A Short-Term Supplementation with a Polyphenol-Rich Extract from Radiata Pine Bark Improves Fatty Acid Profiles in Finishing Lambs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Management
2.2. Experimental Procedure and Dietary Treatments
2.3. Chemical Composition of Diets
2.4. Determination of Dry Matter Intake and Performance
2.5. Blood Collection and Analysis
2.6. Slaughter and Carcass Evaluation
2.7. Lipid Extraction and Analysis
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition of Diets
3.2. Performance, Intake, and Nutrient Conversion
3.3. Hematological and Serum Biochemistry Profile
3.4. Fatty Acid Profiles
4. Discussion
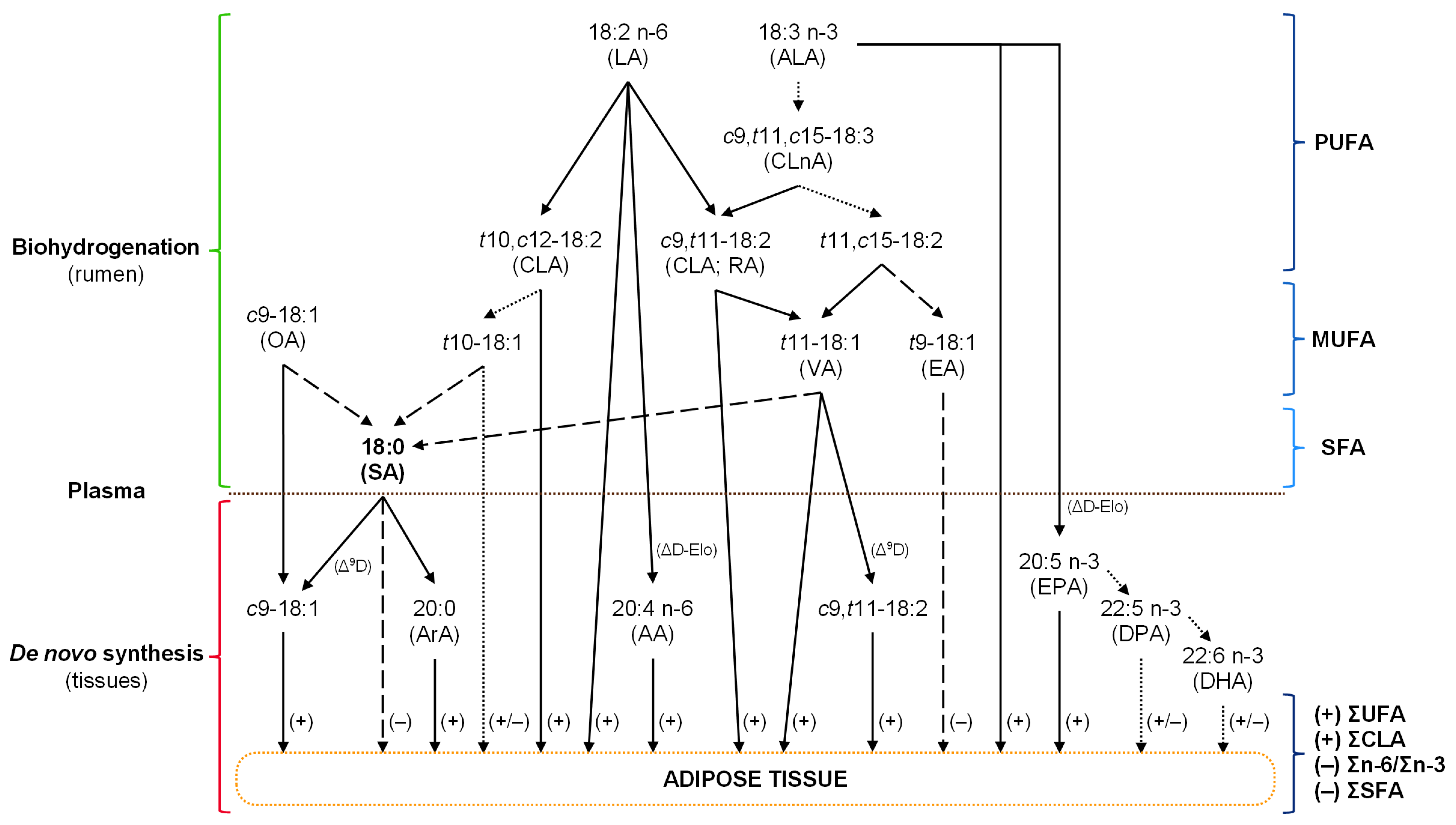
4.1. Effects of Radiata PBE Supplementation on Fatty Acid Profile
4.2. Effects of Radiata PBE Supplementation on Performance and Efficiency
4.3. Effects of Radiata PBE Supplementation on Hemogram and Serum Biochemistry
4.4. Effects of Radiata PBE Supplementation on the Chemical Composition of Diets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and sustainable valorization of bioactive phenolic compounds from pinus by-products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- Romero, R.; Contreras, D.; Segura, C.; Schwederski, B.; Kaim, W. Hydroxyl radical production by a heterogeneous Fenton reaction supported in insoluble tannin from bark of Pinus radiata. Environ. Sci. Pollut. Res. 2017, 24, 6135–6142. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2013, 69, 32–48. [Google Scholar] [CrossRef]
- Torres, R.N.S.; Ghedini, C.P.; Paschoaloto, J.R.; da Silva, D.A.V.; Coelho, L.M.; Almeida Junior, G.A.; Ezequiel, J.M.B.; Machado Neto, O.R.; Almeida, M.T.C. Effects of tannins supplementation to sheep diets on their performance, carcass parameters and meat fatty acid profile: A meta-analysis study. Small Rumin. Res. 2022, 206, 106585. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Gurung, N.; Behrends, J.; Eun, J.-S.; Taha, E.; Rose, J. Effects of pine bark supplementation on performance, rumen fermentation, and carcass characteristics of Kiko crossbred male goats. J. Anim. Sci. 2012, 90, 3556–3567. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Solaiman, S.; Terrill, T.; Ramsay, A.; Mueller-Harvey, I. The effects of tannins–containing ground pine bark diet upon nutrient digestion, nitrogen balance, and mineral retention in meat goats. J. Anim. Sci. Biotechnol. 2015, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Solaiman, S.; Taha, E.; Lee, J. Effect of plant tannin-containing diet on fatty acid profile in meat goats. J. Anim. Res. Nutr. 2015, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, D.; Min, B.R.; Gurung, N.; McElhenney, W.; Lee, J.H.; Solaiman, S.; Bolden-Tiller, O. Influence of tannin–rich pine bark supplementation in the grain mixes for meat goats: Growth performance, blood metabolites, and carcass characteristics. Anim. Nutr. 2020, 6, 85–91. [Google Scholar] [CrossRef]
- Lee, J.H.; Min, B.R.; Lemma, B.B. Quality characteristics of goat meat as influenced by condensed tannins-containing pine bark. Small Rumin. Res. 2017, 146, 28–32. [Google Scholar] [CrossRef]
