Timing of Artificial Insemination Using Sexed or Conventional Semen Based on Automated Activity Monitoring of Estrus in Holstein Heifers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Automated Activity Monitoring System
2.3. Reproductive Management
2.4. Data Collection and Statistical Analyses
3. Results
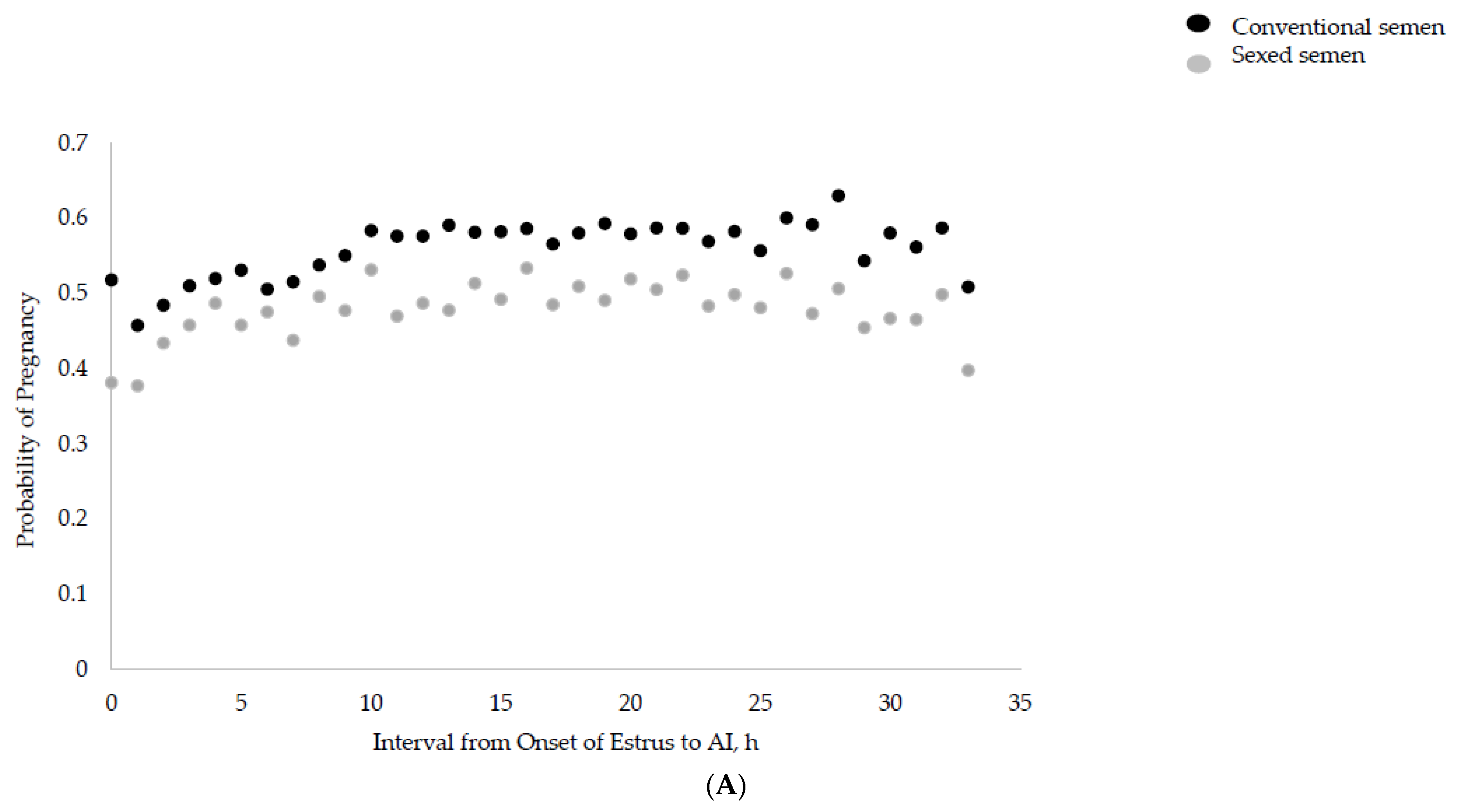
3.1. Interval from Onset of Estrus to AI
3.2. Interval from Peak of Activity to AI
3.3. Interval from End of Estrus to AI
3.4. Factors Associated with P/AI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalton, J.C.; Nadir, S.; Bame, J.H.; Noftsinger, M.; Nebel, R.L.; Saacke, R.G. Effect of time of insemination on number of accessory sperm, fertilization rate, and embryo quality in nonlactating dairy cattle. J. Dairy Sci. 2001, 84, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Saacke, R.G. Insemination factors related to timed AI in cattle. Theriogenology 2008, 70, 479–484. [Google Scholar] [CrossRef]
- Guner, B.; Erturk, M.; Yilmazbas-Mecitoglu, G.; Keskin, A.; Karakaya-Bilen, E.; Cakircali, R.; Serim, E.; Orman, A.; Gumen, A. Effect of delaying the time of insemination with sex-sorted semen on pregnancy rate in Holstein heifers. Reprod. Domest. Anim. 2020, 55, 1411–1417. [Google Scholar] [CrossRef]
- Macmillan, K.; Gobikrushanth, M.; Plastow, G.; Colazo, M.G. Performance and optimization of an ear tag automated activity monitor for estrus prediction in dairy heifers. Theriogenology 2020, 155, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Tippenhauer, C.M.; Plenio, J.-L.; Madureira, A.M.L.; Cerri, R.L.A.; Heuwieser, W.; Borchardt, S. Timing of artificial insemination using fresh or frozen semen after automated activity monitoring of estrus in lactating dairy cows. J. Dairy Sci. 2021, 104, 3585–3595. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Haughian, J.M.; Shaver, R.D.; Rosa, G.; Wiltbank, M.C. Comparison of ovarian function and circulating steroids in estrous cycles of Holstein heifers and lactating cows. J. Dairy Sci. 2004, 87, 905–920. [Google Scholar] [CrossRef]
- Løvendahl, P.; Chagunda, M. On the use of physical activity monitoring for estrus detection in dairy cows. J. Dairy Sci. 2010, 93, 249–259. [Google Scholar] [CrossRef]
- Sales, J.N.S.; Neves, K.A.L.; Souza, A.H.; Crepaldi, G.A.; Sala, R.V.; Fosado, M.; Campos Filho, E.P.; de Faria, M.; Sá Filho, M.F.; Baruselli, P.S. Timing of insemination and fertility in dairy and beef cattle receiving timed artificial insemination using sex-sorted sperm. Theriogenology 2011, 76, 427–435. [Google Scholar] [CrossRef]
- Sá Filho, M.F.; Ayres, H.; Ferreira, R.M.; Nichi, M.; Fosado, M.; Filho, E.P.C.; Baruselli, P.S. Strategies to improve pregnancy per insemination using sex-sorted semen in dairy heifers detected in estrus. Theriogenology 2010, 74, 1636–1642. [Google Scholar] [CrossRef]
- Chebel, R.C.; Cunha, T. Optimization of timing of insemination of dairy heifers inseminated with sex-sorted semen. J. Dairy Sci. 2020, 103, 5591–5603. [Google Scholar] [CrossRef]
