Improving the Aerobic Capacity in Fingerlings of European Sea Bass (Dicentrarchus labrax) through Moderate and Sustained Exercise: A Metabolic Approach
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Fish Collection and Maintenance
2.2. Procedure for a Swimming Test
Determination of a Practical Swimming Speed Using a Group-Swimming Test
2.3. Growth Trial under Sustained and Moderate Swimming Speed: Experimental Set-Up and Protocol
2.4. Preparation of Tissue Samples
2.5. White Muscle Proximate Composition and Isotopic Composition Analysis (δ15N and δ13C)
2.6. Gene Expression (RNA Extraction and cDNA Synthesis)
2.7. Enzymatic Activities
2.8. Protein Expression Using Western Blot
2.9. Statistical Analysis
3. Results
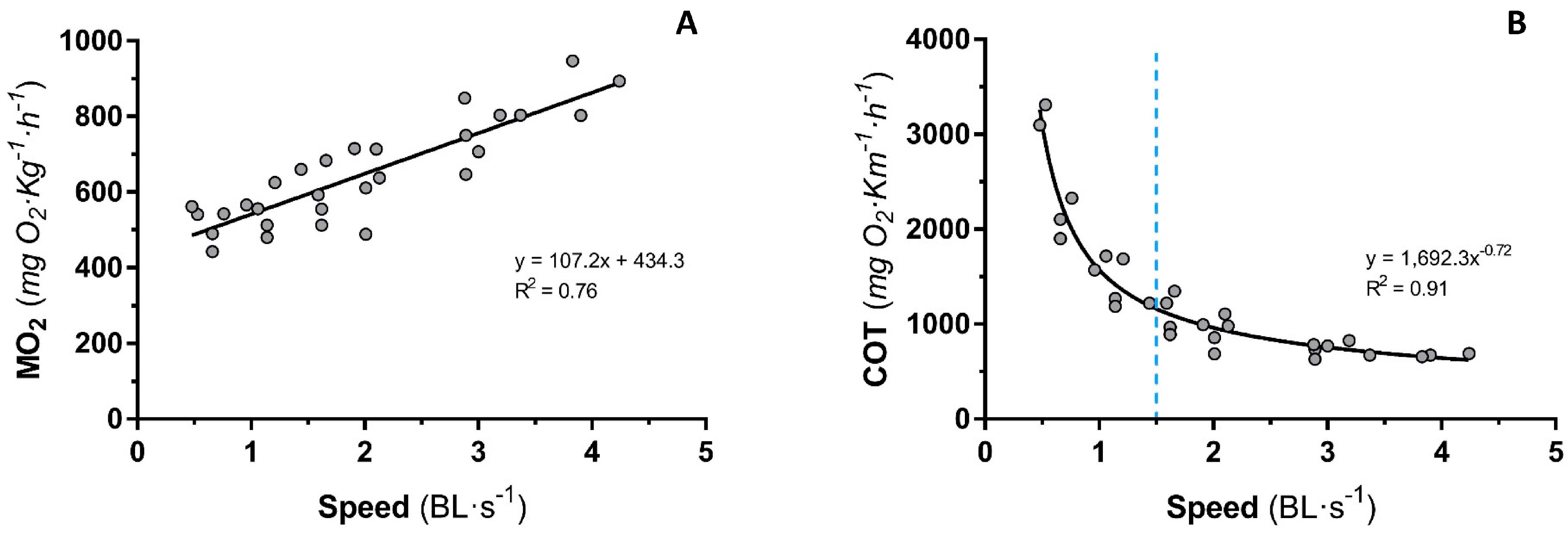
3.1. Group-Swimming Test for Sea Bass Fingerlings
3.2. Growth Trial at Moderate Swimming Speed
3.2.1. Growth Performance, Proximal and Isotopic Composition
3.2.2. Mitochondrial Proteins in White and Red Muscle: Gene and Protein Expression and Enzyme Activities
3.3. Effect of Moderate Swimming Training on the Aerobic Capacity of European Sea Bass
4. Discussion
4.1. Finding a Practical Swimming Speed for Sea Bass Farming
4.2. Growth Trial at Moderate Swimming Speed
4.2.1. Growth Performance, Proximal and Isotopic Composition
4.2.2. Mitochondrial Proteins in White and Red Muscle: Gene and Protein Expression and Enzyme Activities
4.3. Effect of Moderate Swimming Training on the Aerobic Capacity of European Sea Bass
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davison, W. The Effects of Exercise Training on Teleost Fish, a Review of Recent Literature. Comp. Biochem. Physiol. Part A Physiol. 1997, 117, 67–75. [Google Scholar] [CrossRef]
- Palstra, A.P.; Planas, J.V. Swimming Physiology of Fish: Towards Using Exercise to Farm a Fit Fish in Sustainable Aquaculture; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Davison, W.; Herbert, N.H. Swimming-Enhanced Growth. In Swimming Physiology of Fish; Palstra, A.P., Planas, J.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Svendsen, Y.S.; Jobling, M. The Combined Effects of Stocking Density and Sustained Exercise on the Behaviour, Food Intake, and Growth of Juvenile Arctic Charr (Salvelinus alpinus L.). Can. J. Zool. 1992, 70, 115–122. [Google Scholar] [CrossRef]
- Houlihan, D.F.; Laurent, P. Effects of exercise training on the performance, growth, and protein turnover of rainbow trout (Salmo gairdneri). Can. J. Fish. Aquat. Sci. 1987, 44, 1614–1621. [Google Scholar] [CrossRef]
- Alsop, D.H.; Wood, C.M. The interactive effects of feeding and exercise on oxygen consumption, swimming performance and protein usage in juvenile rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 1997, 200, 2337–2346. [Google Scholar] [CrossRef]
- Hernandez, M.D.; Mendiola, P.; de Costa, J.; Zamora, S. Effects of intense exercise training on rainbow trout growth, body composition and metabolic responses. J. Physiol. Biochem. 2002, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Totland, G.K.; Kryvi, H.; Jødestøl, K.A.; Christiansen, E.N.; Tangerås, A.; Slinde, E. Growth and composition of the swimming muscle of adult Atlantic salmon (Salmo salar L.) during long-term sustained swimming. Aquaculture 1987, 66, 299–313. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Jobling, M. The behavior and the relationship between food intake and growth of juvenile Arctic charr, Salvelinus alpinus L., subjected to sustained exercise. Can J. Zool 1990, 68, 2185–2191. [Google Scholar] [CrossRef]