- Berg, A.; Olave, L.; Navarrete, P. Process for Obtaining Low and Medium Molecular Weight Polyphenols and Standardized Solid Fuel from Tree Wood or Bark. U.S. Patent US 20090077871 A1, 26 March 2009. [Google Scholar]
- Vera, N.; Gutiérrez, C.; Allende, R.; Williams, P.; Fuentealba, C.; Ávila-Stagno, J. Dose-response effect of a pine bark extract on in vitro ruminal ammonia and methane formation kinetics. Acta Agric. Scand. A Anim. Sci. 2018, 68, 181–189. [Google Scholar] [CrossRef]
- Vera, N.; Gutiérrez, C.; Williams, P.; Fuentealba, C.; Allende, R.; Ávila-Stagno, J. Low concentrations of a polyphenolic extract from pine bark in high–concentrate diets decrease in vitro rumen ammonia nitrogen but not methane production. J. Appl. Anim. Res. 2021, 49, 413–422. [Google Scholar] [CrossRef]
- Vera, N.; Gutiérrez-Gómez, C.; Williams, P.; Allende, R.; Fuentealba, C.; Ávila-Stagno, J. Comparing the Effects of a Pine (Pinus radiata D. Don) Bark Extract with a Quebracho (Schinopsis balansae Engl.) Extract on Methane Production and In Vitro Rumen Fermentation Parameters. Animals 2022, 12, 1080. [Google Scholar] [CrossRef]
- Peng, K.; Shirley, D.C.; Xu, Z.; Huang, Q.; McAllister, T.A.; Chaves, A.V.; Acharya, S.; Liu, C.; Wang, S.; Wang, Y. Effect of purple prairie clover (Dalea purpurea Vent.) hay and its condensed tannins on growth performance, wool growth, nutrient digestibility, blood metabolites and ruminal fermentation in lambs fed total mixed rations. Anim. Feed Sci. Technol. 2016, 222, 100–110. [Google Scholar] [CrossRef]
- Mezzomo, R.; Paulino, P.V.R.; Detmann, E.; Valadares Filho, S.C.; Paulino, M.F.; Monnerat, J.P.I.S.; Duarte, M.S.; Silva, L.H.P.; Moura, L.S. Influence of condensed tannin on intake, digestibility, and efficiency of protein utilization in beef steers fed high concentrate diet. Livest. Sci. 2011, 141, 1–11. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Bryner, S.F.; Scheeder, M.R.L.; Wettstein, H.-R.; Kreuzer, M.; Soliva, C.R. Evidence for the inhibition of the terminal step of ruminal α-linolenic acid biohydrogenation by condensed tannins. J. Dairy Sci. 2009, 92, 177–188. [Google Scholar] [CrossRef] [Green Version]
- NRC (National Research Council). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids; The National Academic Press: Washington, DC, USA, 2007. [Google Scholar]
- Hall, M.B. Calculation of Non-Structural Carbohydrate Content of Feeds That Contain Non-Protein Nitrogen; University of Florida: Gainesville, FL, USA, 2000. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Hartman, L.; Lago, R.C. Further observations concerning effects of unsaponifiable constituents on the properties of coffee seed oil. J. Am. Oil Chem. Soc. 1973, 50, 99–100. [Google Scholar] [CrossRef]
- Villeneuve, M.-P.; Lebeuf, Y.; Gervais, R.; Tremblay, G.F.; Vuillemard, J.C.; Fortin, J.; Chouinard, P.Y. Milk volatile organic compounds and fatty acid profile in cows fed timothy as hay, pasture, or silage. J. Dairy Sci. 2013, 96, 7181–7194. [Google Scholar] [CrossRef]
- Berry, D.P.; Crowley, J.J. CELL BIOLOGY SYMPOSIUM: Genetics of feed efficiency in dairy and beef cattle. J. Anim. Sci. 2013, 91, 1594–1613. [Google Scholar] [CrossRef] [PubMed]
