Efficacy of Management Efforts to Reduce Food-Related Dingo–Human Interactions and Conflict on K’gari (Fraser Island), Australia
Abstract
Simple Summary
Abstract
1. Introduction

2. Materials and Methods
3. Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conover, M.R. Numbers of human fatalities, injuries, and illnesses in the United States due to wildlife. Hum. Wildl. Interact. 2019, 13, 12. [Google Scholar]
- McLeod, R. Cost of Pest Animals in NSW and Australia, 2013–14; Report Prepared for the NSW Natural Resources Commission; eSYS Development Pty Ltd.: Sydney, NSW, Australia, 2016. [Google Scholar]
- Penteriani, V.; del Mar Delgado, M.; Pinchera, F.; Naves, J.; Fernández-Gil, A.; Kojola, I.; Härkönen, S.; Norberg, H.; Frank, J.; Fedriani, J.M.J.S.R. Human behaviour can trigger large carnivore attacks in developed countries. Sci. Rep. 2016, 6, 20552. [Google Scholar] [CrossRef] [PubMed]
- Lamarque, F.; Anderson, J.; Fergusson, R.; Lagrange, M.; Osei-Owusu, Y.; Bakker, L. Human-Wildlife Conflict in Africa: Causes, Consequences and Management Strategies; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009. [Google Scholar]
- Ripple, W.J.; Chapron, G.; López-Bao, J.V.; Durant, S.M.; Macdonald, D.W.; Lindsey, P.A.; Bennett, E.L.; Beschta, R.L.; Bruskotter, J.T.; Campos-Arceiz, A. Saving the world’s terrestrial megafauna. Bioscience 2016, 66, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Messmer, T.A. The emergence of human–wildlife conflict management: Turning challenges into opportunities. Int. Biodeterior. Biodegrad. 2000, 45, 97–102. [Google Scholar] [CrossRef]
- Richardson, S.; Mill, A.C.; Davis, D.; Jam, D.; Ward, A.I. A systematic review of adaptive wildlife management for the control of invasive, non-native mammals, and other human–wildlife conflicts. Mammal Rev. 2020, 50, 147–156. [Google Scholar] [CrossRef]
- Holder, F.W.; Howe, J.H.; Kirkpatrick, A.A.; Ward, E.; Playford, T.; Moule, J.; Osman, J.J.; White, J.W. Report of the Vermin-Proof Fencing Commission, Together with the Proceedings, Evidence and Appendices (No. 59); South Australian Government Printer: Adelaide, SA, Australia, 1893. [Google Scholar]
- Allen, B.L.; Goullet, M.; Allen, L.R.; Lisle, A.; Leung, L.K.-P. Dingoes at the doorstep: Preliminary data on the ecology of dingoes in urban areas. Landsc. Urban Plan. 2013, 119, 131–135. [Google Scholar] [CrossRef]
- Appleby, R. Dingo-human conflict: Attacks on humans. In The Dingo Debate: Origins, Behaviour and Conservation; Smith, B., Ed.; CSIRO Publishing: Clayton South, VIC, Australia, 2015; pp. 131–158. [Google Scholar]
- Brumm, A. Before Azaria: A historical perspective on dingo attacks. Animals 2022, 12, 1592. [Google Scholar] [CrossRef]
- Fleming, P.J.S.; Allen, B.L.; Allen, L.R.; Ballard, G.; Bengsen, A.J.; Gentle, M.N.; McLeod, L.J.; Meek, P.D.; Saunders, G.R. Management of wild canids in Australia: Free-ranging dogs and red foxes. In Carnivores of Australia: Past, Present and Future; Glen, A.S., Dickman, C.R., Eds.; CSIRO Publishing: Melbourne, VIC, Australia, 2014; pp. 105–149. [Google Scholar]
- Archer-Lean, C.; Wardell-Johnson, A.; Conroy, G.; Carter, J. Representations of the dingo: Contextualising iconicity. Australas. J. Environ. Manag. 2015, 22, 181–196. [Google Scholar] [CrossRef]
- Burns, G.L. The fascination of fur and feathers: Managing human-animal interactions in wildlife tourism settings. Aust. Zool. 2006, 33, 446–457. [Google Scholar] [CrossRef]
- Carter, J.; Wardell-Johnson, A.; Archer-Lean, C. Butchulla perspectives on dingo displacement and agency at K’gari-Fraser Island, Australia. Geoforum 2017, 85, 197–205. [Google Scholar] [CrossRef]