- Tippenhauer, C.M.; Plenio, J.-L.; Madureira, A.M.L.; Cerri, R.L.A.; Heuwieser, W.; Borchardt, S. Factors associated with estrous expression and subsequent fertility in lactating dairy cows using automated activity monitoring. J. Dairy Sci. 2021, 104, 6267–6282. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.M.L.; Silper, B.F.; Burnett, T.A.; Polsky, L.; Cruppe, L.H.; Veira, D.M.; Vasconcelos, J.L.M.; Cerri, R.L.A. Factors affecting expression of estrus measured by activity monitors and conception risk of lactating dairy cows. J. Dairy Sci. 2015, 98, 7003–7014. [Google Scholar] [CrossRef] [PubMed]
- Plenio, J.-L.; Bartel, A.; Madureira, A.; Cerri, R.; Heuwieser, W.; Borchardt, S. Application note: Validation of BovHEAT—An open-source analysis tool to process data from automated activity monitoring systems in dairy cattle for estrus detection. Comput. Electron. Agric. 2021, 188, 106323. [Google Scholar] [CrossRef]
- Norman, H.D.; Wright, J.R.; Kuhn, M.T.; Hubbard, S.M.; Cole, J.B.; VanRaden, P.M. Genetic and environmental factors that affect gestation length in dairy cattle. J. Dairy Sci. 2009, 92, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Neto, A.; Galvão, K.N.; Thatcher, W.W.; Santos, J.E.P. Association among gestation length and health, production, and reproduction in Holstein cows and implications for their offspring. J. Dairy Sci. 2017, 100, 3166–3181. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, M.C.; Baez, G.M.; Cochrane, F.; Barletta, R.V.; Trayford, C.R.; Joseph, R.T. Effect of a second treatment with prostaglandin F2α during the Ovsynch protocol on luteolysis and pregnancy in dairy cows. J. Dairy Sci. 2015, 98, 8644–8654. [Google Scholar] [CrossRef]
- Dohoo, P.J.; Martin, S.W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; University of Prince Edward Island: Charlottetown, PE, Canada, 2009. [Google Scholar]
- Silper, B.F.; Robles, I.; Madureira, A.M.L.; Burnett, T.A.; Reis, M.M.; de Passillé, A.M.; Rushen, J.; Cerri, R.L.A. Automated and visual measurements of estrous behavior and their sources of variation in Holstein heifers. I: Walking activity and behavior frequency. Theriogenology 2015, 84, 312–320. [Google Scholar] [CrossRef]
- Nelson, S.T.; Haadem, C.S.; Nødtvedt, A.; Hessle, A.; Martin, A.D. Automated activity monitoring and visual observation of estrus in a herd of loose housed Hereford cattle: Diagnostic accuracy and time to ovulation. Theriogenology 2017, 87, 205–211. [Google Scholar] [CrossRef]
- Drake, E.; Holden, S.A.; Aublet, V.; Doyle, R.C.; Millar, C.; Moore, S.G.; Maicas, C.; Randi, F.; Cromie, A.R.; Lonergan, P.; et al. Evaluation of delayed timing of artificial insemination with sex-sorted sperm on pregnancy per artificial insemination in seasonal-calving, pasture-based lactating dairy cows. J. Dairy Sci. 2020, 103, 12059–12068. [Google Scholar] [CrossRef]
- Silva, T.V.; Lima, F.S.; Thatcher, W.W.; Santos, J.E.P. Synchronized ovulation for first insemination improves reproductive performance and reduces cost per pregnancy in dairy heifers. J. Dairy Sci. 2015, 98, 7810–7822. [Google Scholar] [CrossRef]