- Young, P.S.; Cech, J.J. Effects of different exercise conditioning velocities on the energy reserves and swimming stress responses in young-of-the-year striped bass (Morone saxatilis). Can. J. Fish Aquat. Sci. 1994, 51, 1528–1534. [Google Scholar] [CrossRef]
- Brown, E.J.; Bruce, M.; Pether, S.; Herbert, N.A. Do swimming fish always grow fast? Investigating the magnitude and physiological basis of exercise-induced growth in juvenile New Zealand yellowtail kingfish, Seriola lalandi. Fish Physiol. Biochem. 2011, 37, 327–336. [Google Scholar] [CrossRef]
- Ibarz, A.; Felip, O.; Fernández-Borràs, J.; Martín-Pérez, M.; Blasco, J.; Torrella, J.R. Sustained swimming improves muscle growth and cellularity in gilthead sea bream. J. Comp. Physiol. B Biochem. System. Environ. Physiol. 2011, 181, 209–221. [Google Scholar] [CrossRef]
- Martin-Perez, M.; Fernandez-Borras, J.; Ibarz, A.; Millan-Cubillo, A.; Felip, O.; De Oliveira, E.; Blasco, J. New insights into fish swimming: A proteomic and isotopic approach in gilthead sea bream. J. Proteome Res. 2012, 11, 3533–3547. [Google Scholar] [CrossRef] [PubMed]
- Bjørnevik, M.; Karlsen, Ø.; Johnston, I.A.; Kiessling, A. Effect of sustained exercise on white muscle structure and flesh quality in farmed cod (Gadus morhua L.). Aquac. Res. 2003, 34, 55–64. [Google Scholar] [CrossRef]
- Kiessling, A.; Higgs, D.A.; Dosanjh, B.S.; Eales, J.G. Influence of sustained exercise at two ration levels on growth and thyroid function of all-female Chinook Salmon (Oncorhynchus tshawytscha) in seawater. Can. J. Fish. Aquat. Sci. 1994, 51, 1975–1984. [Google Scholar] [CrossRef]
- Graziano, M.; Benito, R.; Planas, J.V.; Palstra, A.P. Swimming exercise to control precocious maturation in male seabass (Dicentrarchus labrax). BMC Dev. Biol. 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, J.; Yang, Z.; Gao, X.; Liu, Y.; Wang, C. Sustained swimming training is associated with reversible filet texture changes of european sea bass (Dicentrarchus labrax L.). Front. Physiol. 2019, 10, 725. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Palstra, A.P.; Planas, J.; Mackenzie, S.; Thorarensen, H.; Vandeputte, M.; Mes, D.; Rey, S.; De Boeck, G.; Domenici, P.; et al. Aerobic swimming in intensive finfish aquaculture: Applications for production, mitigation and selection. Rev. Aquacult. 2021, 13, 138–155. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Crespo, D.; Ibarz, A.; Blasco, J.; Fernández-Borràs, J.; Planas, J.V. Effects of sustained swimming on the red and white muscle transcriptome of rainbow trout (Oncorhynchus mykiss) fed a carbohydrate-rich diet. Comp. Biochem. Physiol. A Mol. Int. Physiol. 2013, 166, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Perelló-Amorós, M.; Fernández-Borràs, J.; Sánchez-Moya, A.; Vélez, E.J.; García-Pérez, I.; Gutiérrez, J.; Blasco, J. Mitochondrial Adaptation to Diet and Swimming Activity in Gilthead Seabream: Improved Nutritional Efficiency. Front. Physiol. 2021, 12, 678985. [Google Scholar] [CrossRef]