- ABC Science. The Last of the Pure Dingoes|What Are Dingos? ABC Science: Sydney, NSW, Australia, 2015. [Google Scholar]
- Appleby, R.; Mackie, J.; Smith, B.; Bernede, L.; Jones, D. Human–dingo interactions on Fraser Island: An analysis of serious incident reports. Aust. Mammal. 2018, 40, 146–156. [Google Scholar] [CrossRef]
- Behrendorff, L.; Leung, L.K.-P.; McKinnon, A.; Hanger, J.; Belonje, G.; Tapply, J.; Jones, D.; Allen, B.L. Insects for breakfast and whales for dinner: The diet and body condition of dingoes on Fraser Island (K‘gari). Sci. Rep. 2016, 6, 23469. [Google Scholar] [CrossRef] [PubMed]
- Déaux, E.C.; Crowe, T.; Charrier, I. Recreational fishing alters dingo foraging behavior on Fraser Island. J. Wildl. Manag. 2018, 82, 85–92. [Google Scholar] [CrossRef]
- Allen, B.L.; Higginbottom, K.; Bracks, J.H.; Davies, N.; Baxter, G.S. Balancing dingo conservation with human safety on Fraser Island: The numerical and demographic effects of humane destruction of dingoes. Australas. J. Environ. Manag. 2015, 22, 197–215. [Google Scholar] [CrossRef]
- Newsome, T.; van Eeden, L.M. The effects of food waste on wildlife and humans. Sustainability 2017, 9, 1269. [Google Scholar] [CrossRef]
- Newsome, T.M.; Dellinger, J.A.; Pavey, C.R.; Ripple, W.J.; Shores, C.R.; Wirsing, A.J.; Dickman, C.R. The ecological effects of providing resource subsidies to predators. Glob. Ecol. Biogeogr. 2014, 24, 1–11. [Google Scholar] [CrossRef]
- Newsome, T.M.; Ballard, G.; Crowther, M.S.; Fleming, P.J.S.; Dickman, C.R. Dietary niche overlap of free-roaming dingoes and domestic dogs: The role of human-provided food. J. Mammal. 2014, 95, 392–403. [Google Scholar] [CrossRef][Green Version]
- Newsome, T.M.; Ballard, G.; Dickman, C.R.; Fleming, P.J.S.; Howden, C. Anthropogenic resource subsidies determine space use by Australian arid zone dingoes: An improved resource selection modelling approach. PLoS ONE 2013, 8, e63931. [Google Scholar] [CrossRef]
- Newsome, T.M.; Ballard, G.; Fleming, P.J.S.; van de Ven, R.; Story, G.L.; Dickman, C.R. Human-resource subsidies alter the dietary preferences of a mammalian top predator. Oecologia 2014, 175, 139–150. [Google Scholar] [CrossRef]
- Newsome, T.M.; Howden, C.; Wirsing, A.J. Restriction of anthropogenic foods alters a top predator’s diet and intraspecific interactions. J. Mammal. 2019, 100, 1522–1532. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Timm, R.M. Bad dogs: Why do coyotes and other canids become unruly? In Proceedings of the 12th Wildlife Damage Management Conference, Corpus Christi, TX, USA, 9–12 April 2007; Nolte, D.L., Arjo, W.M., Stalman, D.H., Eds.; The Wildlife Society: Corpus Christi, TX, USA, 2007. [Google Scholar]
- Allen, B.L.; Boswell, J.; Higginbottom, K. Fraser Island Dingo Management Strategy Review: Report to Department of Environment and Heritage Protection; Ecosure Pty Ltd.: West Burleigh, QLD, Australia, 2012. [Google Scholar]
- Behrendorff, L. Best-practice dingo management: Six lessons from K’gari (Fraser Island). Aust. Zool. 2021, 41, 521–533. [Google Scholar] [CrossRef]
- O’Neill, A.J.; Cairns, K.M.; Kaplan, G.; Healy, E. Managing dingoes on Fraser Island: Culling, conflict, and an alternative. Pac. Conserv. Biol. 2017, 23, 4–14. [Google Scholar] [CrossRef]
- Bertsch, P. Review of the Implimentation Plan for the K’gari Wongari (Fraser Island Dingo) Conservation and Risk Management Strategy; Office of the Queensland Chief Scientist: Brisbane, QLD, Australia, 2020. [Google Scholar]
- Corbett, L.K. Fraser Island Dingo Management Strategy Audit; Queensland Parks and Wildlife Service: Brisbane, QLD, Australia, 2009.