- DeJarnette, J.M.; Leach, M.A.; Nebel, R.L.; Marshall, C.E.; McCleary, C.R.; Moreno, J.F. Effects of sex-sorting and sperm dosage on conception rates of Holstein heifers: Is comparable fertility of sex-sorted and conventional semen plausible? J. Dairy Sci. 2011, 94, 3477–3483. [Google Scholar] [CrossRef] [PubMed]
- Maicas, C.; Holden, S.A.; Drake, E.; Cromie, A.R.; Lonergan, P.; Butler, S.T. Fertility of frozen sex-sorted sperm at 4 × 106 sperm per dose in lactating dairy cows in seasonal-calving pasture-based herds. J. Dairy Sci. 2020, 103, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, R.; Moreno, J.F. Review: Semen sexing—Current state of the art with emphasis on bovine species. Animals 2018, 12, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.W.; Gonzalez-Marin, C.; Gilligan, T.B.; DeJarnette, J.M.; Utt, M.D.; Helser, L.A.; Hasenpusch, E.; Evans, K.M.; Moreno, J.F.; Vishwanath, R. 190 SexedULTRA™, A new method of processing sex-sorted bovine sperm improves conception rates. Reprod. Fertil. Dev. 2017, 29, 203–204. [Google Scholar] [CrossRef]
- Reese, S.; Pirez, M.C.; Steele, H.; Kölle, S. The reproductive success of bovine sperm after sex-sorting: A meta-analysis. Sci. Rep. 2021, 11, 17366. [Google Scholar] [CrossRef]
- Colazo, M.G.; Mapletoft, R.J. Pregnancy per AI in Holstein heifers inseminated with sex-selected or conventional semen after estrus detection or timed-AI. Can. Vet. J. 2017, 58, 365–370. [Google Scholar] [PubMed]
- Roelofs, J.; López-Gatius, F.; Hunter, R.; van Eerdenburg, F.; Hanzen, C. When is a cow in estrus? Clinical and practical aspects. Theriogenology 2010, 74, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Guner, B.; Selcuk, G.; Guclu, S.; Sengul, S.; Altun, I.; Dikmen, S.; Gumen, A. Comparison of pregnancy per AI of heifers inseminated with sex-sorted or conventional semen after oestrus detection or timed artificial insemination. Reprod. Domest. Anim. 2021, 56, 1254–1260. [Google Scholar] [CrossRef]
- Hall, J.B.; Kasimanickam, R.K.; Glaze, J.B.; Roberts-Lew, M.C. Impact of delayed insemination on pregnancy rates to gender selected semen in a fixed-time AI system. Theriogenology 2017, 102, 154–161. [Google Scholar] [CrossRef]
- Woelders, H.; van der Lende, T.; Kommadath, A.; te Pas, M.F.W.; Smits, M.A.; Kaal, L.M.T.E. Central genomic regulation of the expression of oestrous behaviour in dairy cows: A review. Animals 2014, 8, 754–764. [Google Scholar] [CrossRef]
- Galvão, K.N.; Santos, J.E.P.; Juchem, S.O.; Cerri, R.L.A.; Coscioni, A.C.; Villaseñor, M. Effect of addition of a progesterone intravaginal insert to a timed insemination protocol using estradiol cypionate on ovulation rate, pregnancy rate, and late embryonic loss in lactating dairy cows. J. Anim. Sci. 2004, 82, 3508–3517. [Google Scholar] [CrossRef] [PubMed]
