The Evanescent Bouquet of Individual Bear Fingerprint
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Anesthesia Protocols
2.3. Extraction and Analysis
2.4. GC-MS Analysis
3. Results
3.1. Brown Bear Individual Compounds Isolation
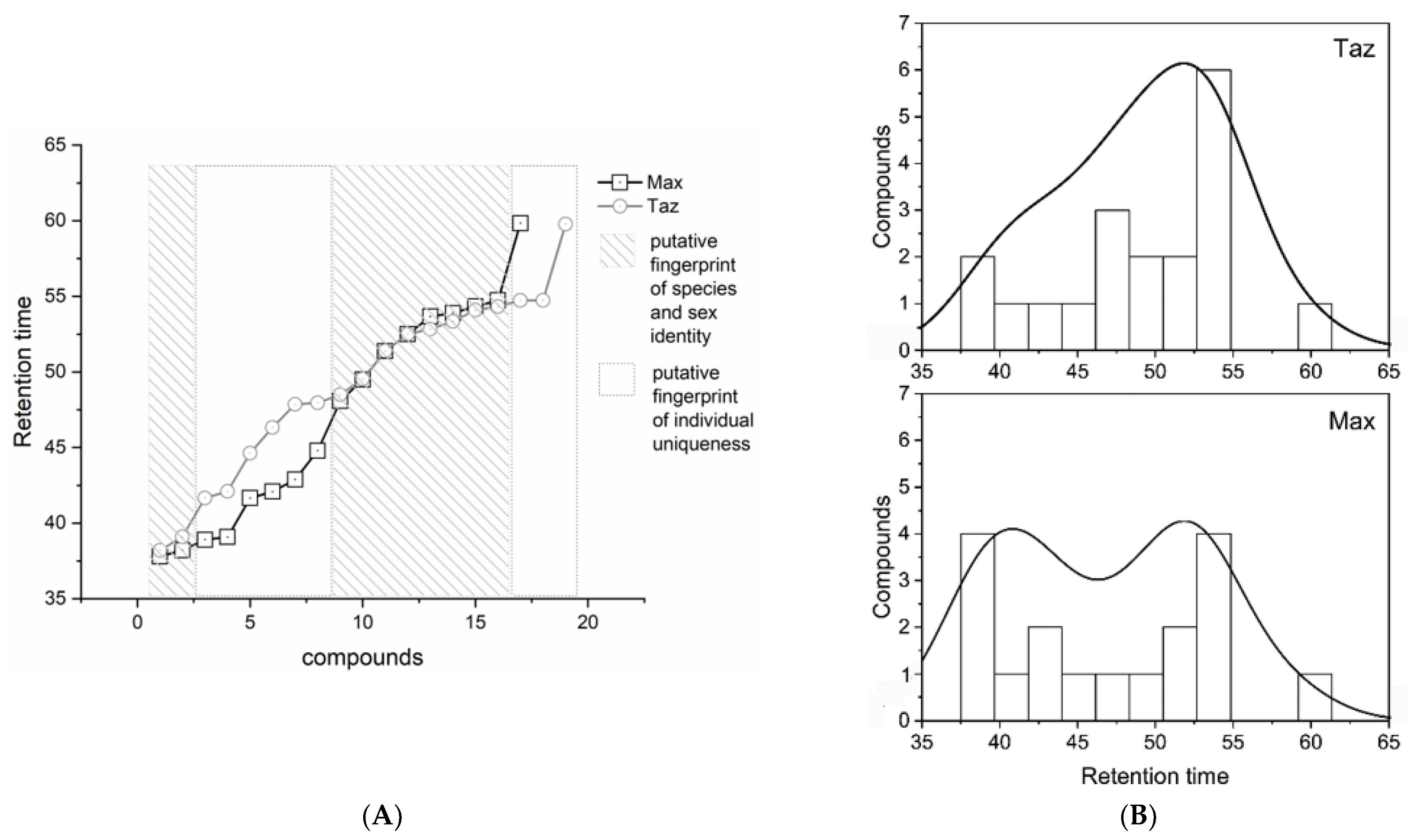
3.2. Brown Bear Putative Species vs. Individual Chemo-Signals
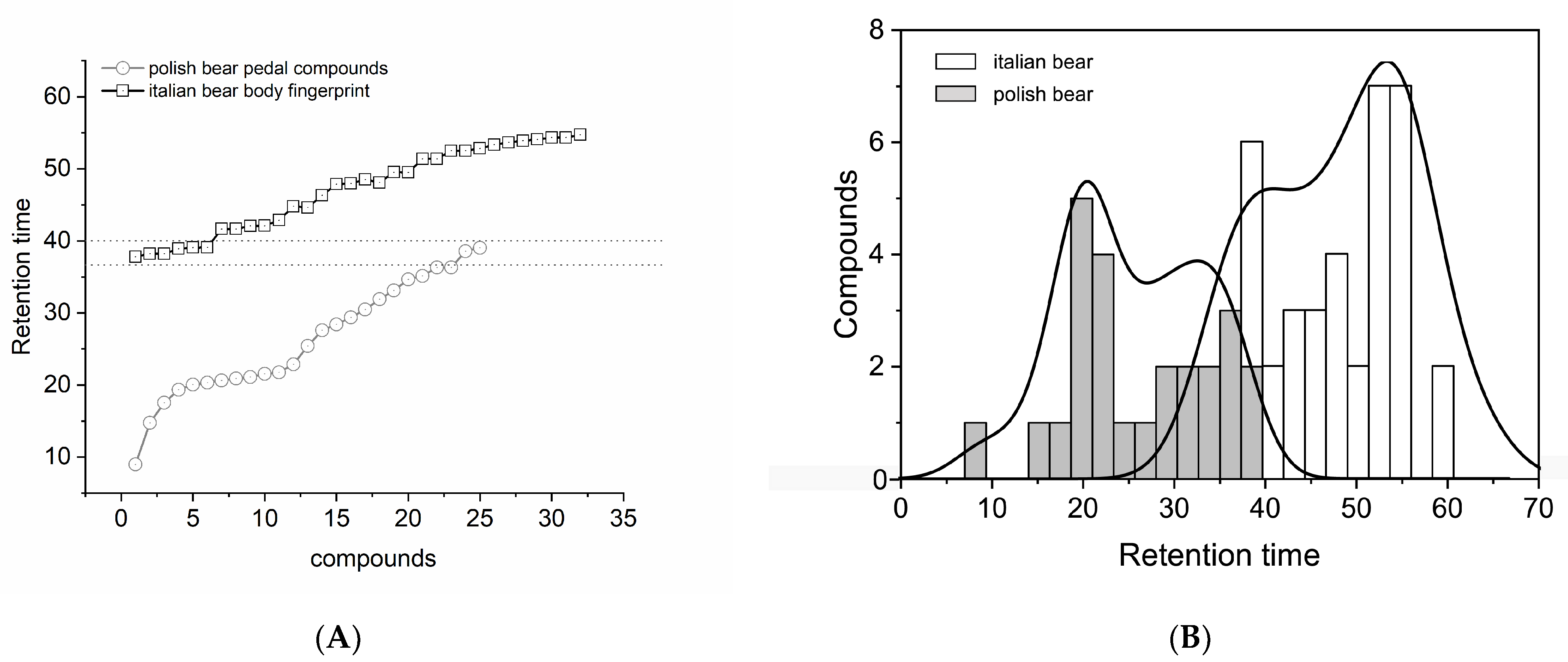
3.3. Brown Bear Population and Body District Putative Pheromones Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wyatt, T.D. Pheromones. Curr. Biol. 2017, 27, R731–R745. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.M.; Teicher, M.H. Suckling. Science 1980, 210, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.P. The harderian gland: A tercentennial review. J. Anat. 1994, 185, 1–49. [Google Scholar] [PubMed]
- Sergiel, A.; Naves, J.; Kujawski, P.; Maślak, R.; Serwa, E.; Ramos, D.; Fernández-Gil, A.; Revilla, E.; Zwijacz-Kozica, T.; Zięba, F.; et al. Histological, chemical, and behavioural evidence of pedal communication in brown bears. Sci. Rep. 2017, 7, 1052. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, Y.; Wang, P.; Guo, X.; Wu, Y.; Zhang, J.X.; Huang, L. Two Preputial Gland-Secreted Pheromones Evoke Sexually Dimorphic Neural Pathways in the Mouse Vomeronasal System. Front. Cell. Neurosci. 2019, 13, 455. [Google Scholar] [CrossRef]
- Cavaliere, R.M.; Silvotti, L.; Percudani, R.; Tirindelli, R. Female mouse tears contain an anti-aggression pheromone. Sci. Rep. 2020, 10, 2510. [Google Scholar] [CrossRef] [Green Version]
- Demir, E.; Li, K.; Bobrowski-Khoury, N.; Sanders, J.I.; Beynon, R.J.; Hurst, J.L.; Kepecs, A.; Axel, R. The pheromone darcin drives a. circuit for innate and reinforced behaviours. Nature 2020, 578, 137–141. [Google Scholar] [CrossRef]