- Tapply, J. Contemporary dingo management on K’gari (Fraser Island, Great Sandy National Park) under the Queensland Parks and Wildlife Service. Australas. J. Environ. Manag. 2018, 25, 119–131. [Google Scholar] [CrossRef]
- Behrendorff, L.; Leung, L.K.-P.; Allen, B.L. Utilisation of stranded marine fauna washed ashore on K’gari (Fraser Island), Australia, by dingoes. Aust. J. Zool. 2018, 66, 128–138. [Google Scholar] [CrossRef]
- Walker, K.E.; Baldwin, C.; Conroy, G.C.; Applegate, G.; Archer-Lean, C.; Arthington, A.H.; Behrendorff, L.; Gilby, B.L.; Hadwen, W.; Henderson, C.J. Ecological and Cultural Understanding as a Basis for Management of a Globally Significant Island Landscape. Coasts 2022, 2, 152–202. [Google Scholar] [CrossRef]
- Team, R. RStudio: Integrated Development Environment for R; Team, R: Boston, MA, USA, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Corbett, L.K. The Dingo in Australia and Asia, 2nd ed.; J.B. Books: Marleston, SA, Australia, 2001. [Google Scholar]
- Allen, L.R. Best practice baiting: Dispersal and seasonal movement of wild dogs (Canis lupus familiaris). In Technical Highlights: Invasive Plant and Animal Research 2008–09; QLD Department of Employment, Economic Development and Innovation: Brisbane, QLD, Australia, 2009; pp. 61–62. [Google Scholar]
- Corbett, L.K. Management of Dingoes on Fraser Island; ERA Environmental Services Pty Ltd.: Darwin, NT, Australia, 1998. [Google Scholar]
- Allen, B.L. Scat happens: Spatiotemporal fluctuation in dingo scat collection rates. Aust. J. Zool. 2012, 60, 137–140. [Google Scholar] [CrossRef]
- Cursino, M.S.; Harriott, L.; Allen, B.L.; Gentle, M.; Leung, L.K.-P. Do female dingo–dog hybrids breed like dingoes or dogs? Aust. J. Zool. 2017, 65, 112–119. [Google Scholar] [CrossRef]
- Purcell, B.V. Dingo. Australian Natural History Series; CSIRO Publishing: Collingwood, VIC, Australia, 2010. [Google Scholar]
- Allen, L.R. Demographic and functional responses of wild dogs to poison baiting. Ecol. Manag. Restor. 2015, 16, 58–66. [Google Scholar] [CrossRef]
- Madsen, C. Butchulla People Join Debate on Fraser Island Dingos—Seven Local News 25 April 2017. 2017. Available online: https://www.youtube.com/watch?v=KubY5_pX5_g (accessed on 15 September 2021).
- Bell, S.J. Warning from Butchulla Rangers about Fraser Island Dingoes during Whelping Season. 2019. Available online: https://www.youtube.com/watch?v=mjqnWwTWugU (accessed on 15 September 2021).
- Department of the Premier and Cabinet. Strong Sentence Shows Dingo Strategy Right; Queensland Government: Brisbane, QLD, Australia, 2010.
- Department of the Premier and Cabinet. New Fencing to Protect K’gari Dingoes, Visitors; Department of the Premier and Cabinet: Adelaide, SA, Australia, 2021.
- Dean, J. Fraser Island Dingoes Heading for Extinction. Available online: https://www.change.org/p/tony-abbott-mp-save-the-fraser-island-dingo/u/9252861 (accessed on 3 August 2021).
- Gunn, I. Death of the Fraser Island Dingo. April 27, 2011. Available online: https://theconversation.com/death-of-the-fraser-island-dingo-793 (accessed on 5 September 2021).
