A Subovulatory Dose of Human Chorionic Gonadotropin (hCG) May Sustain Terminal Follicle Development and Reproductive Efficiency during Anestrus in Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures
2.1.1. Animals and General Management
2.1.2. Estrous Synchronization, Estrous Detection, and Mating Program
2.1.3. Blood Sampling and Endocrine Measures
2.1.4. Follicular and Corpora Lutea (CLs) Measures and Functional Definitions
2.2. Experiment Descriptions
2.2.1. Experiment 1: Reproductive Performance in Progesterone-Based Estrous Synchronized Highlander Ewes during Two Contrasting Periods of an Annual Reproductive Cycle
2.2.2. Experiment 2: Plasma Concentrations of hCG after a Single Administration in Sheep
2.2.3. Experiment 3: Effect of hCG on Terminal Follicular Development, Estrus, and Ovulation in the Anestrous Season
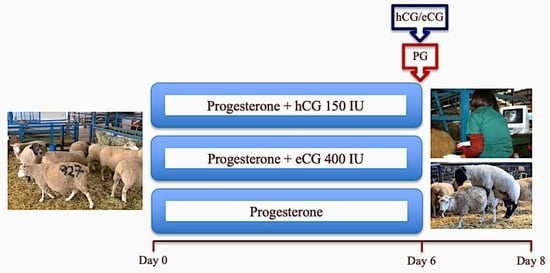
2.2.4. Experiment 4: Comparative Assessment of the Effect of the Administration of hCG vs. eCG on Ovarian Functional Markers, Ovulation, and Fertility in Anestrous Sheep
2.3. Statistical Analysis
3. Results
3.1. Experiment 1: Characterization of Reproductive Performance of Highlander Ewes during an Annual Reproductive Cycle
3.2. Experiment 2: Assessment of Plasma Concentrations of hCG in Ewes Treated with a Single Administration of 150 and 250 IU of hCG Compared to Untreated Controls
3.3. Experiment 3: Assessment of the Effect of the Administration of hCG on Terminal Follicular Development, Estrus, and Ovulation during the Anestrous Season
3.4. Experiment 4: Comparative Assessment of the Effect of the Administration of hCG vs. eCG on Ovarian Functional Markers, Ovulation, and Fertility in Anestrous Sheep
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, R.M.; Notter, D.R.; Hogue, D.E.; Magee, B.H. Ewe Fertility in the STAR Accelerated Lambing System. J. Anim. Sci. 1996, 74, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Lomet, L.; Robert, V.; Decourt, C.; Beltramo, M.; Pellicer-Rubio, M.-T. Seasonal breeding in mammals: From basic science to applications and back. Theriogenology 2016, 86, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Inskeep, E.K. Control of the ovarian cycle of the sheep. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: London, UK, 2015; Volume 2, pp. 1259–1305. [Google Scholar] [CrossRef]
- Sirard, M.A. Folliculogenesis and acquisition of oocyte competence in cows. Anim. Reprod. 2019, 16, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Sirard, M.-A. The two-step process of ovarian follicular growth and maturation in mammals can be compared to a fruit ripening where quality depends on the second step. Biol. Reprod. 2022, 106, 230–234. [Google Scholar] [CrossRef]
- Bartlewski, P.M.; Beard, A.P.; Cook, S.J.; Rawlings, N.C. Ovarian follicular dynamics during anestrus in ewes. J. Reprod. Fertil. 1998, 113, 275–285. [Google Scholar] [CrossRef]
- Seekallu, S.V.; Toosi, B.M.; Ziegler, A.; Reeves, J.J.; Rawling, N.C. Pulsed GnRH secretion and the FSH secretory peaks that initiate ovarian antral follicular wave emergence in anestrous ewes. Anim. Reprod. Sci. 2010, 120, 56–64. [Google Scholar] [CrossRef]
