The Different Fate of the Pyrenean Desman (Galemys pyrenaicus) and the Eurasian Otter (Lutra lutra) under Climate and Land Use Changes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
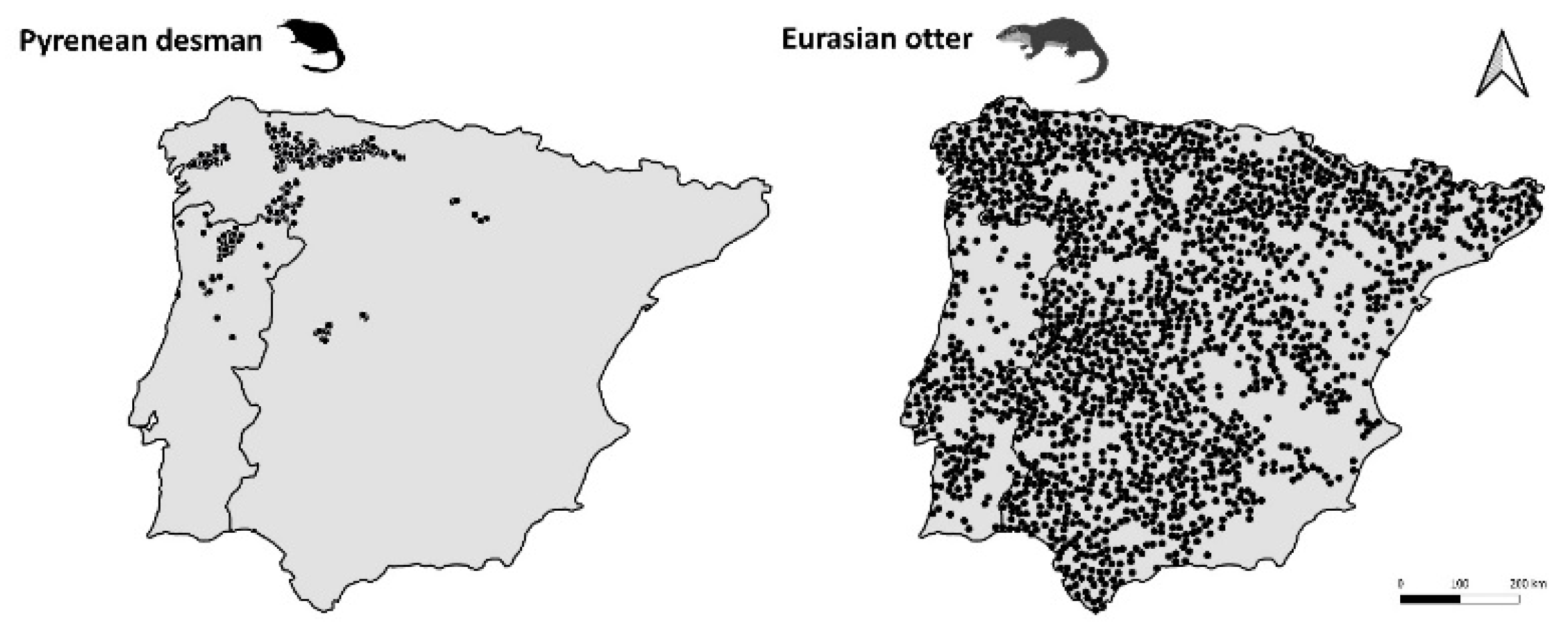
2.1. Occurrence Data