- Tudorache, C.; Blust, R.; Claireaux, G. Forced and preferred swimming speeds of fish: A methodological approach. In Swimming Physiology of Fish; Palstra, A.P., Planas, J.V., Eds.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Johnston, I.A.; Moon, T.W. Exercise training in skeletal muscle of brook trout (Salvelinus fontinalis). J. Exp. Biol. 1980, 87, 177–194. [Google Scholar] [CrossRef]
- McClelland, G.B.; Craig, P.M.; Dhekney, K.; Dipardo, S. Temperature- and Exercise-Induced Gene Expression and Metabolic Enzyme Changes in Skeletal Muscle of Adult Zebrafish (Danio rerio). J. Physiol. 2006, 2, 739–751. [Google Scholar] [CrossRef]
- Lemoine, C.M.R.; Craig, P.M.; Dhekney, K.; Kim, J.J.; McClelland, G.B. Temporal and spatial patterns of gene expression in skeletal muscles in response to swim training in adult zebrafish (Danio rerio). J. Comp. Physiol. B 2010, 180, 151–160. [Google Scholar] [CrossRef]
- McClelland, G.B.; Scott, G.B. Muscle plasticity. In The Physiology of Fishes; Evans, D.H., Claiborne, J.B., Currie, S., Eds.; CRC Press: Boca Raton, EL, USA, 2013. [Google Scholar]
- Blasco, J.; Moya, A.; Millán-Cubillo, A.; Vélez, E.J.; Capilla, E.; Pérez-Sánchez, J.; Gutiérrez, J.; Fernández- Borrás, J. Growth-promoting effects of sustained swimming in fingerlings of gilthead sea bream (Sparus aurata L.). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2015, 185, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Norin, T.; Clark, T.D. Measurement and relevance of maximum metabolic ratein fishes. J. Fish Biol. 2016, 88, 122–151. [Google Scholar] [CrossRef]
- Nelson, J.A.; Chabot, D. General energy metabolism. In Encyclopedia of Fish Physiology: From Genome to Environment; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; Volume 3, pp. 1566–1572. [Google Scholar]
- Chabot, D.; Steffensen, J.F.; Farrell, A.P. The determination of standard metabolic rate in fishes. J. Fish Biol. 2016, 88, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, C.; Blust, R.; De Boeck, G. Swimming capacity and energetics of migrating and non-migrating morphs of three spined stickleback Gasetosteus aculeatus L. and their ecological implications. J. Fish Biol. 2007, 71, 1448–1456. [Google Scholar] [CrossRef]
- Palstra, A.P.; Kals, J.; Böhm, T.; Bastiaansen, J.W.M. Swimming Performance and Oxygen Consumption as Non-lethal Indicators of Production Traits in Atlantic Salmon and Gilthead Seabream. Front. Physiol. 2020, 11, 759. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, P.; Alfonso, S.; Dioguardi, M.; Zupa, W.; Vazzana, M.; Dara, M.; Spedicato, M.T.; Lembo, G.; Cammarat, M. Calibrating accelerometer data, as a promising tool for health and welfare monitoring in aquaculture: Case study in European sea bass (Dicentrarchus labrax) in conventional or organic aquaculture. Aquac. Rep. 2021, 21, 100817. [Google Scholar] [CrossRef]
- Wiwchar, L.D.; Gilbert, M.J.H.; Kasurak, A.V.; Tierney, K.B. Schooling improves critical swimming performance in zebrafish (Danio rerio). Can. J. Fish. Aquat. Sci. 2018, 75, 653–661. [Google Scholar] [CrossRef]
- Handelsman, C.; Claireaux, G.; Nelson, J.A. Swimming Ability and Ecological Performance of Cultured and Wild European Sea Bass (Dicentrarchus labrax) in Coastal Tidal Ponds. Physiol. Biochem. Zool. 2010, 83, 435–445. [Google Scholar] [CrossRef]