- Walton, J.S.; McNeilly, J.R.; McNeilly, A.S.; Cunningham, F.J. Changes in concentration of follicle-stimulating hormone, luteinizing hormone, prolactin and progesterone in the plasma of ewes during the transition from anoestrus to breeding activity. J. Endocrinol. 1977, 75, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.M.; Bartlewski, P.M.; Duggavathi, R.; Davies, K.L.; Rawlings, N.C. Suppression of follicle wave emergence in cyclic ewes by supraphysiologic concentrations of estradiol-17beta and induction with a physiologic dose of exogenous ovine follicle-stimulating hormone. Biol. Reprod. 2006, 75, 633–641. [Google Scholar] [CrossRef]
- Orisaka, M.; Miyazaki, Y.; Shirafuji, A.; Tamamura, C.; Tsuyoshi, H.; Tsang, B.; Yoshida, Y. The role of pituitary gonadotropins and intraovarian regulators in follicle development: A mini-review. Reprod. Med. Biol. 2021, 20, 169–175. [Google Scholar] [CrossRef]
- Campbell, B.K.; Kendall, N.R.; Baird, D.T. The effect of the presence and pattern of Luteinizing Hormone stimulation on ovulatory follicle development in sheep. Biol. Reprod. 2007, 76, 719–727. [Google Scholar] [CrossRef]
- Gong, J.G.; Campbell, B.K.; Webb, R. Defining the gonadotrophin requirement for the selection of a single dominant follicle in cattle. Reprod. Fertil. Dev. 2020, 32, 322–334. [Google Scholar] [CrossRef]
- Weems, P.; Smith, J.; Clarke, I.J.; Coolen, L.M.; Goodman, R.L.; Lehman, M.N. Effects of Season and Estradiol on KNDy Neuron Peptides, Colocalization with D2 Dopamine Receptors, and Dopaminergic Inputs in the Ewe. Endocrinology 2017, 158, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Nestor, C.C.; Bedenbaugh, M.N.; Hileman, S.M.; Coolen, L.M.; Lehman, M.N.; Goodman, R.L. Regulation of GnRH pulsatility in ewes. Reproduction 2018, 156, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.; Forcada, F.; González-Bulnes, A. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 2012, 130, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.D. Equine chorionic gonadotropin: An enigmatic but essential tool. Anim. Reprod. 2012, 9, 223–230. [Google Scholar]
- Cox, J.F.; Allende, R.; Lara, E.; Leiva, A.; Díaz, T.; Dorado, J.; Saravia, S. Follicular Dynamics and Interval to Ovulation in Short-Term Progesterone and PGF2a Based Estrous-Synchronization Protocol in Sheep. Reprod. Domest. Anim. 2012, 47, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Clark, Z.L.; Karl, K.R.; Ruebel, M.L.; Latham, K.E.; Ireland, J.J. Excessive follicle-stimulating hormone during ovarian stimulation of cattle may induce premature luteinization of most ovulatory-size follicles. Biol. Reprod. 2022, 106, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Cognie, I. State of the art in sheep-goat embryo transfer. Theriogenology 1999, 51, 105–116. [Google Scholar] [CrossRef]
- Amaridis, G.S.; Cseh, S. Assisted reproductive technologies in the reproductive management of small ruminants. Anim. Reprod. Sci. 2012, 130, 152–161. [Google Scholar] [CrossRef]
- Mapletoft, R.J.; Bennett Steward, K.; Adams, G.P. Recent advances in superovulation in cattle. Reprod. Nutr. Dev. 2002, 42, 601–611. [Google Scholar] [CrossRef]