2.2. Environmental Variables
2.3. Species Distribution Models
2.4. Climate and Land Use Change Scenarios
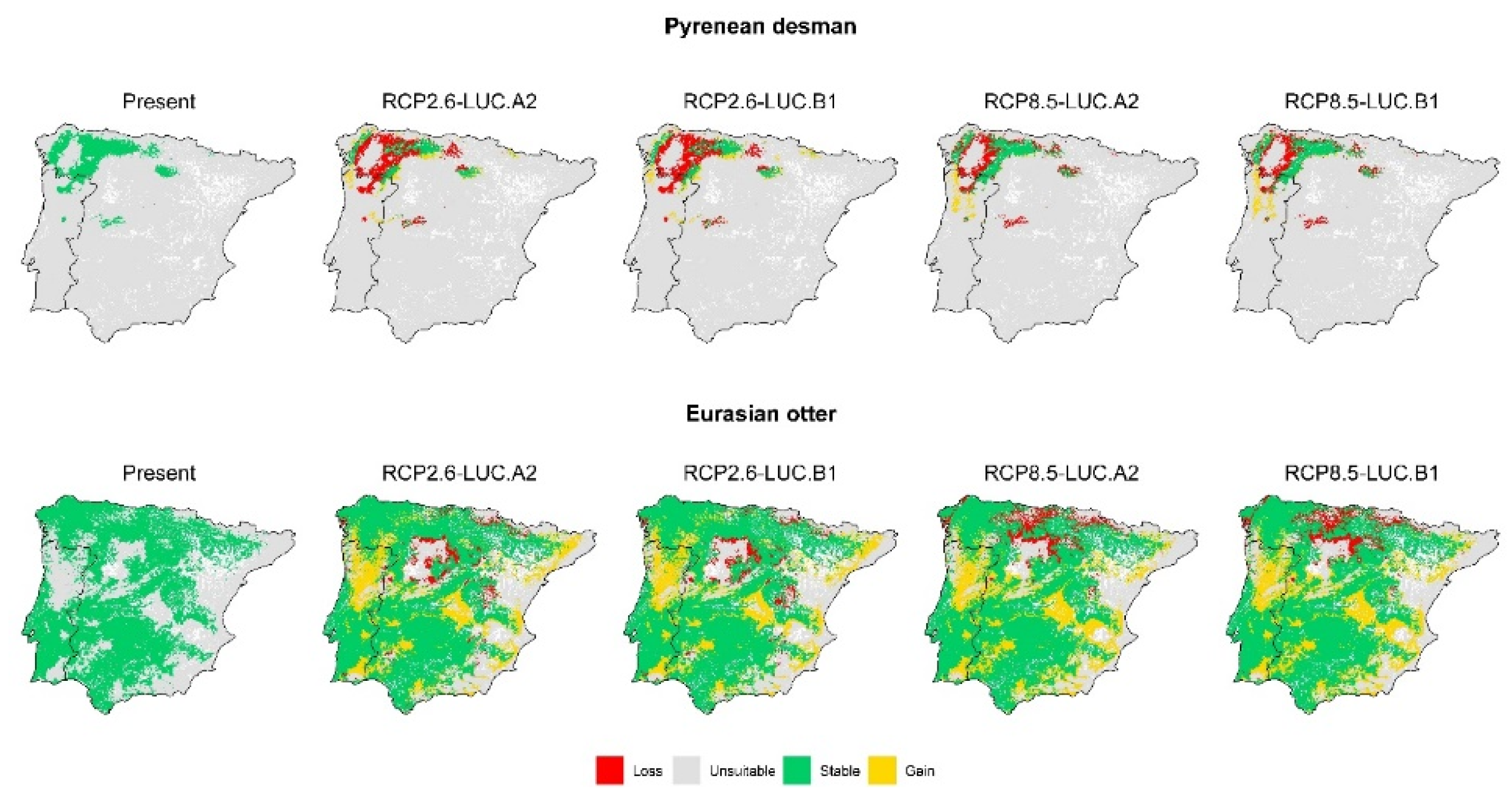
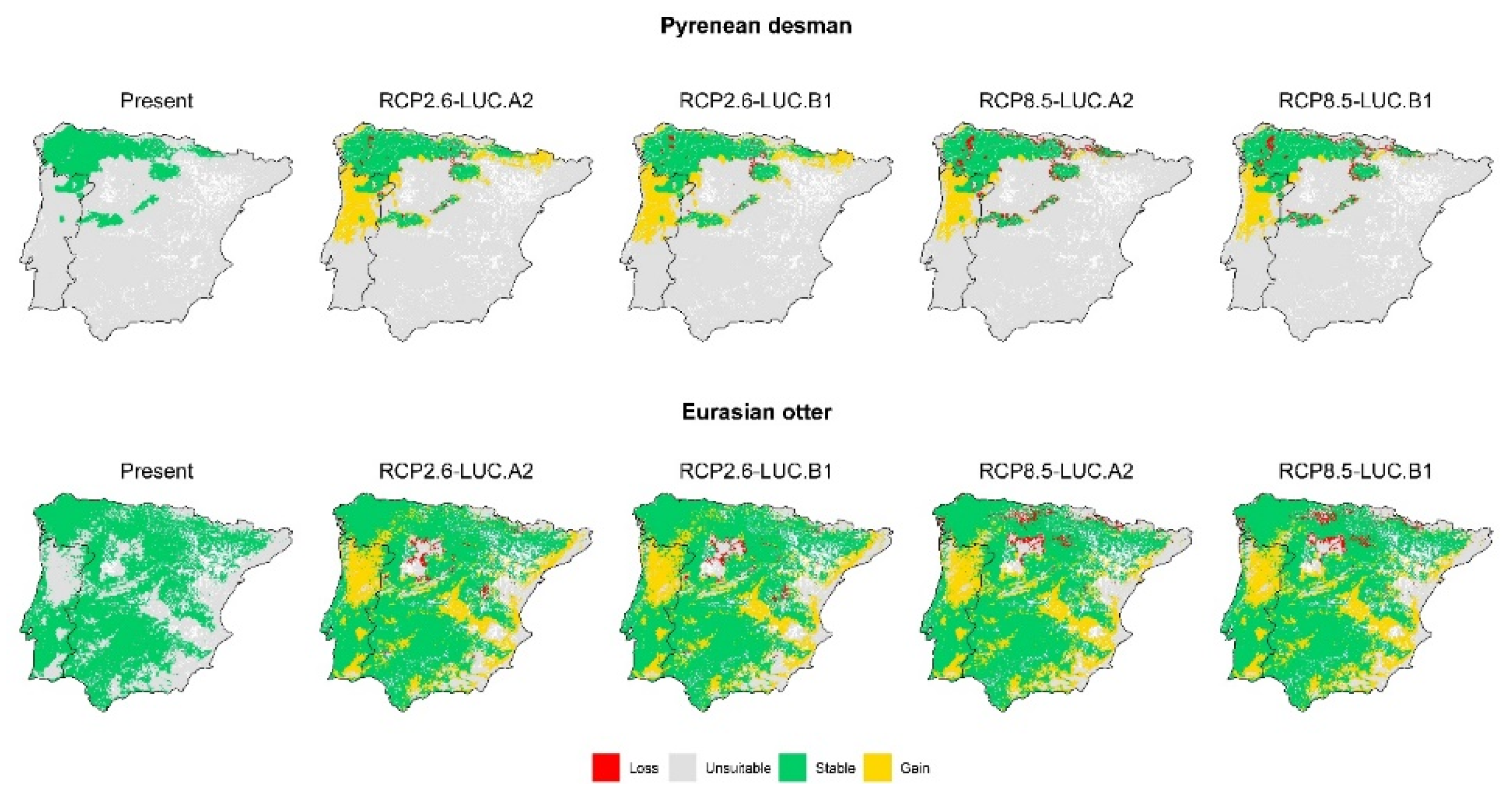