- Vogler, S. Fraser Island Dingoes Dying in Food Crisis. Sunday Mail (QLD), 20 December 2009. [Google Scholar]
- Barnsley, W. Fraser Island Campsite Closures Extended Because of Visitors’ ‘Selfish, Dangerous and Foolish’ Acts with Dingoes; Channel Seven: Eveleigh, NSW, Australia, 2021. [Google Scholar]

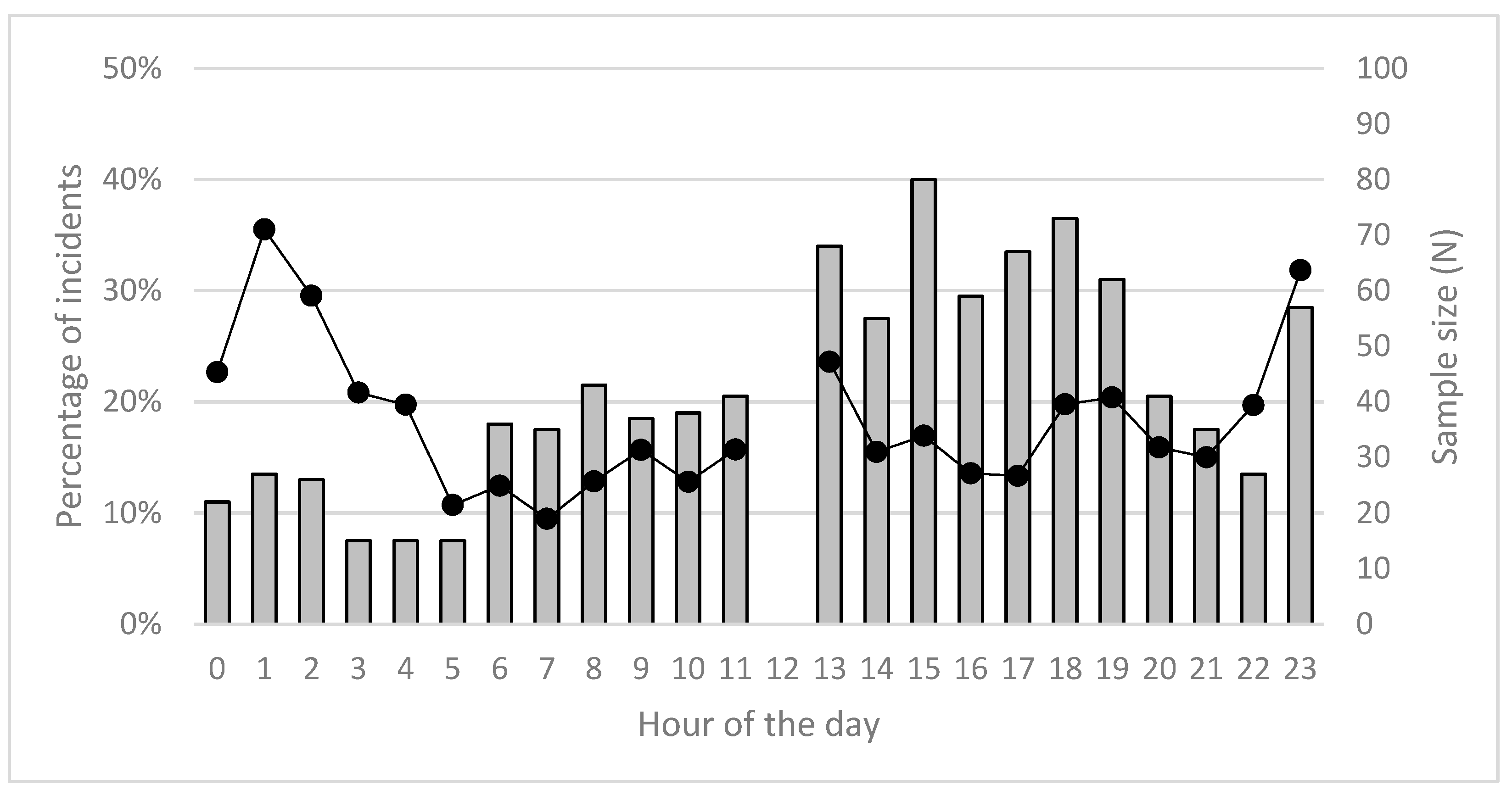

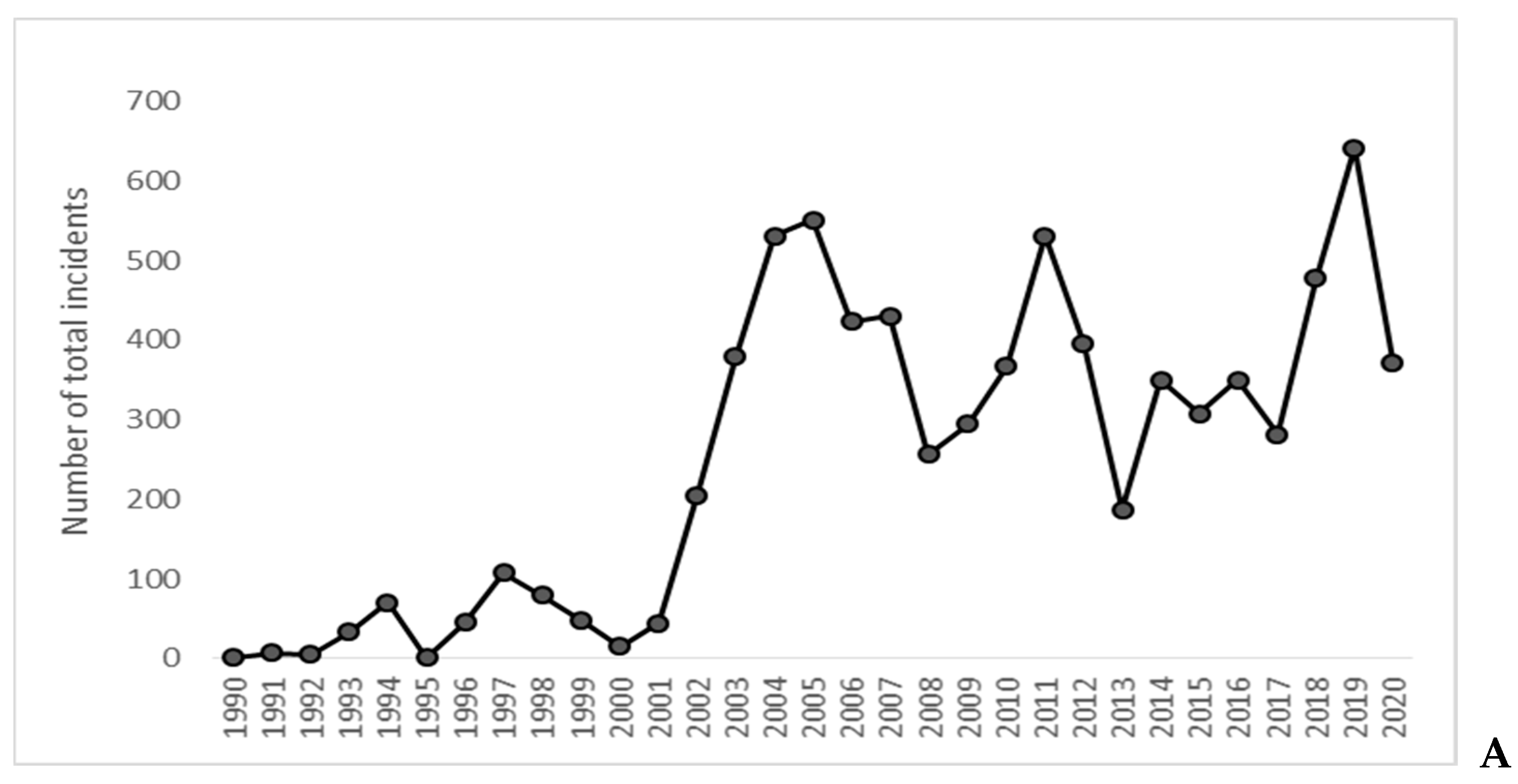

| N Total Interactions | Proportion of Food-Related Interactions | Proportion of Code B and C Interactions | Proportion of Code D and E Interactions | |
|---|---|---|---|---|
| N years with data | 31 | 31 | 27 | 31 |
| N incident records | 7791 | 1307 | 5888 | 1893 |
| Mean | 251.32 | 0.14 | 0.24 | 0.03 |
| Standard error | 35.41 | 0.01 | 0.02 | 0.00 |
| Median | 282 | 0.15 | 0.24 | 0.03 |
| Standard deviation | 197.18 | 0.08 | 0.13 | 0.03 |
| Range | 640 | 0.30 | 0.64 | 0.09 |
| Minimum | 1 | 0 | 0 | 0 |
| Maximum | 641 | 0.30 | 0.64 | 0.09 |
| Incidents | Covariate | Estimate | Std Error | Z | Pr (>|z|) | Odds Ratio (95% CI) |
|---|---|---|---|---|---|---|
| All | Year | −0.04 | 0.01 | −6.94 | <0.001 | 0.96 (0.95, 0.97) |
| Season (Breeding) | 0.26 | 0.09 | 2.91 | 0.004 | 1.29 (1.09, 1.54) | |
| Season (Whelping) | 0.19 | 0.09 | 2.10 | 0.036 | 1.21 (1.01, 1.45) | |
| Season (Dispersal) | 0.26 | 0.09 | 2.87 | 0.004 | 1.30 (1.09, 1.56) | |
| Code B and C | Year | −0.04 | 0.01 | −7.15 | <0.001 | 0.96 (0.95, 0.97) |
| Season (Breeding) | 0.25 | 0.09 | 2.65 | 0.008 | 1.28 (1.07, 1.54) | |
| Season (Whelping) | 0.15 | 0.10 | 1.54 | 0.123 | - | |
| Season (Dispersal) | 0.28 | 0.10 | 2.87 | 0.004 | 1.32 (1.09, 1.60) | |
| Code D and E | Year | 0.01 | 0.03 | 0.37 | 0.708 | - |
| Season (Breeding) | −0.09 | 0.36 | −0.26 | 0.793 | - | |