- Stavrakidis-Zachou, O.; Lika, K.; Pavlidis, M.; Asaad, M.H.; Papandroulakis, N. Metabolic scope, performance and tolerance of juvenile European sea bass Dicentrarchus labrax upon acclimation to high temperatures. PLoS ONE 2022, 17, e0272510. [Google Scholar] [CrossRef]
- Zhang, Y.; Mauduit, F.; Farrell, A.P.; Chabot, D.; Ollivier, H.; Rio-Cabello, A.; LeFloch, S.; Claireaux, G. Exposure of European sea bass (Dicentrarchus labrax) to chemically dispersed oil has a chronic residual effect on hypoxia tolerance but not aerobic scope. Aquat.Toxicol. 2017, 191, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Prinet, A.; Chatain, B.; Grima, L.; Vandeputte, M.; Claireaux, G.; McKenzie, D.J. Physiological mechanisms underlying a trade-off between growth rate and tolerance of feed deprivation in the European sea bass (Dicentrarchus labrax). J. Exp. Biol. 2010, 213, 1143–1152. [Google Scholar] [CrossRef]
- Marras, S.; Claireaux, G.; Mckenzie, D.J.; Nelson, J.A. Individual variation and repeatability in aerobic and anaerobic swimming performance of European sea bass, Dicentrarchus labrax. J. Exp. Biol. 2010, 213, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Johnston, I.A. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc. Natl. Acad. Sci. USA 2012, 109, 14247–14252. [Google Scholar] [CrossRef]
- Felip, O.; Ibarz, A.; Fernández-Borràs, J.; Beltrán, M.; Martín-Pérez, M.; Planas, J.V.; Blasco, J. Tracing metabolic routes of dietary carbohydrate and protein in rainbow trout (Oncorhynchus mykiss) using stable isotopes ([13C] starch and [15N] protein): Effects of gelatinisation of starches and sustained swimming. Br. J. Nutr. 2012, 107, 834–844. [Google Scholar] [CrossRef]
- Felip, O.; Blasco, J.; Ibarz, A.; Martin-Perez, M.; Fernández-Borràs, J. Beneficial effects of sustained activity on the use of dietary protein and carbohydrate traced with stable isotopes 15N and 13C in gilthead sea bream (Sparus aurata). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2013, 183, 223–234. [Google Scholar] [CrossRef]
- Basaran, F.; Ozbilgin, H.; Ozbilgin, Y.D. Effect of lordosis on the swimming performance of juvenile sea bass (Dicentrarchus labrax L.). Aquac. Res. 2007, 38, 870–876. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef]
- Boushel, R.; Lundby, C.; Qvortrup, K.; Sahlin, K. Mitochondrial plasticity with exercise training and extreme environments. Exerc. Sport Sci. Rev. 2014, 42, 169–174. [Google Scholar] [CrossRef]
- Petrick, H.L.; Dennis, K.M.J.H.; Miotto, P.M. The importance of exercise intensity, volume and metabolic signalling events in the induction of mitochondrial biogenesis. J. Physiol. 2018, 596, 4571–4572. [Google Scholar] [CrossRef] [PubMed]
- Parry, H.A.; Roberts, M.D.; Kavazis, A.N. Human Skeletal Muscle Mitochondrial Adaptations following Resistance Exercise Training. Int. J. Sports Med. 2020, 41, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moya, A.; Perelló-Amorós, M.; Vélez, E.J.; Viñuales, J.; García-Pérez, I.; Blasco, J.; Gutiérrez, J.; Fernández-Borràs, J. Interaction between the Effects of Sustained Swimming Activity and Dietary Macronutrient Proportions on the Redox Status of Gilthead Sea Bream Juveniles (Sparus aurata L.). Antioxidants 2022, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pérez, M.; Fernández-Borràs, J.; Ibarz, A.; Felip, O.; Gutiérrez, J.; Blasco, J. Stable isotope analysis combined with metabolic indices discriminates between gilthead sea bream (Sparus aurata) fingerlings produced in various hatcheries. J. Agric. Food. Chem. 2011, 59, 11893. [Google Scholar] [CrossRef]