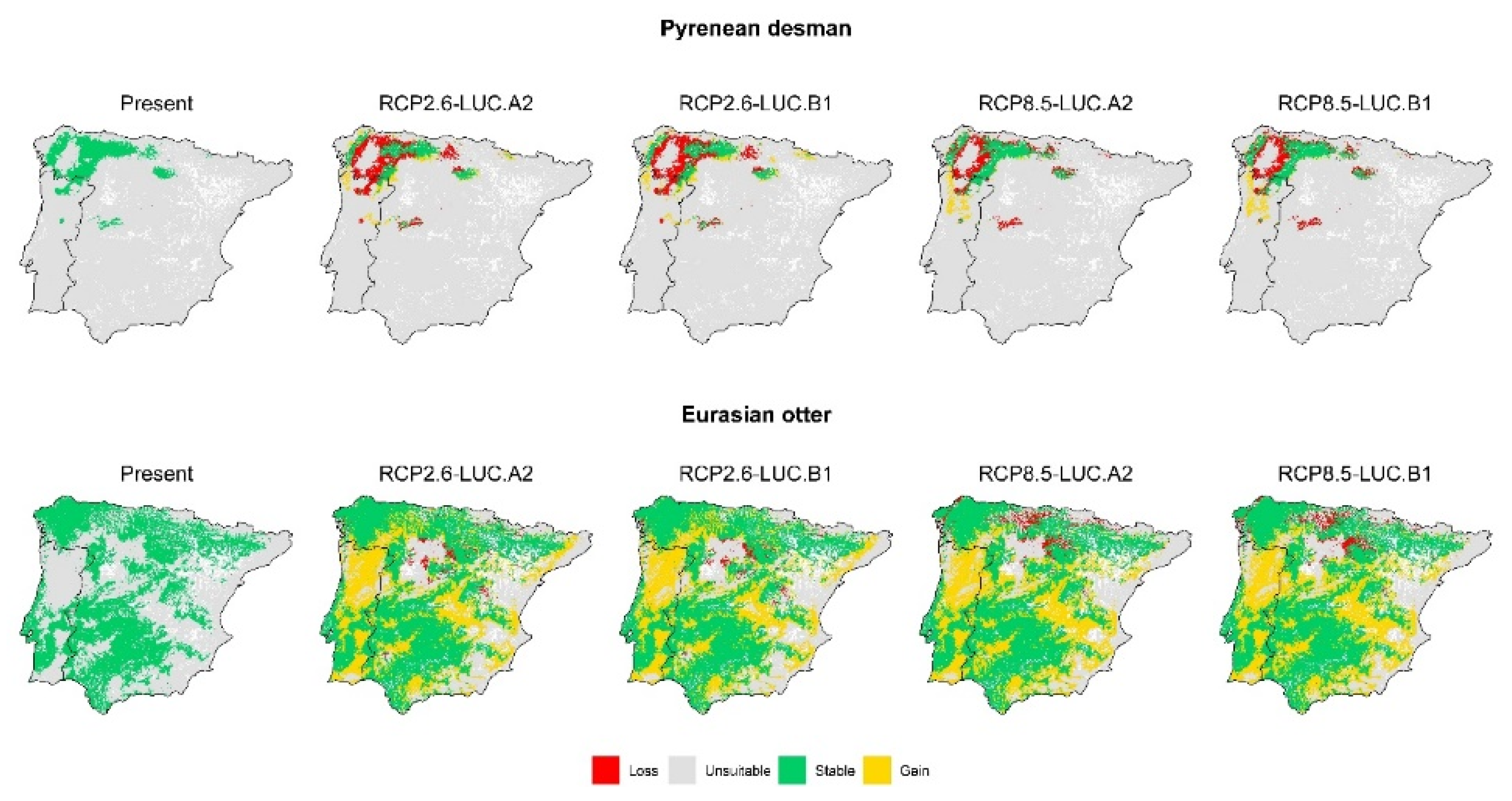
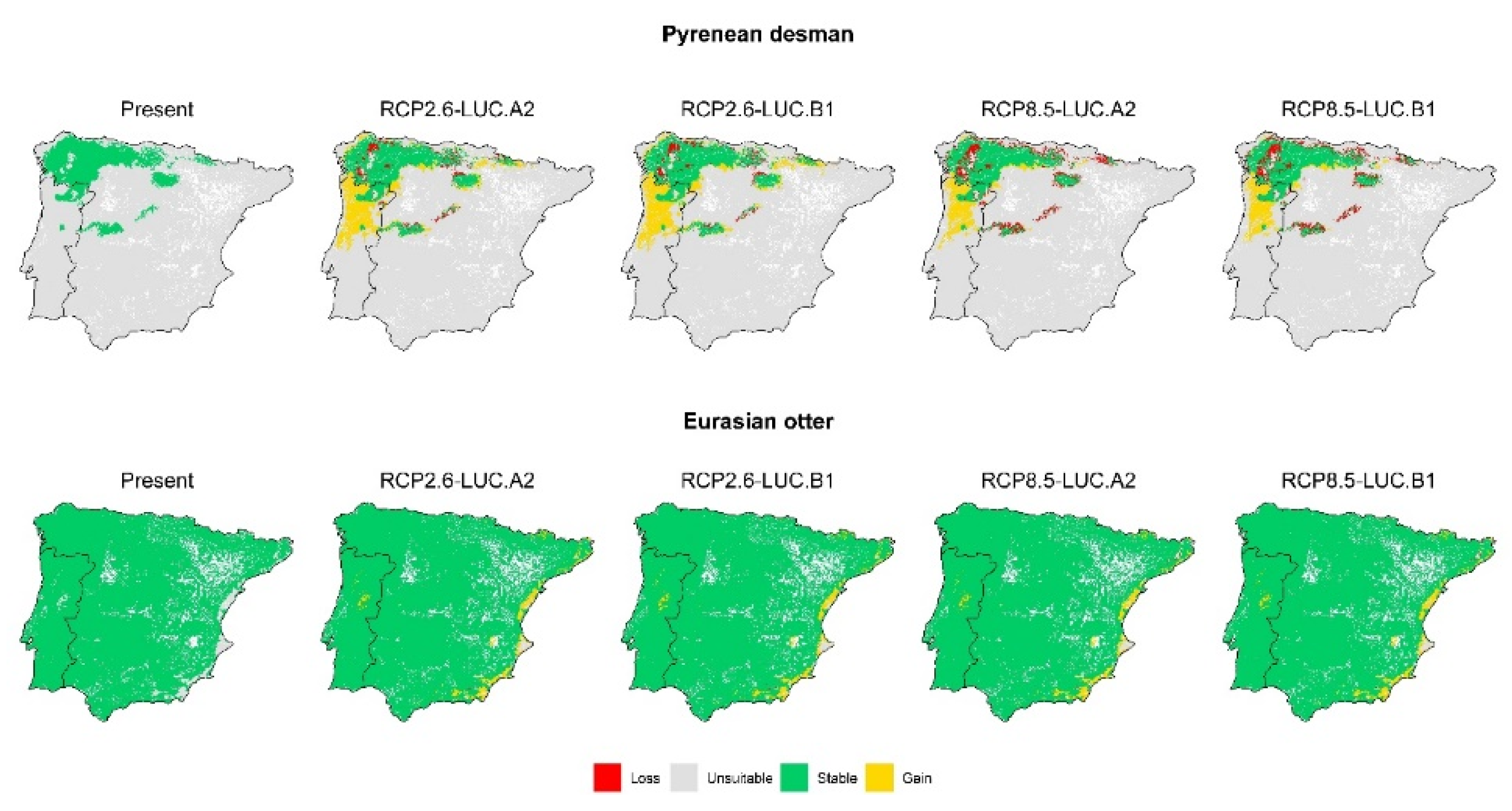