- Septer, A.N.; Lyell, N.L.; Stabb, E.V. The iron-dependent regulator fur controls pheromone signaling systems and luminescence in the squid symbiont Vibrio fischeri ES114. Appl. Environ. Microbiol. 2013, 79, 1826–1834. [Google Scholar] [CrossRef] [Green Version]
- Sbarbati, A.; Osculati, F. Allelochemical Communication in Vertebrates: Kairomones, Allomones and Synomones. Cells Tissues Organs 2006, 183, 206–219. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and signature mixtures: Defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J. Comp. Physiol. A Neur. Sens. Neur. Behav. Physiol. 2010, 196, 685–700. [Google Scholar] [CrossRef]
- Sherman, P.W.; Reeve, H.K.; Pfennig, D.W. Recognition Systems in Behavioural Ecology: An Evolutionary Approach, 4th ed.; Krebs, J.R., Davies, N.B., Eds.; Blackwell Science: Oxford, UK, 1997; pp. 69–96. [Google Scholar]
- Koh, T.-W.; Carlson, J.R. Chemoreception: Identifying friends and foes. Curr. Biol. 2011, 24, 998–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamanlidis, A.A.; Youlatos, D.; Sgardelis, S.; Scouras, Z. Using sign at power poles to document presence of bears in Greece. Ursus 2007, 18, 54–61. [Google Scholar] [CrossRef]
- Clapham, M.; Nevin, O.T.; Ramsey, A.D.; Rosell, F. A hypothetico-deductive approach to assessing the social function of chemicalsignaling in a non-territorial solitary carnivore. PLoS ONE 2012, 7, 35404. [Google Scholar] [CrossRef] [PubMed]
- Clapham, M.; Nevin, O.T.; Ramsey, A.D.; Rosell, F. The function of strategic tree selectivity in the chemical signaling of brownbears. Anim. Behav. 2013, 85, 1351–1357. [Google Scholar] [CrossRef]