- Fitzhugh, H.A.; Taylor, C.S. Genetic analysis of degree of maturity. J. Anim. Sci. 1971, 33, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Moritz, A. Quality Control and Laboratory Techniques. In Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley Blackwell Publishing: Ames, IA, USA, 2010; pp. 1019–1175. [Google Scholar]
- Smith, B.P. Large Animal Internal Medicine, 5th ed.; Elsevier Mosby: St. Louis, MO, USA, 2015. [Google Scholar]
- MINAGRI (Ministerio de Agricultura). Aprueba Reglamento Sobre Estructura y Funcionamiento de Mataderos, Establecimientos Frigoríficos, Cámaras Frigoríficas y Plantas de Desposte y fija Equipamiento Mínimo de Tales Establecimientos; Decreto N° 94; Publicado en el Diario Oficial: Santiago, Chile, 2009. (In Spanish) [Google Scholar]
- MINAGRI (Ministerio de Agricultura). Aprueba Reglamento Sobre Protección de los Animales que Provean de Carne, Pieles, Plumas y Otros Productos al Momento del Beneficio en Establecimientos Industriales; Decreto N° 28; Publicado en el Diario Oficial: Santiago, Chile, 2013. (In Spanish) [Google Scholar]
- Maciel, M.V.; Carvalho, F.F.R.; Batista, Â.M.V.; Guim, A.; Souza, E.J.O.; Maciel, L.P.A.A.; Pereira Neto, J.D.; Lima Júnior, D.M. Carcass and non-carcass characteristics of sheep fed on cassava (Manihot pseudoglaziovii Pax & K. Hoffm.). Chil. J. Agric. Res. 2015, 75, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.H.S. Simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Avila-Stagno, J.; Chaves, A.V.; Graham, A.S.; McAllister, T.A. Effects of replacing barley grain with wheat dry distillers’ grains on growth performance, eating behavior, and subcutaneous fatty acid profiles of lambs. Acta Agric. Scand. A Anim. Sci. 2013, 63, 3–100. [Google Scholar] [CrossRef]
- David, F.; Sandra, P.; Vickers, A.K. Column selection for the analysis of fatty acid methyl esters. Agil. Technol. 2005, 19, 19. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.; Mendes, I.A.; Bessa, R.J.B. The effect of genotype, feeding system and slaughter weight on the quality of light lambs: 1. Growth, carcass composition and meat quality. Livest. Prod. Sci. 2002, 76, 17–25. [Google Scholar] [CrossRef]
- Rhee, K.S. Fatty acids in meats and meat products. In Fatty Acids in Foods and Their Health Implications; Chow, C.K., Ed.; Marcel Dekker: New York, NY, USA, 1992; pp. 65–93. [Google Scholar]
- Smet, S.; Raes, K.; Demeyer, D. Meat fatty acid composition as affected by fatness and genetic factors: A review. Anim. Res. 2004, 53, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Avila-Stagno, J.; Chaves, A.V.; He, M.L.; Harstad, O.M.; Beauchemin, K.A.; McGinn, S.M.; McAllister, T.A. Effects of increasing concentrations of glycerol in concentrate diets on nutrient digestibility, methane emissions, growth, fatty acid profiles, and carcass traits of lambs. J. Anim. Sci. 2013, 91, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Gama, K.V.M.F.; Pereira Filho, J.M.; Soares, R.F.; Cordão, M.A.; Cézar, M.F.; Batista, A.S.M.; de Azevedo Silva, A.M.; Madruga, M.S.; Oliveira, R.L.; Bezerra, L.R. Fatty acid, chemical, and tissue composition of meat comparing Santa Inês breed sheep and Boer crossbreed goats submitted to different supplementation strategies. Trop. Anim. Health Prod. 2020, 52, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Frutos, P.; Hervás, G.; Natalello, A.; Luciano, G.; Fondevila, M.; Priolo, A.; Toral, P.G. Ability of tannins to modulate ruminal lipid metabolism and milk and meat fatty acid profiles. Anim. Feed Sci. Technol. 