- Drion, P.V.; Furtoss, V.; Baril, G.; Manfredi, E.; Bouvier, F.; Pougnard, J.-L.; Bernelas, D.; Caugnon, P.; McNamara, E.M.; Remy, B.; et al. Four years of induction/synchronization of estrus in dairy goats: Effect on the evolution of eCG binding rate in relation with the parameters of reproduction. Reprod. Nutr. Dev. 2001, 41, 401–412. [Google Scholar] [CrossRef] [PubMed]
- González-Bulnes, A.; Menchaca, A.; Martin, G.B.; Martínez-Ros, P. Seventy years of progestagen treatments for the management of the sheep estrous cycle: Where we are and where we should go. Reprod. Fertil. Dev. 2020, 32, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, X.M.; De Briyne, N.; Beaver, B.; Turner, P.V. Horse welfare during equine chorionic gonadotropin (eCG) production. Animals 2019, 9, 1053. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.M.K.; Sales, J.N.S.; Viau, P.; Barros, B.P.; Nicolau, S.S.; Simões, L.M.S.; Alves, N.G.; Alonso, M.A.; Valentim, R.; Oliveira, C.A. Although it induces synchronized ovulation, hCG reduces the fertility of Santa Ines ewes submitted to TAI. Arq. Bras. Vet. Zootec. 2018, 70, 122–130. [Google Scholar] [CrossRef]
- Bruno-Galarraga, M.; Cano-Moreno, V.; Lago-Cruz, B.; Encinas, T.; González-Bulnes, A.; Martínez-Ros, P. The Use of hCG for Inducing Ovulation in Sheep Estrus Synchronization Impairs Ovulatory Follicle Growth and Fertility. Animals 2021, 11, 984. [Google Scholar] [CrossRef]
- Santos-Jiménez, Z.; Meza-Herrera, C.A.; Calderón-Leyva, G.; Martínez-Ros, P.; Guillen-Muñoz, J.M.; González-Bulnes, A. Efficiency of hCG for Inducing Resumption of Ovarian Cyclicity and Synchronized Ovulations during the Seasonal Anestrous in Sheep. Animals 2021, 11, 3159. [Google Scholar] [CrossRef]
- Dehkordi, R.S.; Mirzaei, A.; Boostani, A. Reproductive efficiency of treated Karakul ewes with short-term progesterone and hCG injections during the non-breeding and breeding seasons. Anim. Reprod. Sci. 2022, 239, 106969. [Google Scholar] [CrossRef]
- Choi, J.; Smitz, J. Luteinizing hormone and human chorionic gonadotropin: Origins of difference. Mol. Cell. Endocrinol. 2014, 383, 203–213. [Google Scholar] [CrossRef]
- Saleh, M.; Shahin, M.; Wuttke, W.; Gauly, M.; Holtz, W. Pharmacokinetics of human chorionic gonadotropin after i.m. administration in goats (Capra hircus). Reproduction 2012, 144, 77–81. [Google Scholar] [CrossRef]
- Cox, J.F.; Jeria, E.; Bocic, A.; Soto-Saravia, R.; Dorado, J.; Saravia, F. Characterization of the productive performance of Highlander sheep in Southern Chile. I. Female reproductive traits. Small Rumin. Res. 2015, 130, 183–188. [Google Scholar] [CrossRef]
- McNatty, K.P.; Heath, D.A.; Hudson, N.L.; Reader, K.L.; Quirke, L.; Lun, S.; Juengel, J.L. The conflict between hierarchical ovarian follicular development and superovulation treatment. Reproduction 2010, 140, 287–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cox, J.; Carrasco, A.; Navarrete, F.; Saravia, F.; Dorado, J. Validation of an immunoradiometric assay (IRMA) to characterize plasma concentrations of hCG after treatment in sheep and goats. In Proceedings of the XLVII Congress of the Chilean Society of Animal Production, Coyhaique, Chile, 29–30 November 2022; p. 1076. [Google Scholar]