- Clapham, M.; Nevin, O.T.; Ramsey, A.D.; Rosell, F. Scent-marking investment and motor patterns are affected by the age and sex of wild brown bears. Anim. Behav. 2014, 94, 107–116. [Google Scholar] [CrossRef]
- Taylor, A.P.; Allen, M.L.; Gunther, M.S. Black bear marking behavior at rub trees during the breeding season in northern California. Behaviour 2015, 152, 1097–1111. [Google Scholar] [CrossRef]
- Hagey, L.; MacDonald, E. Chemical cues identify gender and individuality in giant pandas (Ailuropoda melanoleuca). J. Chem. Ecol. 2003, 29, 1479–1488. [Google Scholar] [CrossRef]
- Owen, M.A.; Swaisgood, R.R.; Slocomb, C.; Amstrup, S.C.; Durner, G.M.; Simac, K.; Pessier, A.P. An experimental investigation of chemical communication in the polar bear. J. Zool. 2014, 295, 36–43. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Liu, D.; Sun, L.; Wei, R.; Zhang, G.; Wu, H.; Zhang, H.; Zhao, C. Potential Chemosignals in the Anogenital Gland Secretion of Giant Pandas, Ailuropoda melanoleuca, Associated with Sex and Individual Identity. J. Chem. Ecol. 2008, 34, 398–407. [Google Scholar] [CrossRef]
- Hurst, J.L.; Beynon, R.J. Making progress in genetic kin recognition among vertebrates. J. Biol. 2010, 9, 1–4. [Google Scholar] [CrossRef]
- Holekamp, K.E.; Dloniak, S.M. Intraspecific variation in the behavioural ecology of a tropical carnivore, the spotted hyena. Adv. Stud. Behav. 2010, 42, 189–229. [Google Scholar]
- European Commission, Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee on the European Union Strategy for the Protection and Welfare of Animals 2012–2015, Brussels, 15 February 2012. Available online: http://ec.europa.eu/food/animal/welfare/actionplan/docs/aw_strategy_19012012_en.pdf (accessed on 21 November 2022).