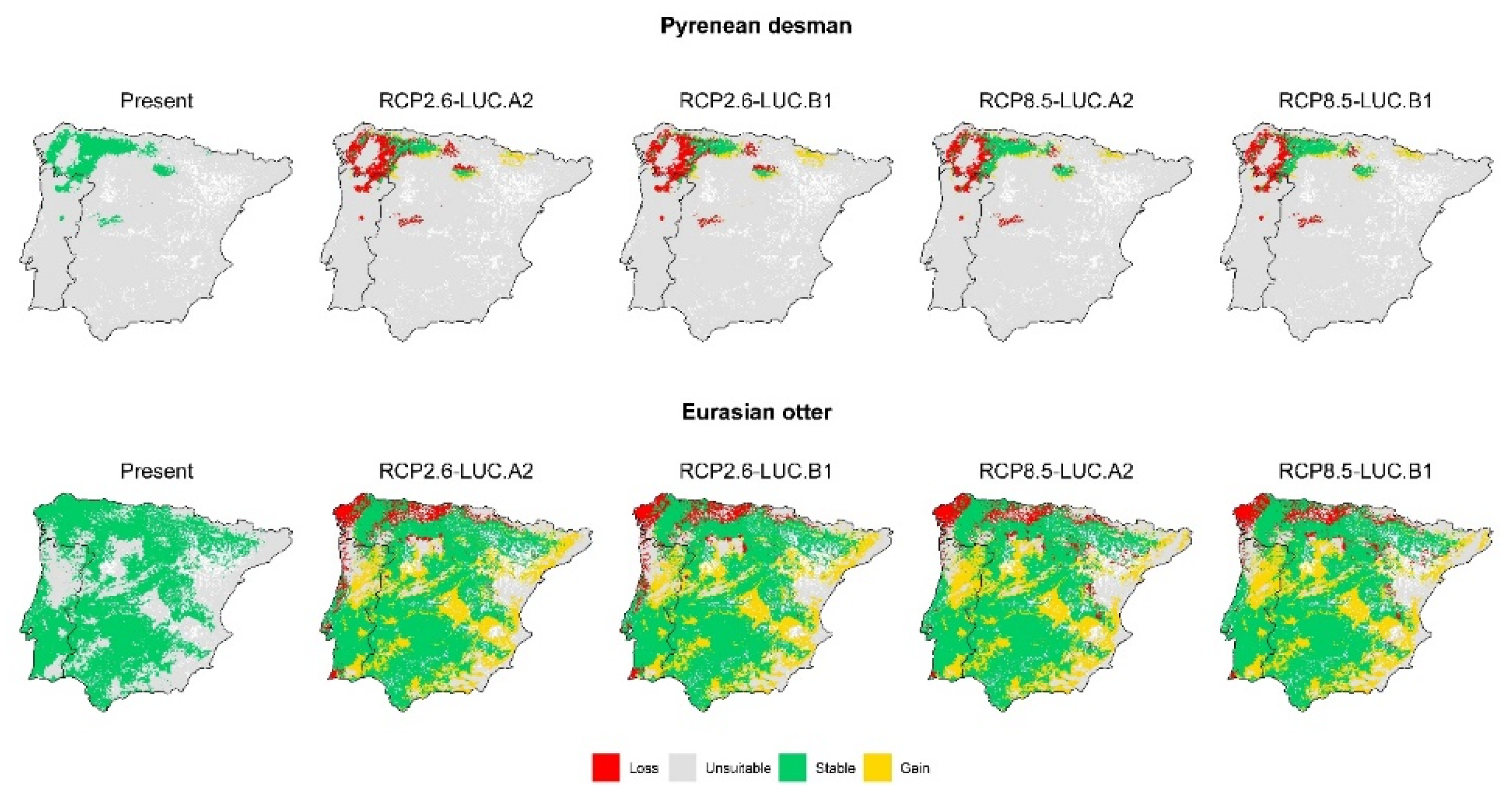
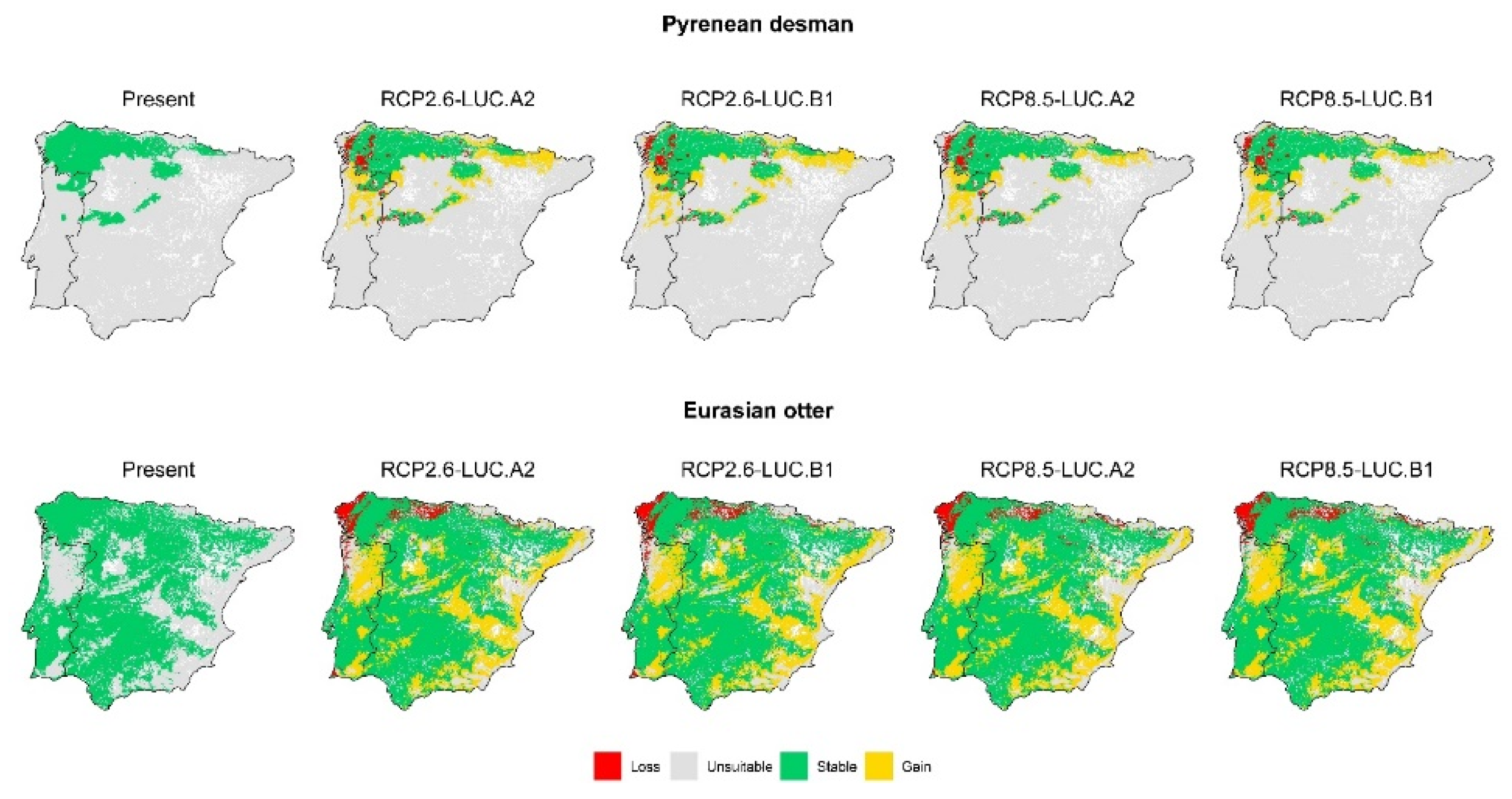
