)-15-Methyl prostaglandin A2 | Com_2092_pos | −0.34 | 0.04 |
| Pyridoxal 5′-phosphate hydrate | Com_5949_pos | −0.28 | 0.04 |
| Docosapentaenoic acid | Com_21_pos | −0.23 | 0.18 |
| Prostaglandin F2α-1-glyceryl ester | Com_3467_pos | −0.2 | 0.26 |
| 7Z,10Z,13Z,16Z,19Z-docosapentaenoic acid | Com_11503_pos | −0.2 | 0.73 |
| Thromboxane A2 | Com_7080_pos | −0.1 | 0.73 |
| all-cis-4,7,10,13,16-Docosapentaenoic acid | Com_778_pos | −0.09 | 0.67 |
| d-threo-Isocitric acid | Com_12240_pos | −0.09 | 0.81 |
| Glycerol-3-phosphate | Com_483_neg | −0.07 | 0.83 |
| Prostaglandin A1 ethyl ester | Com_1353_neg | −0.01 | 0.95 |
| Arachidonic acid | Com_22_neg | 0.00 | 0.99 |
| Citric acid | Com_168_neg | 0.06 | 0.94 |
| Palmitoyl sphingomyelin | Com_1814_pos | 0.17 | 0.54 |
| Levalbuterol | Com_11294_pos | 0.24 | 0.66 |
| 1-Palmitoylglycerol | Com_10_pos | 0.25 | 0.40 |
| l-Palmitoyl carnitine | Com_13685_pos | 0.38 | 0.27 |
| N-Palmitoyl taurine | Com_3290_pos | 0.45 | 0.01 |
| 2-Arachidonyl Glycerol ether | Com_1450_pos | 0.56 | 0.00 |
| Myristic acid | Com_8920_pos | 1.10 | 0.00 |
| Oleamide | Com_1_pos | 1.25 | 0.03 |
| 12-Oxo phytodienoic acid | Com_669_pos | 1.46 | 0.03 |
| 4-acetyl-4-(ethoxycarbonyl)heptanedioic acid | Com_2516_pos | 2.31 | 0.00 |
| N-Tetradecanamide | Com_3122_pos | 2.99 | 0.00 |
| (R)-3-Hydroxy myristic acid | Com_2141_neg | 3.19 | 0.00 |
| Hexadecanamide | Com_148_pos | 3.66 | 0.00 |
| Glucose metabolism | |||
| Phenyl pyruvic acid | Com_735_neg | −1.28 | 0.45 |
| Guanosine5′-diphosphate (GDP) | Com_2804_neg | −0.91 | 0.02 |
| Trehalose-6-phosphate | Com_12250_pos | −0.63 | 0.33 |
| Hydroxyglutaric acid | Com_28_neg | −0.60 | 0.02 |
| Phosphoenolpyruvic acid | Com_1634_neg | −0.6 | 0.35 |
| Citraconic acid | Com_2432_pos | −0.52 | 0.14 |
| d-Galactosamine | Com_904_pos | −0.33 | 0.03 |
| Malic acid | Com_82_neg | −0.20 | 0.49 |
| Uridine 5′-monophosphate | Com_2039_pos | −0.15 | 0.46 |
| Glycerol-3-phosphate | Com_177_neg | −0.15 | 0.68 |
| Fumaric acid | Com_322_neg | −0.15 | 0.51 |
| d-Glucose-1-phosphate | Com_6290_pos | −0.02 | 0.96 |
| d-Glucose-6-phosphate | Com_237_neg | 0.10 | 0.74 |
| N-Acetyl-d-glucosamine 6-phosphate | Com_7268_neg | 0.11 | 0.64 |
| Glycerol-1-hexadecanoate | Com_1112_pos | 0.29 | 0.04 |
| Benzyl 6-O-beta-d-glucopyranosyl-beta-d-glucopyranoside | Com_15594_pos | 0.34 | 0.04 |
| 6-phospho-d-glucono-1,5-lactone | Com_4851_pos | 0.34 | 0.47 |
| d-Glucosamine-6-phosphate | Com_10302_neg | 0.95 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Chen, G.; Zhang, J.; Yang, Q.; Dong, X.; Xie, S.; Liang, W.; Tan, B.; Chi, S. Integrated Metabolome and Transcriptome Analyses Reveal the Efficacy of Steroidal Saponins for Glucose and Lipid Metabolism in Hybrid Grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatu) Fed Higher-Lipid Diets. Animals 2023, 13, 2894. https://doi.org/10.3390/ani13182894
Deng H, Chen G, Zhang J, Yang Q, Dong X, Xie S, Liang W, Tan B, Chi S. Integrated Metabolome and Transcriptome Analyses Reveal the Efficacy of Steroidal Saponins for Glucose and Lipid Metabolism in Hybrid Grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatu) Fed Higher-Lipid Diets. Animals. 2023; 13(18):2894. https://doi.org/10.3390/ani13182894
Chicago/Turabian StyleDeng, Hongjin, Guiqiong Chen, Jiacheng Zhang, Qihui Yang, Xiaohui Dong, Shiwei Xie, Weixing Liang, Beiping Tan, and Shuyan Chi. 2023. "Integrated Metabolome and Transcriptome Analyses Reveal the Efficacy of Steroidal Saponins for Glucose and Lipid Metabolism in Hybrid Grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatu) Fed Higher-Lipid Diets" Animals 13, no. 18: 2894. https://doi.org/10.3390/ani13182894