- EFSA. Statement on the use of animal-based measures to assess the welfare of animals. EFSA J. 2012, 10, 2767. [Google Scholar] [CrossRef]
- NIST11. Mass Spectral Library (2011/EPA/NIH). Available online: https://chemdata.nist.gov/ (accessed on 18 June 2021).
- Rosell, F.; Jojola, S.M.; Ingdal, K.; Lassen, B.A.; Swenson, J.E.; Arnemo, J.M.; Zedrosser, A. Brown bears possess anal sacs and secretions may code for sex. J. Zool. 2011, 283, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Yuan, H.; Tian, H.; Wei, R.; Zhang, G.; Sun, L.; Wang, L.; Sun, R. Do anogenital gland secretions of giant panda code for their sexual ability? Chin. Sci. Bull. 2006, 51, 1986–1995. [Google Scholar] [CrossRef]
- Liu, D.; Wei, R.; Zhang, G.; Yuan, H.; Wang, Z.; Sun, L.; Zhang, J.; Zhang, H. Male panda (Ailuropoda melanoleuca) urine contains kinship information. Chin. Sci. Bull. 2008, 53, 2793–2800. [Google Scholar] [CrossRef] [Green Version]
- Theis, K.R.; Venkataraman, A.; Dycus, J.A.; Koonter, K.D.; Schmitt-Matzen, E.N.; Wagner, A.P.; Holekamp, K.E.; Schmidt, T.M. Symbiotic bacteria appear to mediate hyena social odors. Proc. Natl. Acad. Sci. USA 2013, 110, 19832–19837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, D.C. Molecular weight, and volatility. Nature 1954, 4424, 323–324. [Google Scholar] [CrossRef]
- Haze, S.; Gozu, Y.; Nakamura, S.; Kohno, Y.; Sawano, K.; Ohta, H.; Yamazaki, K. 2-Nonenal Newly Found in Human Body Odor Tends to Increase with Aging. J. Investig. Dermatol. 2001, 116, 520–524. [Google Scholar] [CrossRef] [Green Version]
- Pojmanová, P.; Ladislavová, N.; Škeříková, V.; Kania, P. Human scent samples for chemical analysis. Chem. Pap. 2020, 74, 1383–1393. [Google Scholar] [CrossRef] [Green Version]
- Hissa, R.; Hohtola, E.; Tuomala-Saramaki, T.; Laine, T.; Kallio, H. Seasonal changes in fatty acid and leptin contents in the plasma of the European brown bears (Ursus arctos arctos). Ann. Zool. Fenn. 1998, 35, 215–224. [Google Scholar]