- Delgadillo, J.A.; Hernández, H.; Abecia, J.A.; Keller, M.; Chemineau, P. Is it time to reconsider the relative weight of sociosexual relationships compared with photoperiod in the control of reproduction of small ruminant females? Domest. Anim. Endocrin. 2020, 73, 106468. [Google Scholar] [CrossRef] [PubMed]
- Gomez-León, V.E.; Ginther, O.J.; Domingues, R.; Guimaraes, J.D.; Wiltbank, M.C. Necessity for LH in selection and continued growth of the bovine dominant follicle. Reproduction 2020, 159, 559–569. [Google Scholar] [CrossRef]
- Ben Saïd, S.; Lomet, D.; Chesneau, D.; Lardic, L.; Canepa, S.; Guillaume, D.; Briant, C.; Fabre-Nys, C.; Caraty, A. Differential estradiol requirement for the induction of estrus behavior and the Luteinizing Hormone surge in two breeds of sheep. Biol. Reprod. 2007, 76, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Herbison, A.E.; Lehman, M.N.; Navarro, V.M. Neuroendocrine control of gonadotropin-releasing hormone: Pulsatile and surge modes of secretion. J. Neuroendocrinol. 2022, 34, e13094. [Google Scholar] [CrossRef] [PubMed]
- Chemineau, P.; Guillaume, D.; Migaud, M.; Thiery, J.C.; Pellicer-Rubio, M.T.; Malpaux, B. Seasonality of Reproduction in Mammals: Intimate Regulatory Mechanisms and Practical Implications. Reprod. Domest. Anim. 2008, 43, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Landry, D.A.; Sirard, M.-A. Follicle capacitation: A meta-analysis to investigate the transcriptome dynamics following follicle-stimulating hormone decline in bovine granulosa cells. Biol. Reprod. 2018, 99, 877–887. [Google Scholar] [CrossRef]
- Casarini, L.; Simoni, M. Recent advances in understanding gonadotropin signaling. Fac. Rev. 2021, 10, 41. [Google Scholar] [CrossRef]
- Hattori, K.; Orisaka, M.; Fukuda, S.; Tajima, K.; Yamazaki, Y.; Mizutani, T.; Yoshida, Y. Luteinizing Hormone Facilitates Antral Follicular Maturation and Survival via Thecal Paracrine Signaling in Cattle. Endocrinology 2018, 159, 2337–2347. [Google Scholar] [CrossRef]
- Cox, J.F.; Dorado, J.; Saravia, F. Effect of hCG at low dosage on follicular dynamics and ovulation in estrous synchronized sheep during anestrus. Anim. Reprod. 2016, 13, 387. [Google Scholar]
- Dorado, J.; Cox, J.F.; Saravia, F. GnRH may increase the control of ovulation in ewes synchronized by short-term progesterone treatment. Anim. Reprod. 2016, 13, 417. [Google Scholar]
- Burutaran, M.; Fierro, S.; Negrín, F.; Mintehuiaga, M.; Gil, J.; Olivera-Muzante, J. Estrous, ovulation and reproductive responses of ewes synchronized with a long interval prostaglandin-based protocol for timed AI. Theriogenology 2024, 214, 187–191. [Google Scholar] [CrossRef]
- Romano, J.E.; Keisler, D.H.; Amstalden, M. Effect of copulation on estrus duration, LH response, and ovulation in Boer goats. Theriogenology 2018, 121, 62–66. [Google Scholar] [CrossRef]