- Martin-Perez, M.; Fernandez-Borras, J.; Ibarz, A.; Felip, O.; Fontanillas, R.; Gutierrez, J.; Blasco, J. Naturally Occurring Stable Isotopes Reflect Changes in Protein. J. Agric. Food Chem. 2013, 61, 8924–8933. [Google Scholar] [CrossRef] [PubMed]
- Martínez Del Rio, C.; Wolf, N.; Carleton, S.A.; Gannes, L.Z. Isotopic ecology ten years after a call for more laboratory experiments. Biol. Rev. 2009, 84, 91–111. [Google Scholar] [CrossRef]
- Brett, J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish Res. Board Can. 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Steffensen, J.F. Respiratory Systems and Metabolic Rates. Fish Physiol. 2005, 22, 203–238. [Google Scholar]
- Roche, D.G.; Binning, S.A.; Bosiger, Y.; Johansen, J.L.; Rummer, J.L. Finding the best estimates of metabolic rates in a coral reef fish. J. Exp. Biol. 2013, 216, 2103–2110. [Google Scholar] [CrossRef]
- Steinhausen, M.F.; Steffensen, J.F.; Andersen, N.G. The Effects of Swimming Pattern on the Energy Use of Gilthead Seabream (Sparus aurata L.). Mar. Freshw. Behav. Physiol. 2010, 43, 227–241. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Good, C.A.; Kramer, H.; Somogyi, M. The determination of glycogen. J. Biol. Chem. 1993, 100, 485–491. [Google Scholar] [CrossRef]
- Fraga, F. Determinación de glucógeno en moluscos con el reactivo de antrona. Inv. Pesq. 1956, 3, 69–74. [Google Scholar]
- Buckley, L.J.; Bulow, F.J. Techniques for the estimation of RNA, DNA and protein. In Age and Growth of Fish; Summerfelt, R.C., Hall, G.E., Eds.; University Press: Ames, IA, USA, 1987; pp. 345–357. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Salmerón, C.; De La Serrana, D.G.; Jimenez-Amilburu, V.; Fontanillas, R.; Navarro, I.; Johnston, I.A.; Gutierrez, J.; Capilla, E. Characterisation and expression of calpain family members in relation to nutritional status, diet composition and flesh texture in gilthead sea bream (Sparus aurata). PLoS ONE 2013, 8, e75349. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Srere, P.A. Citrate synthase. In Methods of Enzymology; Lowenstein, J.M., Ed.; Academic Press: New York, NY, USA, 1969; Volume 13, pp. 3–5. [Google Scholar]
- Palstra, A.P.; Mes, D.; Kusters, K.; Roques, J.A.C.; Flik, G.; Kloet, K.; Blonk, R.J.W. Forced sustained swimming exercise at optimal speed enhances growth of juvenile yellowtail kingfish (Seriola lalandi). Front. Physiol. 2015, 6, 506. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, J.; Rašković, B.; Blust, R.; De Boeck, G. Exercise improves growth, alters physiological performance and gene expression in common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 226, 38–48. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Höglund, E.; Dupont-Prinet, A.; Larsen, B.k.; Skov, P.V.; Pedersen, P.B.; Jokumsen, A. Effects of stocking density and sustained aerobic exercise on growth, energetics and welfare of rainbow trout. Aquaculture 2012, 338–341, 216–222. [Google Scholar] [CrossRef]
- Skov, P.V.; Lund, I.; Pargana, A.M. No evidence for a bioenergetics advantage from forced swimming in rainbow trout under a restrictive feeding regime. Front. Physiol. 2015, 6, 31. [Google Scholar] [CrossRef]