- Buhi, W.C. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction 2002, 123, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Aungier, S.P.M.; Roche, J.F.; Duffy, P.; Scully, S.; Crowe, M.A. The relationship between activity clusters detected by an automatic activity monitor and endocrine changes during the periestrous period in lactating dairy cows. J. Dairy Sci. 2015, 98, 1666–1684. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.L.; Allrich, R.D. Relationship between endogenous estradiol-17b and estrous behavior in heifers. J. Anim. Sci. 1989, 67, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.T.; Hutchison, J.L.; Wiggans, G.R. Characterization of Holstein heifer fertility in the United States. J. Dairy Sci. 2006, 89, 4907–4920. [Google Scholar] [CrossRef] [PubMed]
- Veronese, A.; Marques, O.; Moreira, R.; Belli, A.L.; Bilby, T.R.; Chebel, R.C. Estrous characteristics and reproductive outcomes of Holstein heifers treated with 2 prostaglandin formulations and detected in estrus by an automated estrous detection or mounting device. J. Dairy Sci. 2019, 102, 6649–6659. [Google Scholar] [CrossRef]
- Fodor, I.; Baumgartner, W.; Abonyi-Tóth, Z.; Lang, Z.; Ózsvári, L. Associations between management practices and major reproductive parameters of Holstein-Friesian replacement heifers. Anim. Reprod. Sci. 2018, 188, 114–122. [Google Scholar] [CrossRef]
- Archbold, H.; Shalloo, L.; Kennedy, E.; Pierce, K.; Buckley, F. Influence of age, body weight and body condition score before mating start date on the pubertal rate of maiden Holstein–Friesian heifers and implications for subsequent cow performance and profitability. Animals 2012, 6, 1143–1151. [Google Scholar] [CrossRef]
- Adamiak, S.J.; Mackie, K.; Watt, R.G.; Webb, R.; Sinclair, K.D. Impact of nutrition on oocyte quality: Cumulative effects of body composition and diet leading to hyperinsulinaemia in cattle. Biol. Reprod. 2005, 73, 918–926. [Google Scholar] [CrossRef]
- Mann, G.E.; Green, M.P.; Sinclair, K.D.; Demmers, K.J.; Fray, M.D.; Gutierrez, C.G.; Garnsworthy, P.C.; Webb, R. Effects of circulating P4 and insulin on early embryo development in beef heifers. Anim. Reprod. Sci. 2003, 79, 71–79. [Google Scholar] [CrossRef]
- Micke, G.C.; Sullivan, T.M.; Magalhaes, R.J.S.; Rolls, P.J.; Norman, S.T.; Perry, V.E.A. Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight. Anim. Reprod. Sci. 2010, 117, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, K.; Yamazaki, T.; Yamaguchi, S.; Abe, H.; Bai, H.; Takahashi, M.; Kawahara, M. Effects of use of conventional and sexed semen on the conception rate in heifers: A comparison study. Theriogenology 2019, 135, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Rosa, G.; Wiltbank, M.C. Ovarian structures and circulating steroids in heifers and lactating cows in summer and lactating and dry cows in winter. J. Dairy Sci. 2002, 85, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, A.; Bisinotto, R.S.; Peñagaricano, F. Genetic analysis of fetal loss in Holstein cattle. J. Dairy Sci. 2022, 105, 9012–9020. [Google Scholar] [CrossRef]
- Reese, S.T.; Pereira, M.H.C.; Edwards, J.L.; Vasconcelos, J.L.M.; Pohler, K.G. Pregnancy diagnosis in cattle using pregnancy associated glycoprotein concentration in circulation at day 24 of gestation. Theriogenology 2018, 106, 178–185. [Google Scholar] [CrossRef]