- Adib, A.; Freret, S.; Touze, J.-L.; Lomet, D.; Lardic, L.; Chesneau, D.; Estienne, A.; Papillier, P.; Monniaux, D.; Pellicer-Rubio, M.-T. Progesterone improves the maturation of male-induced preovulatory follicles in anoestrous ewes. Reproduction 2014, 148, 403–416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gordon, I. Reproductive Technology in Farm Animals, 2nd ed.; CABI: Boston, MA, USA, 2017; pp. 175–190. [Google Scholar]
- Jackson, C.G.; Neville, T.L.; Mercadante, V.R.G.; Waters, K.M.; Lamb, G.C.; Dahlen, C.R.; Redden, R.R. Efficacy of various five-day estrous synchronization protocols in sheep. Small Rumin. Res. 2014, 120, 100–107. [Google Scholar] [CrossRef]
- Abecia, J.A.; Araya, J.; Chemineau, P.; Palacios, C.; Keller, M.; Delgadillo, J.A. Photoperiod-melatonin-induced, sexually-activated rams increase pregnancy rate and number of lambs per ewe in a ram effect. Large Anim. Rev. 2018, 24, 31–35. [Google Scholar]


| Parameters | Annual Reproductive Cycle | p-Value | ||
|---|---|---|---|---|
| Breeding | Anestrus | |||
| Number of ewes (n) | 37 | 42 | ||
| Follicles ≥ 3.5 at day 0: | ||||
| Number (n) | 2.2 ± 0.19 | 2.3 ± 0.13 | 0.602 | |
| Diameter (mm) | 4.6 ± 0.09 | 4.4 ± 0.06 | 0.140 | |
| Follicles ≥ 4.0 at day 2: | ||||
| Number (n) | 2.4 ± 0.11 | 2.2 ± 0.15 | 0.175 | |
| Diameter (mm) | 5.5 ± 0.08 | 5.0 ± 0.08 | <0.001 | |
| Estrous presentation (%) | 100 (37/37) | 88.1 (37/42) | 0.057 | |
| Interval CIDR–estrus (h) | 35.9 ± 1.62 | 41.1 ± 1.53 | 0.021 | |
| Ovulated ewes (%) | 97.3 (36/37) | 97.6 (41/42) | >0.999 | |
| Ovulating efficiency (%) | 83.5 (71/85) | 77.2 (71/92) | 0.347 | |
| Corpora lutea at day 7: | ||||
| Number (n) | 1.9 ± 0.10 | 1.7 ± 0.10 | 0.076 | |
| Total luteal area (mm) | 173.0 ± 7.24 | 126.7 ± 7.72 | <0.001 | |
| Plasma progesterone (ng/mL) | 6.5 ± 0.46 | 5.4 ± 0.24 | 0.018 | |
| Pregnancy rate (%) | 97.3 (36/37) | 76.2 (32/42) | 0.008 | |
| Lambing rate (%) | 88.9 (32/36) | 96.9 (31/32) | 0.361 | |
| Fecundity rate (%) | 156.8 (58/37) | 97.6 (41/42) | <0.001 | |
| Parameters | hCG-150 | hCG-250 | Control | |
|---|---|---|---|---|
| Number of treatments (n) | 40 | 41 | 37 | |
| Follicles ≥ 3.0 at day 0: | ||||
| Number (n) | 1.6 ± 0.18 | 1.3 ± 0.16 | 1.5 ± 0.19 | |
| Diameter (mm) | 4.6 ± 0.13 | 4.8 ± 0.11 | 4.5 ± 0.12 | |
| Follicles ≥ 4.0 at day 2: | ||||
| Number (n) | 2.5 ± 0.10 a1 | 2.4 ± 0.15 a | 1.9 ± 0.12 b | |
| Diameter (mm) | 5.7 ± 0.09 a | 5.8 ± 0.10 a | 5.2 ± 0.10 b | |
| Estrous presentation (%) | 97.5 (39/40) | 87.8 (36/41) | 91.9 (29/32) | |
| Interval CIDR–estrus (h) | 32.4 ± 1.50 a | 39.1 ± 2.41 b | 41.4 ± 1.91 b | |
| Ovulated ewes (%): | 97.5 (39/40) | 90.2 (37/41) | 97.3 (36/37) | |
| Corpora lutea at day 6: | ||||
| Number (n) | 2.3 ± 0.12 a | 2.0 ± 0.16 a | 1.5 ± 0.07 b | |
| Total luteal area (mm) | 157.3 ± 9.35 a | 143.3 ± 13.48 a | 92.8 ± 5.95 b | |
| Pregnancy rate (%): | 94.7 (18/19) | 76.2 (16/21) | 89.5 (17/19) | |
| Lambing rate (%): | 89.5 (17/19) | 71.4 (15/21) | 84.2 (16/19) | |
| Fecundity rate (n): | 168.4 (32/19) a | 133.3 (28/21) ab | 115.8 (22/19) b | |
| Variables | hCG-150 | hCG-GnRH | eCG-400 | |
|---|---|---|---|---|
| Number of ewes (replicates) | 29 (4) | 20 (3) | 31 (4) | |