2020, 269, 114623. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Paengkoum, P.; Paengkoum, S. The links between supplementary tannin levels and conjugated linoleic acid (CLA) formation in ruminants: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0216187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, J.; Pereira, F.J.; Menezes, D.; Caldas, A.C.; Cavalcante, I.; Oliveira, J.; Silva, J.J.; Cézar, M.; Bezerra, L. Carcass and meat quality in lambs receiving natural tannins from Mimosa tenuiflora hay. Small Rumin. Res. 2021, 198, 106362. [Google Scholar] [CrossRef]
- Yusuf, A.L.; Adeyemi, K.D.; Roselina, K.; Alimon, A.R.; Goh, Y.M.; Samsudin, A.A.; Sazili, A.Q. Dietary supplementation of different parts of Andrographis paniculata affects the fatty acids, lipid oxidation, microbiota, and quality attributes of longissimus muscle in goats. Food Res. Int. 2018, 111, 699–707. [Google Scholar] [CrossRef]
- Kamel, H.E.M.; Al-Dobaib, S.N.; Salem, A.Z.M.; López, S.; Alaba, P.A. Influence of dietary supplementation with sunflower oil and quebracho tannins on growth performance and meat fatty acid profile of Awassi lambs. Anim. Feed Sci. Technol. 2018, 235, 97–104. [Google Scholar] [CrossRef]
- Vasta, V.; Priolo, A.; Scerra, M.; Hallett, K.G.; Wood, J.D.; Doran, O. Δ9 Desaturase protein expression and fatty acid composition of longissimus dorsi muscle in lambs fed green herbage or concentrate with or without added tannins. Meat Sci. 2009, 82, 357–364. [Google Scholar] [CrossRef]
- Vasta, V.; Yáñez-Ruiz, D.R.; Mele, M.; Serra, A.; Luciano, G.; Lanza, M.; Biondi, L.; Priolo, A. Bacterial and protozoal communities and fatty acid profile in the rumen of sheep fed a diet containing added tannins. Appl. Environ. Microbiol. 2010, 76, 2549–2555. [Google Scholar] [CrossRef] [Green Version]
- Mapiye, C.; Vahmani, P.; Aalhus, J.L.; Rolland, D.C.; Baron, V.S.; Block, H.C.; McAllister, T.A. Fatty acid composition of beef steers as affected by diet and fat depot. S. Afr. J. Anim. Sci. 2015, 45, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Costa, E.I.D.S.; Ribeiro, C.V.D.M.; Silva, T.M.; Batista, A.S.M.; Vieira, J.F.; Barbosa, A.M.; da Silva Júnior, J.M.; Bezerra, L.R.; Pereira, E.S.; Oliveira, R.L. Effect of dietary condensed tannins inclusion from Acacia mearnsii extract on the growth performance, carcass traits and meat quality of lambs. Livest. Sci. 2021, 253, 104717. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Diet, Nutrition and the Prevention of Chronic Diseases; Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series No. 916; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Seoni, E.; Battacone, G.; Silacci, P.; Kragten, S.A.; Chelali, J.M.; Dohme-Meier, F.; Bee, G. Effect of condensed tannins from Birdsfoot trefoil and dietary protein level on growth performance, carcass composition and meat quality of ram lambs. Small Rumin. Res. 2018, 169, 118–126. [Google Scholar] [CrossRef]