- Grisdale-Helland, B.; Takle, H.; Helland, S.J. Aerobic exercise increases the utilization efficiency of energy and protein for growth in Atlantic salmon post-smolts. Aquaculture 2013, 406–407, 43–51. [Google Scholar] [CrossRef]
- Webb, P.W. Composition and mechanics of routine swimming of rainbow trout, Oncorhynchus mykiss. Can. J. Fish Aquat. Sci. 1991, 48, 583–590. [Google Scholar] [CrossRef]
- LØkkeborg, S.; Fernö, A. Diel activity pattern and food search behaviour in cod, Gadus morhua. Environ. Biol. Fish. 1999, 54, 345–353. [Google Scholar] [CrossRef]
- Steinhausen, M.F.; Andersen, N.G.; Steffensen, J.F. The effect of external dummy transmitters on oxygen consumption and performance of swimming Atlantic cod. J. Fish Biol. 2006, 69, 951–956. [Google Scholar] [CrossRef]
- Webber, D.M.; Boutilier, R.G.; Kerr1and, S.R.; Smale, M.J. Caudal differential pressure as a predictor of swimming speed of cod (Gadus morhua). J. Exp. Biol. 2001, 204, 3561–3570. [Google Scholar] [CrossRef]
- Palstra, A.P.; Planas, J.V. Fish under exercise. Fish Physiol. Biochem. 2011, 37, 259–272. [Google Scholar] [CrossRef]
- Palstra, A.P.; Roque, A.; Kruijt, L.; Jéhannet, P.; Pérez-Sánchez, J.; Dirks, R.P. Physiological Effects of Water Flow Induced Swimming Exercise in Seabream Sparus Aurata. Front. Physiol. 2020, 11, 610049. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Cataldi, E.; Romano, P.; Owen, S.F.; Taylor, E.W.; Bronzi, P. Effects of acclimation to brackish water on the growth, respiratory metabolism, and swimming performance of young-of-the-year Adriatic sturgeon (Acipenser naccarii). Can. J. Fish. Aquat. Sci. 2001, 58, 1104–1112. [Google Scholar] [CrossRef]
- Claireaux, G.; Couturier, C.; Groison, A.L. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J. Exp. Biol. 2006, 209, 3420–3428. [Google Scholar] [CrossRef]
- Chatelier, A.; McKenzie, D.J.; Prinet, A.; Galois, R.; Robin, J.; Zambonino, J.; Claireaux, G. Associations between tissue fatty acid composition and physiological traits of performance and metabolism in the seabass (Dicentrarchus labrax). J. Exp. Biol. 2006, 209, 3429–3439. [Google Scholar] [CrossRef] [PubMed]
- Luna-Acosta, A.; Lefrançois, C.; Millot, S.; Chatain, B.; Bégout, M.L. Physiological response in different strains of sea bass (Dicentrarchus labrax): Swimming and aerobic metabolic capacities. Aquaculture 2011, 317, 162–167. [Google Scholar] [CrossRef]
- Killen, S.S.; Marras, S.; McKenzie, D.J. Fast growers sprint slower: Effects of food deprivation and re-feeding on sprint swimming performance in individual juvenile European sea bass. J. Exp. Biol. 2014, 217, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Vitor, A.L.; Greene, N.P. Exercise training-induced Regulation of Mitochondrial Quality. Exerc. Sport Sci. Rev. 2012, 40, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Nogales, A.; Benedito-Palos, L.; Calduch-Giner, J.A.; Pérez-Sánchez, J. Feed restriction up-regulates uncoupling protein 3 (UCP3) gene expression in heart and red muscle tissues of gilthead sea bream (Sparus aurata L.). New insights in substrate oxidation and energy expenditure. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 159, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Battaglia, V.; Brunati, A.M.; La Rocca, N.; Tibaldi, E.; Pietrangeli, P.; Marcocci, L.; Mondovi, B.; Rossi, C.A.; Toninello, A. Catalase Takes Part in Rat Liver Mitochondria Oxidative Stress Defense. J. Biol. Chem. 2007, 282, 24407–24415. [Google Scholar] [CrossRef]