| Season (Whelping) | −0.58 | 0.46 | −0.13 | 0.201 | - | |
| Season (Dispersal) | 0.03 | 0.36 | 0.07 | 0.943 | - |
| Year | Management Action |
|---|---|
| 1990 | QPWS become responsible for dingo management on Fraser Island |
| 1994 | Closure of the dump at Waddy Point |
| 2000 | ‘Dingo smart’ education campaign initiated |
| 2001 | Campground facilities upgraded at Coolooloi, Central Station, Ungowa |
| Fencing began on QPWS residences at Eurong, Ungowa, Waddy Point, Central Station | |
| Dingo-specific fines implemented for feeding and disturbing dingoes (On-the-spot $225, court imposed $3000) | |
| Dingo ranger program initiated | |
| Quarterly risk assessment reporting commenced | |
| 2002 | Fencing completed at Waddy Point day-use area |
| Campground facilities upgraded at Central Station, Lake McKenzie, Dundubara, Waddy Point, Ungowa, Wathumba, Lake Allom and Lake Wabby | |
| ‘Be dingo-safe!’ education campaign initiated | |
| Instant dismissal policy implemented for resort staff caught feeding dingoes | |
| Permit conditions amended requiring all CTOs to lock food containers | |
| Restrictions implemented for fish cleaning and offal disposal | |
| Stainless steel lids installed on all public-use gas BBQ plates | |
| 2003 | Fencing completed at Central Station, Dilli Village, Waddy Point, Garawongera |
| Campground facilities upgraded at Lake Bowarrady, Moon Point, North Wathumba Spit | |
| 2004 | Fencing completed at Kingfisher Bay and Dundubara |
| 2005 | Beach bins removed, replaced with fenced rubbish compounds |
| 2006 | Food storage facilities installed at all hiker camps |
| 2008 | Fencing completed at Happy Valley and waste station, Eurong Resort and Residential Valley |
| Dingo-specific fines increased (On-the-spot $300, court imposed $4000) | |