| Follicles ≥ 3.0 at day 0: | ||||
| Number (n) | 1.9 ± 0.20 | 2.3 ± 0.22 | 2.3 ± 0.23 | |
| Diameter (mm) | 4.7 ± 0.11 | 4.8 ± 0.13 | 4.8 ± 0.11 | |
| Follicles ≥ 4.0 at day 2: | ||||
| Number (n) | 2.3 ± 0.14 | 2.3 ± 0.19 | 2.5 ± 0.16 | |
| Diameter (mm) | 6.2 ± 0.14 | 6.0 ± 0.17 | 6.0 ± 0.12 | |
| Estrous presentation (%) | 89.7 (26/29) | 85.0 (17/20) | 87.1 (27/31) | |
| Interval CIDR–estrus (h) | 32.6 ± 1.52 | 32.2 ± 1.36 | 33.1 ± 1.46 | |
| Ovulated ewes (%): | 93.1 (27/29) | 85.0 (17/20) | 90.3 (28/31) | |
| Interval CIDR–ovulation (h) | 67.3 ± 2.45 a1 | 55.9 ± 1.27 b | 65.8 ± 1.90 a | |
| Corpora lutea at day 6: | ||||
| Number (n) | 2.2 ± 0.16 | 1.7 ± 0.21 | 1.6 ± 0.16 | |
| Total luteal area (mm) | 166.0 ± 9.78 | 146.3 ± 14.23 | 153.7 ± 10.0 | |
| Progesterone I (ng/mL) 2 | 3.5 ± 0.26 | NA | 4.1 ± 0.46 | |
| Progesterone II (ng/mL) | 4.8 ± 0.45 | NA | 6.9 ± 0.78 | |
| Farm | Reproductive Parameters | Experimental Groups | ||
|---|---|---|---|---|
| hCG-150 | eCG-420 | Control | ||
| 1 | Mating activity (%) | 11/13 (84.6) | 11/13 (84.6) | 5/12 (41.7) |
| Lambing rate (%) | 6/11 (54.5) | 8/11 (72.7) | 3/5 (60.0) | |
| Prolificacy rate (n) | 13/6 (2.17) | 16/8 (2.0) | 3/3 (1.0) | |
| Fecundity rate (%) | 13/13 (100) | 16/13 (123.1) | 3/12 (25.0) | |
| 2 | Mating activity (%) | 22/26 (84.6) | 25/26 (96.2) | 9/22 (40.9) |
| Lambing rate (%) | 12/22 (54.5) | 12/25 (48.0) | 6/9 (66.7) | |
| Prolificacy rate (n) | 20/12 (1.67) | 18/12 (1.5) | 6/6 (1.0) | |
| Fecundity rate (%) | 20/26 (76.9) | 18/26 (69.2) | 6/22 (27.3) | |
| Total | Mating activity (%) | 33/39 (84.6) a1 | 36/39 (92.3) a | 10/34 (29.4) b |
| Lambing rate (%) | 18/33 (54.5) | 20/36 (55.5) | 9/14 (64.3) | |
| Prolificacy rate (n) | 33/18 (1.83) a | 34/20 (1.70) a | 9/9 (1.0) b | |
| Fecundity rate (%) | 33/39 (84.6) a | 34/39 (87.2) a | 9/34 (26.5) b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, J.F.; Carrasco, A.; Navarrete, F.; Bocic, A.; Saravia, F.; Dorado, J. A Subovulatory Dose of Human Chorionic Gonadotropin (hCG) May Sustain Terminal Follicle Development and Reproductive Efficiency during Anestrus in Sheep. Animals 2024, 14, 1096. https://doi.org/10.3390/ani14071096
Cox JF, Carrasco A, Navarrete F, Bocic A, Saravia F, Dorado J. A Subovulatory Dose of Human Chorionic Gonadotropin (hCG) May Sustain Terminal Follicle Development and Reproductive Efficiency during Anestrus in Sheep. Animals. 2024; 14(7):1096. https://doi.org/10.3390/ani14071096
Chicago/Turabian StyleCox, José Francisco, Albert Carrasco, Felipe Navarrete, Antonio Bocic, Fernando Saravia, and Jesús Dorado. 2024. "A Subovulatory Dose of Human Chorionic Gonadotropin (hCG) May Sustain Terminal Follicle Development and Reproductive Efficiency during Anestrus in Sheep" Animals 14, no. 7: 1096. https://doi.org/10.3390/ani14071096
APA StyleCox, J. F., Carrasco, A., Navarrete, F., Bocic, A., Saravia, F., & Dorado, J. (2024). A Subovulatory Dose of Human Chorionic Gonadotropin (hCG) May Sustain Terminal Follicle Development and Reproductive Efficiency during Anestrus in Sheep. Animals, 14(7), 1096. https://doi.org/10.3390/ani14071096