- Ibarz, A.; Blasco, J.; Gallardo, M.A.; Fernández-Borràs, J. Energy reserves and metabolic status affect the acclimation of gilthead sea bream (Sparus aurata) to cold. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 155, 319–326. [Google Scholar] [CrossRef]
- Vanderplancke, G.; Claireaux, G.; Quazuguel, P.; Huelvan, C.; Corporeau, C.; Mazurais, D.; Zambonino-Infante, J.L. Exposure to chronic moderate hypoxia impacts physiological and developmental traits of European sea bass (Dicentrarchus labrax) larvae. Fish Physiol. Biochem. 2015, 41, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Claireaux, G.; McKenzie, D.J.; Genge, A.G.; Chatelier, A.; Aubin, J.; Farrell, A.P. Linking swimming performance, cardiac pumping ability and cardiac anatomy in rainbow trout. J. Exp. Biol. 2005, 208, 1775–1784. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Wong, S.; Randall, D.J.; Egginton, S.; Taylor, E.W.; Farrell, A.P. The effects of sustained exercise and hypoxia upon oxygen tensions in the red muscle of rainbow trout. J. Exp. Biol. 2004, 207, 3629–3637. [Google Scholar] [CrossRef]
- Anttila, K.; Jäntti, M.; Mänttäri, S. Effects of training on lipid metabolism in swimming muscles of sea trout (Salmo trutta). J. Comp. Physiol. B 2010, 180, 707–714. [Google Scholar] [CrossRef]
- Alfonso, S.; Zupa, W.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Using Telemetry Sensors Mapping the Energetic Costs in European Sea Bass (Dicentrarchus labrax), as a Tool for Welfare Remote Monitoring in Aquaculture. Front. Anim. Sci. 2022, 3, 885850. [Google Scholar] [CrossRef]
- Zupa, W.; Carbonara, P.; Spedicato, M.T.; Lembo, G. Modelling swimming activities and energetic costs in European sea bass (Dicentrarchus labrax L., 1758) during critical swimming tests. Mar. Freshw. Behav. Physiol. 2015, 48, 341–357. [Google Scholar] [CrossRef]
- Soofiani, N.M.; Priede, I.G. Aerobic metabolic scope and swimming performance in juvenile cod, Gadus morhua L. J. Fish Biol. 1985, 26, 127–138. [Google Scholar] [CrossRef]
- Claireaux, G.; Lefrançois, C. Linking environmental variability and fish performance: Integration through the concept of scope for activity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 2031–2041. [Google Scholar] [CrossRef]
- Zhanga, Y.; Timmerhaus, G.; Anttila, K.; Mauduit, F.; Jørgensen, S.M.; Kristensen, T.; Claireaux, G.; Takle, H.; Farrell, A.P. Domestication compromises athleticism and respiratory plasticity in response to aerobic exercise training in Atlantic salmon (Salmo salar). Aquaculture 2016, 463, 79–88. [Google Scholar] [CrossRef]





| Gene | Sequences 5′-3′ | Ta (°C) | Accession Number |
|---|---|---|---|
| cox4a | F: CATTGTACCGCATCAGCTTC R: GGCCAGTGAAGCCAATTAAG | 60 | XM_051380547 |
| cs | F: ATTGGGCACAAGGTCTGAAC R: AAGCAGGAAAGCTTGAGACG | 60 | XM_051425289 |
| pgc1a | F: GCAAACTCCCAGCTCAGCTA R: GCTTCACTGGCTTTGGTGTG | 60 | XM_051429129 |
| tfam | F: CCTGCACAAGTACTCTGGCA R: ACGGCTCTTTCTGTTCTGGG | 60 | XM_051417177 |
| ucp2a | F: AAGGAGGAAGGCATTCGTGG R: CCCTGCACCAAAGGCTGATA | 60 | XM_051401934 |
| ucp3 | F: TGATGACGGACAACATGCCA R: GGAGCCCAGACGAAGATACG | 60 | XM_051397440 |