| 2009 | Fencing completed at Eurong waste station |
| Wildlife photographer conviction for long term dingo feeding | |
| 2012 | Fencing completed at K’gari camp |
| 2014 | Fencing completed at Cathedral Beach |
| 2015 | Commercial Tour Operators begin using temporary electric fencing around camps |
| 2019 | Dingo-specific fines increased (On-the-spot $2135, court imposed $10,676) |
| 2020 | Beachside campground fencing completed at Cornwalls, Wongai, Eli Creek and One Tree Rocks |
| 2021 | Dingo-specific fines increased (On-the-spot $2205, court imposed $16,138) |
| Funding announced for fencing at Orchid Beach completed 2022 | |
| Funding announced for fencing at Wathumba Campground completed 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behrendorff, L.; King, R.; Allen, B.L. Efficacy of Management Efforts to Reduce Food-Related Dingo–Human Interactions and Conflict on K’gari (Fraser Island), Australia. Animals 2023, 13, 204. https://doi.org/10.3390/ani13020204
Behrendorff L, King R, Allen BL. Efficacy of Management Efforts to Reduce Food-Related Dingo–Human Interactions and Conflict on K’gari (Fraser Island), Australia. Animals. 2023; 13(2):204. https://doi.org/10.3390/ani13020204
Chicago/Turabian StyleBehrendorff, Linda, Rachel King, and Benjamin L. Allen. 2023. "Efficacy of Management Efforts to Reduce Food-Related Dingo–Human Interactions and Conflict on K’gari (Fraser Island), Australia" Animals 13, no. 2: 204. https://doi.org/10.3390/ani13020204
APA StyleBehrendorff, L., King, R., & Allen, B. L. (2023). Efficacy of Management Efforts to Reduce Food-Related Dingo–Human Interactions and Conflict on K’gari (Fraser Island), Australia. Animals, 13(2), 204. https://doi.org/10.3390/ani13020204