- Hameed, I.H.; Hussein, J.; Kareem, M.A.; Hamad, N.S. Identification of five newly described bioactive chemical compounds in methanolic extract of Mentha viridis by using gas chromatography–mass spectrometry (GC-MS). J. Pharmacogn. Phytother. 2015, 7, 107–125. [Google Scholar]
- Priesner, E.; Bestmann, H.-J.; Vostrowsky, O.; Rösel, P. Sensory Efficacy of Alkyl-Branched Pheromone Analogues in Noctuid and Tortricid Lepidoptera. Zeit. Nat. 1977, 32, 979–991. [Google Scholar] [CrossRef]
- Rizvi, S.; Lin, T.; Zeng, X. Chemical composition of essential oil obtained from (Artemesia absinthium L.) grown under the climatic condition of Skardu Baltistan of Pakistan. Pak. J. Bot. 2018, 50, 599–604. [Google Scholar]
- Abubacker, M.N.; Palaniyappan, K.V. In vitro Antifungal Potentials of Bioactive Compounds Heptadecane, 9- hexyl and Ethyl iso-allocholate isolated from Lepidagathis cristata Willd. (Acanthaceae) leaf. Br. Biomed. Bull. 2015, 3, 336–343. [Google Scholar]
- Daffodil, E.; Uthayakumari, F.; Mohan, V. GC-MS determination of bioactive compounds of Curculigo orchioides gaertn. Sci. Res. Rep. 2012, 2, 198–201. [Google Scholar]
- Sarada, K.; Margret, R.J.; Mohan, V. GC-MS Determination of Bioactive Components of Naringi crenulata (Roxb) Nicolson. Int. J. Chem. Tech. Res. 2011, 3, 1548–1555. [Google Scholar]
- Abubacker, M.N.; Devi, P.K. In vitro antifungal potentials of bioactive compound oleic acid,3-(octadecyloxy) propyl ester isolated from Lepidagathis cristata Willd. (Acanthaceae) inflorescence. Asian Pac. J. Trop. Biomed. 2014, 4, S661–S664. [Google Scholar] [CrossRef]
- Chauhan, V.; Sheikh, A.; Chauhan, A.; Tsiouris, J.; Malik, M.; Vaughan, M. Changes during hibernation in different phospholipid and free and esterified cholesterol serum levels in black bears. Biochimie 2002, 84, 1031–1034. [Google Scholar] [CrossRef]