- Hur, S.J.; Kim, H.S.; Bahk, Y.Y.; Park, Y. Overview of conjugated linoleic acid formation and accumulation in animal products. Livest. Sci. 2017, 195, 105–111. [Google Scholar] [CrossRef]
- Pimentel, P.R.S.; Pellegrini, C.B.; Lanna, D.P.D.; Brant, L.M.S.; Ribeiro, C.V.D.M.; Silva, T.M.; Barbosa, A.M.; da Silva Júnior, J.M.; Bezerra, L.R.; Oliveira, R.L. Effects of Acacia mearnsii extract as a condensed-tannin source on animal performance, carcass yield and meat quality in goats. Anim. Feed Sci. Technol. 2021, 271, 114733. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Norouzian, M.A.; Ghiasi, S.E. Carcass performance and meat mineral content in Balouchi lamb fed pistachio by-products. Meat Sci. 2012, 92, 157–159. [Google Scholar] [CrossRef]
- Krueger, W.K.; Gutierrez-Bañuelos, H.; Carstens, G.E.; Min, B.R.; Pinchak, W.E.; Gomez, R.R.; Anderson, R.C.; Krueger, N.A.; Forbes, T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 2010, 159, 1–9. [Google Scholar] [CrossRef]
- Foiklang, S.; Wanapat, M.; Norrapoke, T. Effect of Grape Pomace Powder, Mangosteen Peel Powder and Monensin on Nutrient Digestibility, Rumen Fermentation, Nitrogen Balance and Microbial Protein Synthesis in Dairy Steers. Asian-Australas. J. Anim. Sci. 2016, 29, 1416–1423. [Google Scholar] [CrossRef] [Green Version]
- Dschaak, C.M.; Williams, C.M.; Holt, M.S.; Eun, J.-S.; Young, A.J.; Min, B.R. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Ortiz, F.A.; Sandoval-Castro, C.A.; Ventura-Cordero, J.; Sarmiento-Franco, L.A.; Torres-Acosta, J.F.J. Condensed tannin intake and sheep performance: A meta–analysis on voluntary intake and live weight change. Anim. Feed Sci. Technol. 2018, 245, 67–76. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Yang, W.Z.; Wang, H. Effects of substituting corn with steam-flaked sorghum on growth, digestion and blood metabolites in young cattle fed feedlot diets. Anim. Prod. Sci. 2016, 58, 299–306. [Google Scholar] [CrossRef]
- Orlandi, T.; Kozloski, G.V.; Alves, T.P.; Mesquita, F.R.; Ávila, S.C. Digestibility, ruminal fermentation and duodenal flux of amino acids in steers fed grass forage plus concentrate containing increasing levels of Acacia mearnsii tannin extract. Anim. Feed Sci. Technol. 2015, 210, 37–45. [Google Scholar] [CrossRef]
- Ahnert, S.; Dickhoefer, U.; Schulz, F.; Susenbeth, A. Influence of ruminal Quebracho tannin extract infusion on apparent nutrient digestibility, nitrogen balance, and urinary purine derivatives excretion in heifers. Livest. Sci. 2015, 177, 63–70. [Google Scholar] [CrossRef]
- Acharya, M.; Burke, J.M.; Coffey, K.P.; Kegley, E.B.; Miller, J.E.; Huff, G.R.; Smyth, E.; Terrill, T.H.; Mosjidis, J.A.; Rosenkrans, C. Changes in hematology, serum biochemistry, and gastrointestinal nematode infection in lambs fed sericea lespedeza with or without dietary sodium molybdate. J. Anim. Sci. 2015, 93, 1952–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roland, L.; Drillich, M.; Iwersen, M. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Investig. 2014, 26, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, G.E.; McGilchrist, P.; Pethick, D.W. Ruminant glycogen metabolism. Anim. Prod. Sci. 2014, 54, 1575–1583. [Google Scholar] [CrossRef]