| Parameter | Farm | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Average farm size | - | 716 | 427 | 581 | 594 | 515 |
| Average 305 d milk yield (kg) | - | 9677 | 9461 | 9105 | 9500 | 9975 |
| Average number of replacement heifers | 868 | 729 | 395 | 453 | 487 | 495 |
| Age at first service (months, ±SD) | 15.0 ± 1.3 | 14.1 ± 1.7 | 15.5 ± 1.4 | 14.2 ± 0.9 | 14.0 ± 0.7 | 16.1 ± 0.7 |
| Pregnancy per AI at 1st service (%) | 63 | 50 | 57 | 46 | 54 | 49 |
| Overall pregnancy per AI (%) | 59 | 40 | 62 | 49 | 49 | 53 |
| Heat detection rate (%) | 69 | 78 | 41 | 75 | 62 | 77 |
| Day of pregnancy diagnosis after AI 2 | 42 ± 3 | 28 ± 3 | 38 ± 3 | 32 ± 3 | 30 ± 3 | 30 ± 3 |
| Method of pregnancy diagnosis | Alert of the AAM 1 system | Transrectal ultrasound | Transrectal palpation | Transrectal ultrasound | Transrectal ultrasound | Transrectal ultrasound |
| Age at first calving (months, ±SD) | 26.5 ± 1.7 | 25.0 ± 1.7 | 25.5 ± 1.6 | 23.6 ± 1.3 | 23.0 ± 0.6 | 26.0 ± 0.9 |
| Farm | 1st AI | 2nd AI | ≥3rd AI | |||
|---|---|---|---|---|---|---|
| Conventional Semen | Sexed Semen | Conventional Semen | Sexed Semen | Conventional Semen | Sexed Semen | |
| 1 | 10.7 (70/657) | 89.3 (587/657) | 13.0 (41/315) | 87.0 (274/315) | 78.3 (76/97) | 21.7 (21/97) |
| 2 | 60.3 (414/686) | 39.7 (272/686) | 79.6 (219/275) | 20.4 (56/275) | - | - |
| 3 | 19.5 (69/354) | 80.5 (285/354) | 35.5 (54/152) | 64.5 (98/152) | 85.6 (83/97) | 14.4 (14/97) |
| 4 | 22.9 (128/559) | 77.1 (431/559) | 52.7 (129/245) | 47.3 (116/245) | 77.3 (167/216) | 22.7 (49/216) |
| 5 | 12.9 (21/163) | 87.1 (142/163) | 4.7 (2/43) | 95.3 (41/43) | 27.3 (3/11) | 72.7 (8/11) |
| 6 | 34.0 (52/153) | 66.0 (101/153) | 59.3 (51/86) | 40.7 (35/86) | 88.0 (44/50) | 12.0 (6/50) |
| Total | 28.9 (759/2623) | 71.1 (1864/2623) | 43.3 (500/1154) | 56.7 (654/1154) | 78.9 (385/488) | 21.1 (103/488) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tippenhauer, C.M.; Plenio, J.-L.; Madureira, A.; Heuwieser, W.; Borchardt, S. Timing of Artificial Insemination Using Sexed or Conventional Semen Based on Automated Activity Monitoring of Estrus in Holstein Heifers. Animals 2023, 13, 2994. https://doi.org/10.3390/ani13192994
Tippenhauer CM, Plenio J-L, Madureira A, Heuwieser W, Borchardt S. Timing of Artificial Insemination Using Sexed or Conventional Semen Based on Automated Activity Monitoring of Estrus in Holstein Heifers. Animals. 2023; 13(19):2994. https://doi.org/10.3390/ani13192994
Chicago/Turabian StyleTippenhauer, Christie Marie, Jan-Lukas Plenio, Augusto Madureira, Wolfgang Heuwieser, and Stefan Borchardt. 2023. "Timing of Artificial Insemination Using Sexed or Conventional Semen Based on Automated Activity Monitoring of Estrus in Holstein Heifers" Animals 13, no. 19: 2994. https://doi.org/10.3390/ani13192994
APA StyleTippenhauer, C. M., Plenio, J.-L., Madureira, A., Heuwieser, W., & Borchardt, S. (2023). Timing of Artificial Insemination Using Sexed or Conventional Semen Based on Automated Activity Monitoring of Estrus in Holstein Heifers. Animals, 13(19), 2994. https://doi.org/10.3390/ani13192994