- Weiß, B.M.; Kücklich, M.; Thomsen, R.; Henkel, S.; Jänig, S.; Kulik, L.; Birkemeyer, C.; Widdig, A. Chemical composition of axillary odorants reflects social and individual attributes in rhesus macaques. Behav. Ecol. Sociobiol. 2018, 72, 65. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, B. Adipose Tissue. In Lipid Metabolism in Mammals. Monographs in Lipid Research; Snyder, F., Ed.; Springer: Boston, MA, USA, 1977; pp. 287–316. [Google Scholar]
- Karlson, P.; Lüscher, M. ‘Pheromones’: A New Term for a Class of Biologically Active Substances. Nature 1959, 183, 55–56. [Google Scholar] [CrossRef]
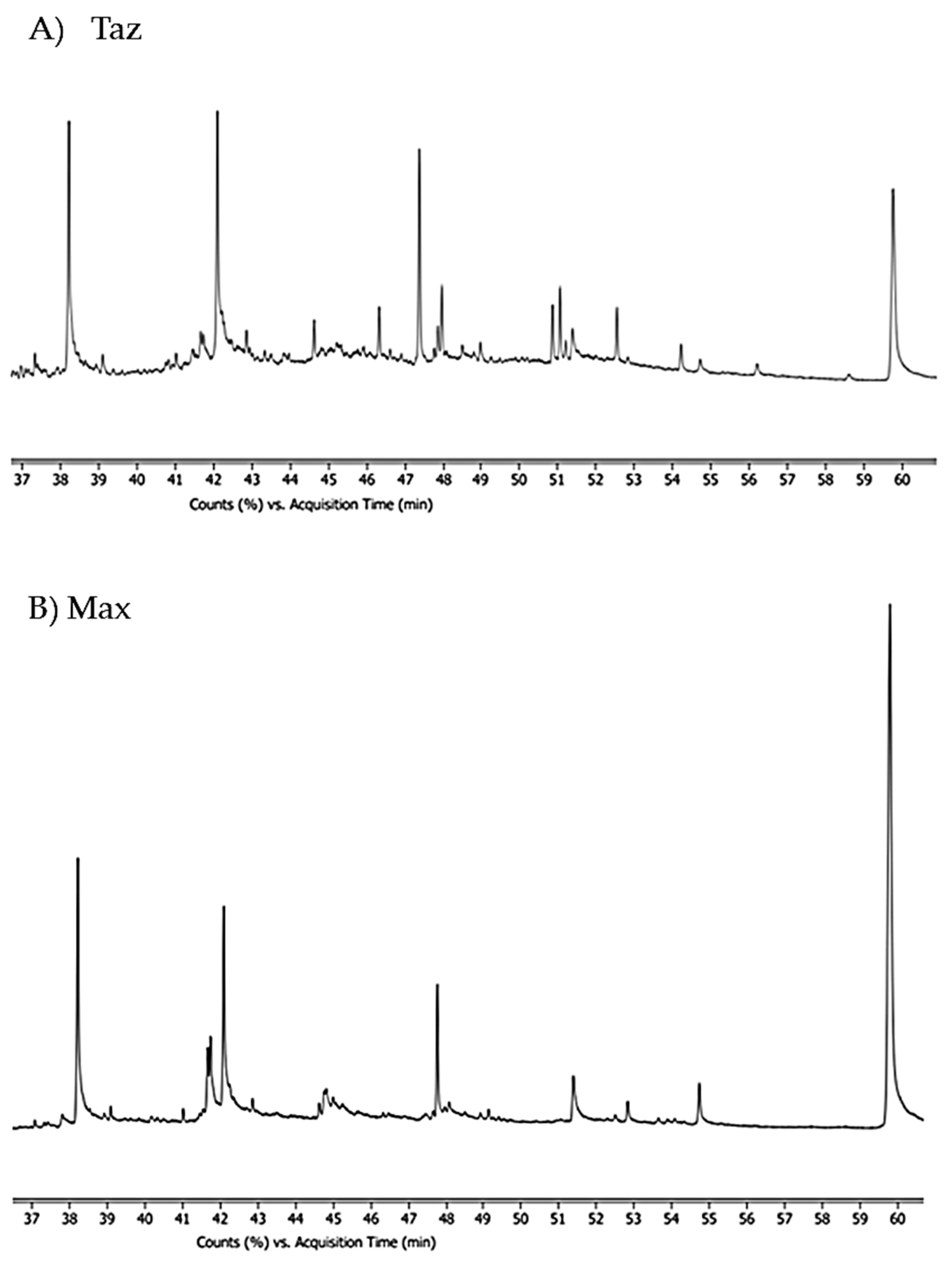
| R.T. (min) | Identified Compounds (TAZ Bear) | R.T. (min) | Identified Compounds (MAX Bear) | M.W. | Matching Percentage (NIST Library) | Chemical Class |
|---|---|---|---|---|---|---|
| 37.793 | 9(Z)-hexadecenoic acid (palmitoleic acid) (C16:1) | 254 | 20 | Carboxylic acid | ||
| 38.202 | Palmitic acid (n-hexadecenoic acid) (C16:0) | Palmitic acid (n-hexadecenoic acid) (C16:0) | 256 | 70.3 | Carboxylic acid | |
| 38.907 | Ethylpalmitate (C18) | 284 | 40 | Carboxylic acid ester | ||
| 39.207 | 7-methyl-Z-tetradecen-1-ol-acetate (C17) | 39.211 | 7-methyl-Z-tetradecen-1-ol-acetate (C17) | 268.43 | 13 | Acetate ester |
| 41.617 | cis-vaccenic acid (C18:1) (cis-11-octadecenoic acid) | 282 | 15 | Carboxylic acid | ||
| 41.655 | Oleic acid (C18) | 41.736 | Oleic acid (18:1n-9) (cis-9-octadecenoic acid) | 282 | 20 | Carboxylic acid |
| 42.093 | Octadecanoic acid (stearic acid) (C18:0) | 42.088 | Octadecanoic acid (stearic acid) (C18:0) | 284 | 70.6 | Carboxylic acid |
| 44.626 | Heptacosane (C27) | 44.626 | Heptacosane (C27) | 380.7 | 12.5 | Alkane |
| 44.689 | (Z)-13-docosenoic acid (C22) | 44.793 | (Z)-13-docosenoic acid (C22) | 338 | 35 | Carboxylic acid |
| 46.326 | Tetracosane (C24) | 338 | 41.3 | Alkane | ||
| 47.964 | Pentacosane (C25) | 352 | 13 | Alkane | ||
| 48.507 | 3-(octadecyloxy) propyl ester oleic acid (C39) | 592 | 38.8 | Carboxylic acid ester | ||
| 49.495 | Ethylisoallocholate (C26) | 436 | 46.9 | Steroid | ||
| 51.384 | 2-hydroxy-1-(hydroxymethyl) ethyl ester (octadecanoic acid) (C21) | 358 | 40 | Carboxylic acid ester | ||