- Antunović, Z.; Novoselec, J.; Šperanda, M.; Vegara, M.; Pavić, V.; Mioč, B.; Djidara, M. Changes in biochemical and hematological parameters and metabolic hormones in Tsigai ewes blood in the first third of lactation. Arch. Anim. Breed. 2011, 54, 535–545. [Google Scholar] [CrossRef]
- Braun, J.P.; Trumel, C.; Bézille, P. Clinical biochemistry in sheep: A selected review. Small Rumin. Res. 2010, 92, 10–18. [Google Scholar] [CrossRef]
| Item | Radiata PBE, % DM | SEM | p-Value 1 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | Diet | Linear 2 | 0 vs. PBE | ||
| Ingredients, g/kg DM | |||||||
| Alfalfa hay | 437.0 | 432.6 | 428.3 | – | – | – | – |
| Soybean meal | 146.0 | 144.5 | 143.1 | – | – | – | – |
| Wheat bran | 175.0 | 173.3 | 171.5 | – | – | – | – |
| Corn grain | 233.0 | 230.7 | 228.3 | – | – | – | – |
| Mineral premix 3 | 9.0 | 8.9 | 8.8 | – | – | – | – |
| Pine bark extract | – | 10.0 | 20.0 | – | – | – | – |
| Chemical composition, % DM (n = 3) | |||||||
| Dry matter, % FW | 89.2 a | 87.5 ab | 87.1 b | 0.48 | 0.032 | 0.015 | 0.001 |
| Organic matter | 92.6 | 93.0 | 93.1 | 0.18 | 0.083 | – | – |
| Crude protein | 20.2 a | 18.5 b | 18.7 b | 0.34 | 0.014 | 0.013 | 0.003 |
| Ether extract | 2.29 | 2.26 | 2.22 | 0.058 | 0.741 | – | – |
| Neutral detergent fiber | 33.4 | 35.1 | 35.2 | 0.90 | 0.247 | – | – |
| Acid detergent fiber | 20.9 b | 23.7 ab | 24.4 a | 0.77 | 0.017 | 0.007 | 0.012 |
| Hemicellulose | 12.5 | 11.5 | 10.3 | 1.37 | 0.566 | – | – |
| Nonfibrous carbohydrates 4 | 36.7 | 37.2 | 37.4 | 0.99 | 0.881 | – | – |
| Phenolic constituents, % DM | |||||||
| Total polyphenols | 0.64 b | 1.35 b | 2.61 a | 0.197 | <0.001 | <0.001 | 0.014 |
| Total tannins | 0.19 b | 0.26 ab | 0.34 a | 0.027 | 0.004 | 0.001 | 0.016 |
| Fatty acids, g/100 g fatty acids | |||||||
| 16:0 | 20.2 | 19.9 | 19.7 | 0.03 | 0.083 | – | – |
| 18:0 | 3.59 | 3.55 | 3.52 | 0.005 | 0.102 | – | – |
| c9–18:1 | 14.5 | 14.3 | 14.2 | 0.05 | 0.149 | – | – |
| c11–18:1 | 0.50 | 0.49 | 0.49 | 0.002 | 0.274 | – | – |
| 18:2 n-6 | 20.8 | 20.5 | 20.3 | 0.05 | 0.156 | – | – |
| 18:3 n-3 | 16.7 | 16.5 | 16.3 | 0.04 | 0.140 | – | – |
| Item | Radiata PBE, % DM | SEM | p-Value 1 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | Diet | Linear 2 | 0 vs. PBE | ||
| Performance | |||||||
| Initial live weight, kg | 35.7 | 36.1 | 36.3 | 1.22 | 0.923 | – | – |
| Final live weight, kg | 42.2 | 43.3 | 42.7 | 1.57 | 0.873 | – | – |
| Average daily gain 3, g/d | 292 | 325 | 334 | 39.8 | 0.679 | – | – |
| Dry matter intake, g/d | 1718 | 1732 | 1796 | 45.9 | 0.402 | – | – |
| Crude protein intake, g/d | 340 | 320 | 321 | 8.7 | 0.144 | – | – |
| Efficiency | |||||||
| Feed conversion ratio 4, g/g | 5.39 | 4.74 | 4.65 | 0.587 | 0.615 | – | – |
| Crude protein conversion 5, g/g | 1.35 a | 0.89 b | 0.85 b | 0.124 | 0.006 | 0.003 | 0.002 |
| Relative growth rate 6 | 0.26 b | 0.36 ab | 0.41 a | 0.045 | 0.026 | 0.008 | 0.007 |
| Carcass characteristics | |||||||
| Hot carcass weight, kg | 21.7 | 22.5 | 21.3 | 0.83 | 0.546 | – | – |