| mit1 | F: AAAGAGGTGCTCAACTCCCG R: GTTGATGGCGTCCATGATGC | 60 | XM_051375872 |
| mit2 | F: TCAGGAAGCTCCATGTGCTG R: CACTGACGAGGAACCAGCTT | 60 | XM_051426021 |
| fis1 | F: TTGTTGAAGGGAGCCGTCTC R: AGTCTGTAGTTGGCCACTGC | 60 | XM_051429174 |
| Tmem20a | F: ACCACCTGACCAATGCGATT R: GTGGGCAGTTTTGTGAGCAG | 60 | XM_051429207 |
| Tmem20b | F: TGCTGGCTCAGGGAGACTAT R: TGGTGAGCAGCATCTGGAAG | 60 | XM_051381355 |
| sod | F: GTTGGAGACCTGGGAGATGT R: CTCCTCATTGCCTCCTTTTC | 60 | FJ_860004 |
| cat | F: ATGGTGTGGGACTTCTGGAG R: AGTGGAACTTGCAGTAGAAACG | 60 | FJ_860003 |
| gpx | F: AGTTAATCCGGAATTCGTGAGA R: TGAGTGTAGTCCCTGGTTGTTG | 60 | FM_013606.1c |
| gshr | F: TGCACCAAAGAACTGCAGAA R: ACGAGTGTCACCTCCAGTCC | 60 | FM_020412 |
| ef1a | F: CAAGGAGGGCAATGCCAGT R: GAGCGAAGGTGACGACCAT | 60 | XM_051391262 |
| rpl13a | F: TCTGGAGGACTGTCAGGGGCATGC R: AGACGCACAATCTTGAGAGCAG | 60 | XM_051389730 |
| rpl17 | F: TTGAAGACAACGCAGGAGTCA R: CAGCGCATTCTTTTGCCACT | 60 | AF_139590.1 |
| fau | F: GACACCCAAGGTTGACAAGCAG R: GGCATTGAAGCACTTAGGAGTTG | 68 | XM_051408030 |
| Control | Exercise | |
|---|---|---|
| Initial BW (g) | 3.93 ± 0.10 | 3.94 ± 0.11 |
| Final BW (g) | 14.76 ± 0.51 | 13.5 ± 0.46 |
| CF a | 1.70 ± 0.04 | 1.72 ± 0.03 |
| SGR b | 3.02 ± 0.09 | 2.95 ± 0.18 |
| HSI c | 1.91 ± 0.10 | 1.92 ± 0.13 |
| MSI d | 38.31 ± 0.69 | 40.15 ± 0.76 |
| MFI e | 3.29 ± 0.23 | 3.31 ± 0.33 |
| Control | Exercise | |
|---|---|---|
| Composition | ||
| Wet weight (%) | 76.8 ± 0.15 | 76.46 ± 1.39 |
| Protein (% w.w) | 19.65 ± 0.4 | 19.57 ± 1.24 |
| Lipids (% w.w) | 1.28 ± 0.19 | 1.55 ± 0.26 |
| Glycogen (% w.w) | 0.10 ± 0.02 | 0.10 ± 0.02 |
| RNA (µg/mg prot.) | 14.18 ± 0.98 | 16.66 ± 0.88 |
| DNA (µg/mg prot.) | 7.27 ± 0.68 | 5.78 ± 0.55 |
| RNA/DNA | 2.28 ± 0.22 | 3.10 ± 0.25 * |
| Control | Exercise | |
|---|---|---|
| δ13C-muscle | −19.75 ± 0.12 | −19.68 ± 0.16 |
| δ15N-muscle | 12.32 ± 0.04 | 12.04 ± 0.06 *** |
| δ13C-protein | −19.81 ± 0.02 | −19.84 ± 0.03 |
| δ15N-protein | 12.59 ± 0.08 | 12.51 ± 0.03 |
| δ13C-glycogen | −23.15 ± 0.33 | −23.39 ± 0.20 |
| Δ15N-muscle 1 | 1.97 ± 0.04 | 1.69 ± 0.06 *** |
| Control | Exercise | |
|---|---|---|
| RMR 1 | 223.58 ± 26.50 | 254.25 ± 20.33 |
| MMR 1 | 691.28 ± 34.40 | 788.55 ± 21.11 * |
| AMS | 467.69 ± 46.32 | 534.31 ± 18.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perelló-Amorós, M.; Fernández-Borràs, J.; Yu, S.; Sánchez-Moya, A.; García de la serrana, D.; Gutiérrez, J.; Blasco, J. Improving the Aerobic Capacity in Fingerlings of European Sea Bass (Dicentrarchus labrax) through Moderate and Sustained Exercise: A Metabolic Approach. Animals 2024, 14, 274. https://doi.org/10.3390/ani14020274
Perelló-Amorós M, Fernández-Borràs J, Yu S, Sánchez-Moya A, García de la serrana D, Gutiérrez J, Blasco J. Improving the Aerobic Capacity in Fingerlings of European Sea Bass (Dicentrarchus labrax) through Moderate and Sustained Exercise: A Metabolic Approach. Animals. 2024; 14(2):274. https://doi.org/10.3390/ani14020274
Chicago/Turabian StylePerelló-Amorós, Miquel, Jaume Fernández-Borràs, Shengnan Yu, Albert Sánchez-Moya, Daniel García de la serrana, Joaquín Gutiérrez, and Josefina Blasco. 2024. "Improving the Aerobic Capacity in Fingerlings of European Sea Bass (Dicentrarchus labrax) through Moderate and Sustained Exercise: A Metabolic Approach" Animals 14, no. 2: 274. https://doi.org/10.3390/ani14020274