| 51.388 | 2,3-dihydroxypropyl ester octadecanoid acid (1α-monostearin) (C21) | 358 | 45.2 | Carboxylic acid ester | ||
| 52.508 | Cholesterol (C27) | 386 | 49.2 | Steroid | ||
| 52.826 | Ethyl iso allocholate (C26) | 436 | 35 | Steroid ester | ||
| 53.346 | 5α, 14 β cholest 9(11)-ene (C27) | 370 | 27.8 | Steroid | ||
| 53.650 | Cholest-1-ene (C27) | 370 | 57.6 | Steroid | ||
| 53.889 | Cholest-3-ene (C27) | 370 | 30 | Steroid | ||
| 53.893 | Cholest-5-ene (C27) | 370 | 30 | Steroid | ||
| 54.089 | Cholest-5-ene-3-ol (3β), 9-octadecenoate (Z) (C45) | 650 | 11.4 | Steroid ester | ||
| 54.332 | Cholesta-4,6-dien-3-ol (3β) (C27) | 54.331 | Cholesta-4,6-dien-3-ol (3β) (C27) | 384 | 40 | Steroid |
| 54.689 | Cholesteryl formate (C34) | 490 | 14 | Steroid ester | ||
| 54.736 | Cholesta-3,5-diene (C27) | 54.735 | Cholesta-3,5-diene (C27) | 368 | 35 | Steroid |
| 54.736 | Cholesteryl benzoate (C34) | 490 | 20 | Steroid ester | ||
| 54.741 | 5-cholesten-(3β)-yl-isobutyl carbonate (C32) | 486 | 14.9 | Steroid ester | ||
| 59.770 | Psi-cholesterol (C27) | 59.836 | Psi-cholesterol (C27) | 386 | 36.2 | Steroid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzatenta, A.; Fiorito, S.; Guadagnini, R.; Genovese, S.; Valentini, A.; Bonadiman, F.; Guadagnini, S.; Epifano, F.; Mollica, A. The Evanescent Bouquet of Individual Bear Fingerprint. Animals 2023, 13, 220. https://doi.org/10.3390/ani13020220
Mazzatenta A, Fiorito S, Guadagnini R, Genovese S, Valentini A, Bonadiman F, Guadagnini S, Epifano F, Mollica A. The Evanescent Bouquet of Individual Bear Fingerprint. Animals. 2023; 13(2):220. https://doi.org/10.3390/ani13020220
Chicago/Turabian StyleMazzatenta, Andrea, Serena Fiorito, Roberto Guadagnini, Salvatore Genovese, Alberto Valentini, Federica Bonadiman, Sofia Guadagnini, Francesco Epifano, and Adriano Mollica. 2023. "The Evanescent Bouquet of Individual Bear Fingerprint" Animals 13, no. 2: 220. https://doi.org/10.3390/ani13020220