| Hot carcass yield 7, % | 50.3 | 51.2 | 50.0 | 0.70 | 0.116 | – | – |
| Cold carcass weight, kg | 21.1 | 22.0 | 20.7 | 0.79 | 0.537 | – | – |
| Cold carcass yield 8, % | 49.5 | 50.0 | 48.3 | 0.58 | 0.118 | – | – |
| Weight losses by cooling 9, % | 2.67 | 2.73 | 2.75 | 0.108 | 0.864 | – | – |
| Item | RI 1 | Radiata PBE, % DM | SEM | p-Value 2 | ||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | Diet | |||
| Hemogram | ||||||
| Hematology | ||||||
| Red blood cells, 106/μL | 9–15 | 12.5 | 12.2 | 12.5 | 0.42 | 0.720 |
| Hemoglobin, g/dL | 9–15 | 11.2 | 11.2 | 11.5 | 0.30 | 0.701 |
| Hematocrit, % | 27–45 | 32.6 | 32.4 | 33.5 | 0.85 | 0.503 |
| MCV, fL | 28–40 | 26.1 | 26.7 | 26.7 | 0.53 | 0.634 |
| MCH, g/dL | 8–12 | 9.0 | 9.2 | 9.1 | 0.17 | 0.703 |
| MCHC, % | 31–34 | 34.4 | 34.5 | 34.1 | 0.36 | 0.713 |
| Platelets, 103 | 100–800 | 530 | 584 | 557 | 86.7 | 0.891 |
| White blood cells, 103/μL | 4–12 | 10.7 | 11.3 | 10.1 | 1.62 | 0.789 |
| Differential, % | ||||||
| Segmented neutrophils | 10–50 | 24.1 | 27.4 | 21.2 | 3.57 | 0.473 |
| Lymphocytes | 40–75 | 67.4 | 74.3 | 68.3 | 3.58 | 0.341 |
| Eosinophils | 0–10 | 2.0 | 0.7 | 1.6 | 0.50 | 0.155 |
| Monocytes | 0–6 | 2.6 | 2.6 | 2.8 | 0.33 | 0.834 |
| Basophils | 0–3 | 1.5 | 0.3 | 0.0 | 0.65 | 0.278 |
| Serum biochemistry | ||||||
| Metabolites | ||||||
| Total bilirubin, mg/dL | 0.1–0.5 | 0.03 | 0.04 | 0.03 | 0.005 | 0.229 |
| Glucose, mg/dL | 50–80 | 88.7 | 88.6 | 88.0 | 2.71 | 0.980 |
| Total proteins, g/dL | 6.0–7.9 | 6.7 | 6.5 | 6.4 | 0.12 | 0.412 |
| Albumin, g/dL | 2.4–3.0 | 3.7 | 3.8 | 3.7 | 0.04 | 0.366 |
| Globulins, g/dL | 3.5–5.7 | 3.0 | 2.7 | 2.7 | 0.13 | 0.378 |
| Albumin:globulin ratio | – | 1.27 | 1.44 | 1.38 | 0.068 | 0.217 |
| Creatinine, mg/dL | 1.2–1.9 | 1.02 | 0.96 | 1.02 | 0.038 | 0.382 |
| BUN, mg/dL | 8–20 | 31.6 | 28.8 | 31.1 | 1.65 | 0.448 |
| Enzymes, IU/L | ||||||
| Alkaline phosphatase | 68–387 | 153 | 154 | 153 | 11.8 | 0.998 |
| Aspartate aminotransferase | 60–280 | 123 | 113 | 112 | 6.7 | 0.440 |
| Alanine aminotransferase | ≤188 | 20.3 | 17.3 | 18.0 | 1.03 | 0.106 |
| Minerals, mEq/L | ||||||
| Calcium | 11.5–12.8 | 9.7 | 10.1 | 9.9 | 0.12 | 0.160 |
| Phosphorus | 5.0–7.3 | 7.0 | 6.1 | 6.3 | 0.42 | 0.350 |
| Item | Radiata PBE, % DM | SEM | p-Value 1 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | Diet | Linear 2 | 0 vs. PBE | ||
| Saturated fatty acids | |||||||
| 10:0 | 0.55 | 0.56 | 0.55 | 0.021 | 0.764 | – | – |
| 12:0 | 0.35 | 0.34 | 0.31 | 0.035 | 0.642 | – | – |
| 14:0 (MA) | 3.80 a | 2.70 b | 2.51 b | 0.287 | 0.011 | 0.005 | 0.003 |
| 15:0 | 1.35 | 1.34 | 1.41 | 0.132 | 0.898 | – | – |
| 16:0 (PA) | 26.1 a | 25.1 a | 22.2 b | 0.53 | <0.001 | <0.001 | 0.013 |
| 17:0 | 2.14 | 1.90 | 2.14 | 0.101 | 0.206 | – | – |
| 18:0 (SA) | 18.7 a | 14.9 b | 14.6 b | 0.79 | 0.001 | 0.001 | <0.001 |
| 20:0 (ArA) | 1.30 b | 1.42 ab | 1.51 a | 0.051 | 0.031 | 0.010 | 0.013 |
| ∑SFA | 53.6 a | 47.8 b | 46.4 b | 1.07 | <0.001 | <0.001 | <0.001 |
| Monounsaturated fatty acids | |||||||
| 16:1 | 1.97 | 1.84 | 1.83 | 0.122 | 0.596 | – | – |
| t6,t8–18:1 | 0.28 | 0.21 | 0.28 | 0.042 | 0.354 | – | – |
| t9–18:1 (EA) | 0.43 a | 0.34 b | 0.34 b | 0.018 | 0.032 | 0.018 | 0.004 |
| t10–18:1 | 1.25 | 1.29 | 1.37 | 0.080 | 0.287 | – | – |
| t11–18:1 (VA) | 2.16 b | 2.46 ab | 2.52 a | 0.103 | 0.025 | 0.010 | 0.002 |
| c9–18:1 (OA) | 31.4 b | 34.9 a | 35.7 a | 0.98 | 0.006 | 0.002 | 0.001 |
| c11–18:1 | 1.53 | 1.53 | 1.54 | 0.036 | 0.991 | – | – |
| ∑MUFA | 39.3 b | 42.8 ab | 44.0 a | 1.14 | 0.010 | 0.003 | 0.001 |
| Polyunsaturated fatty acids | |||||||
| 18:2 n-6 (LA) | 2.94 b | 4.41 a | 4.52 a | 0.299 | 0.002 | 0.001 | <0.001 |
| 18:3 n-3 (ALA) | 0.25 b | 0.28 ab | 0.41 a | 0.041 | 0.029 | 0.013 | 0.045 |
| CLA t10,c12–18:2 | 0.58 b | 0.72 ab | 0.90 a | 0.098 | 0.046 | 0.015 | 0.037 |
| CLA c9,t11–18:2 (RA) | 0.64 b | 0.79 ab | 0.88 a | 0.072 | 0.041 | 0.016 | 0.008 |
| 20:4 n-6 (AA) | 0.78 b | 0.92 ab | 1.10 a | 0.098 | 0.046 | 0.015 | 0.037 |
| 20:5 n-3 (EPA) | 0.05 b | 1.02 a | 1.11 a | 0.193 | 0.012 | 0.006 | 0.046 |
| 22:5 n-3 (DPA) | 0.25 | 0.31 | 0.35 | 0.060 | 0.353 | – | – |
| 22:6 n-3 (DHA) | 0.09 | 0.08 | 0.10 | 0.054 | 0.935 | – | – |
| ∑n-3 | 1.04 b | 1.88 ab | 2.11 a | 0.271 | 0.034 | 0.009 | 0.001 |
| ∑n-6 | 3.43 b | 5.37 a | 5.62 a | 0.320 | 0.002 | <0.001 | <0.001 |
| TFA—(CLA + VA) 3 | 1.25 | 1.10 | 1.12 | 0.119 | 0.247 | – | – |
| ∑PUFA | 5.74 b | 8.48 a | 9.30 a | 0.613 | 0.001 | <0.001 | <0.001 |
| ∑UFA 4 | 44.6 b | 51.0 a | 53.0 a | 1.61 | 0.001 | <0.001 | <0.001 |
| Ratio | |||||||
| ∑n-6/∑n-3 5 | 3.84 a | 2.75 ab | 2.37 b | 0.264 | 0.020 | <0.001 | 0.008 |
| ∑PUFA/∑SFA | 0.10 b | 0.17 a | 0.20 a | 0.146 | 0.002 | <0.001 | <0.001 |
| Nutraceutical compounds | |||||||
| Desirable fatty acids 6 | 61.2 c | 65.1 b | 69.2 ª | 0.92 | 0.001 | 0.001 | 0.001 |
| Atherogenicity index 7 | 0.92 a | 0.72 b | 0.64 b | 0.469 | < 0.001 | <0.001 | <0.001 |
| Thrombogenic index 8 | 1.76 a | 1.27 b | 1.12 b | 0.055 | < 0.001 | <0.001 | <0.001 |
| h:H ratio 9 | 1.23 c | 1.52 b | 1.75 a | 0.670 | < 0.001 | <0.001 | <0.001 |
| Delta-9-desaturase C16 10 | 6.90 | 6.69 | 7.75 | 0.406 | 0.112 | – | – |
| Delta-9-desaturase C18 11 | 62.4 b | 70.9 a | 71.6 a | 1.393 | 0.001 | 0.001 | <0.001 |
| Elongase 12 | 65.7 b | 66.4 b | 69.5 a | 0.668 | < 0.001 | 0.015 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera, N.; Suescun-Ospina, S.T.; Allende, R.; Gutiérrez-Gómez, C.; Junod, T.; Williams, P.; Fuentealba, C.; Ávila-Stagno, J. A Short-Term Supplementation with a Polyphenol-Rich Extract from Radiata Pine Bark Improves Fatty Acid Profiles in Finishing Lambs. Animals 2023, 13, 188. https://doi.org/10.3390/ani13020188
Vera N, Suescun-Ospina ST, Allende R, Gutiérrez-Gómez C, Junod T, Williams P, Fuentealba C, Ávila-Stagno J. A Short-Term Supplementation with a Polyphenol-Rich Extract from Radiata Pine Bark Improves Fatty Acid Profiles in Finishing Lambs. Animals. 2023; 13(2):188. https://doi.org/10.3390/ani13020188
Chicago/Turabian StyleVera, Nelson, Sandra Tatiana Suescun-Ospina, Rodrigo Allende, Constanza Gutiérrez-Gómez, Tania Junod, Pamela Williams, Cecilia Fuentealba, and Jorge Ávila-Stagno. 2023. "A Short-Term Supplementation with a Polyphenol-Rich Extract from Radiata Pine Bark Improves Fatty Acid Profiles in Finishing Lambs" Animals 13, no. 2: 188. https://doi.org/10.3390/ani13020188