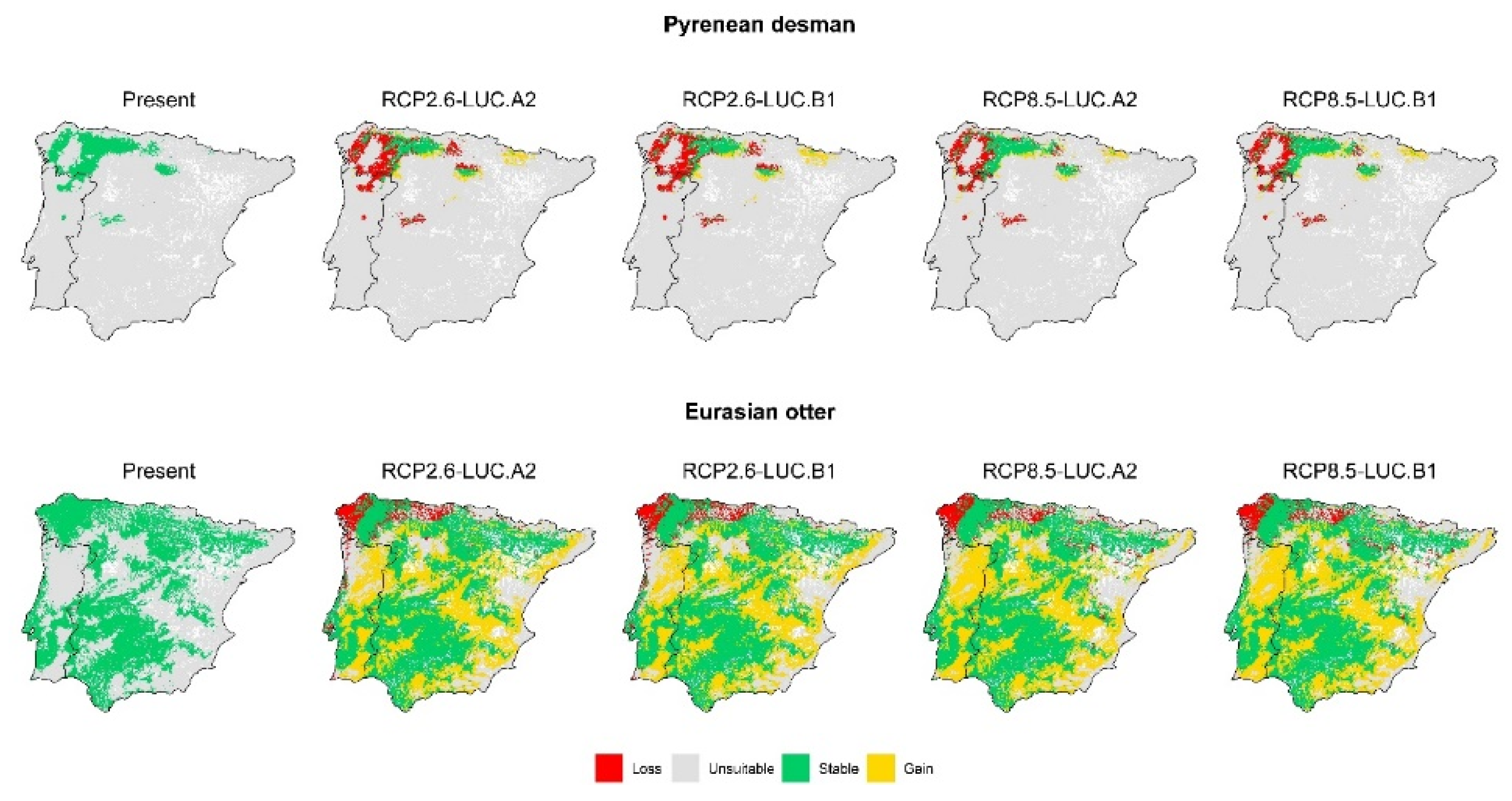
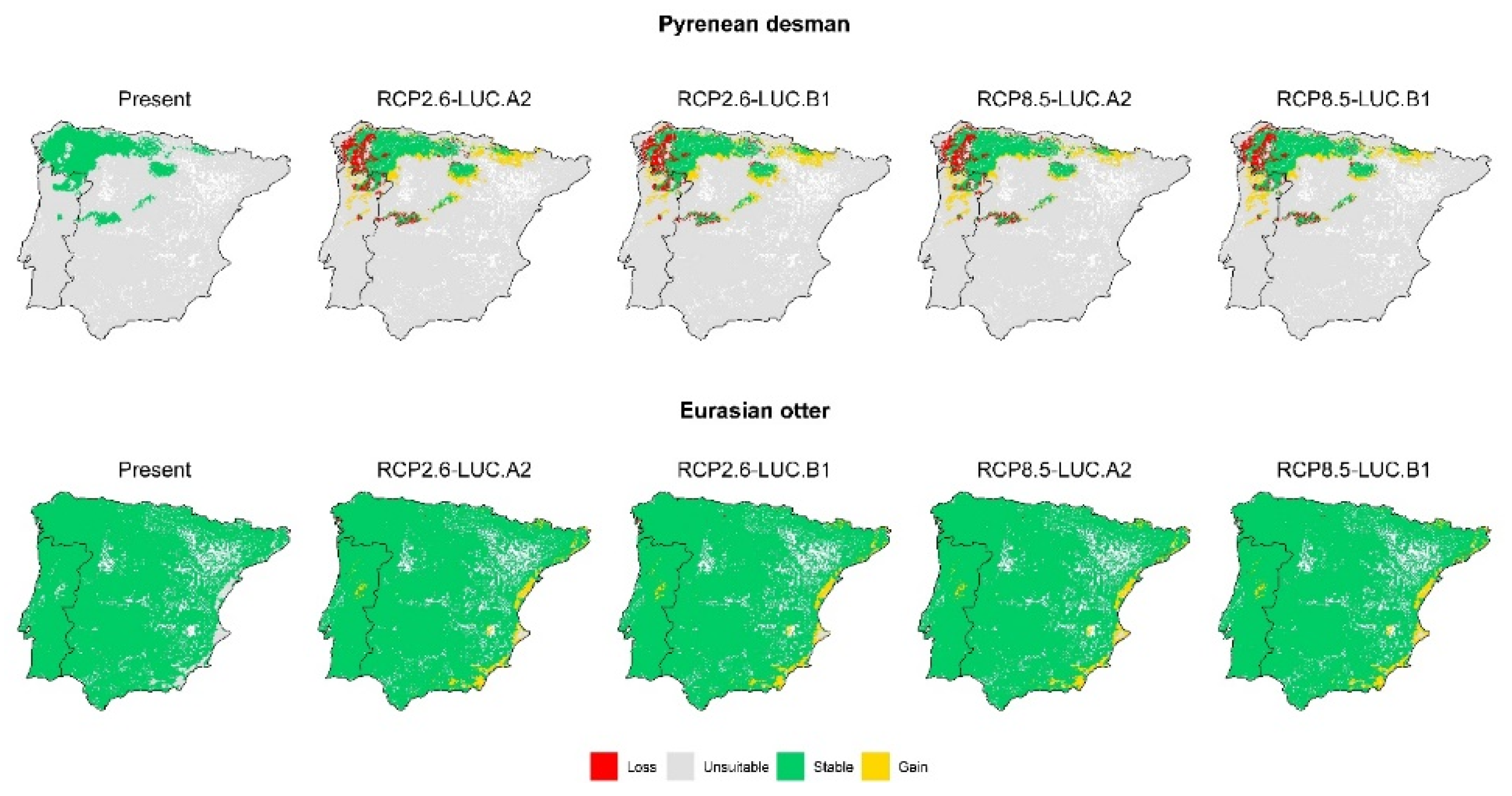
3. Results
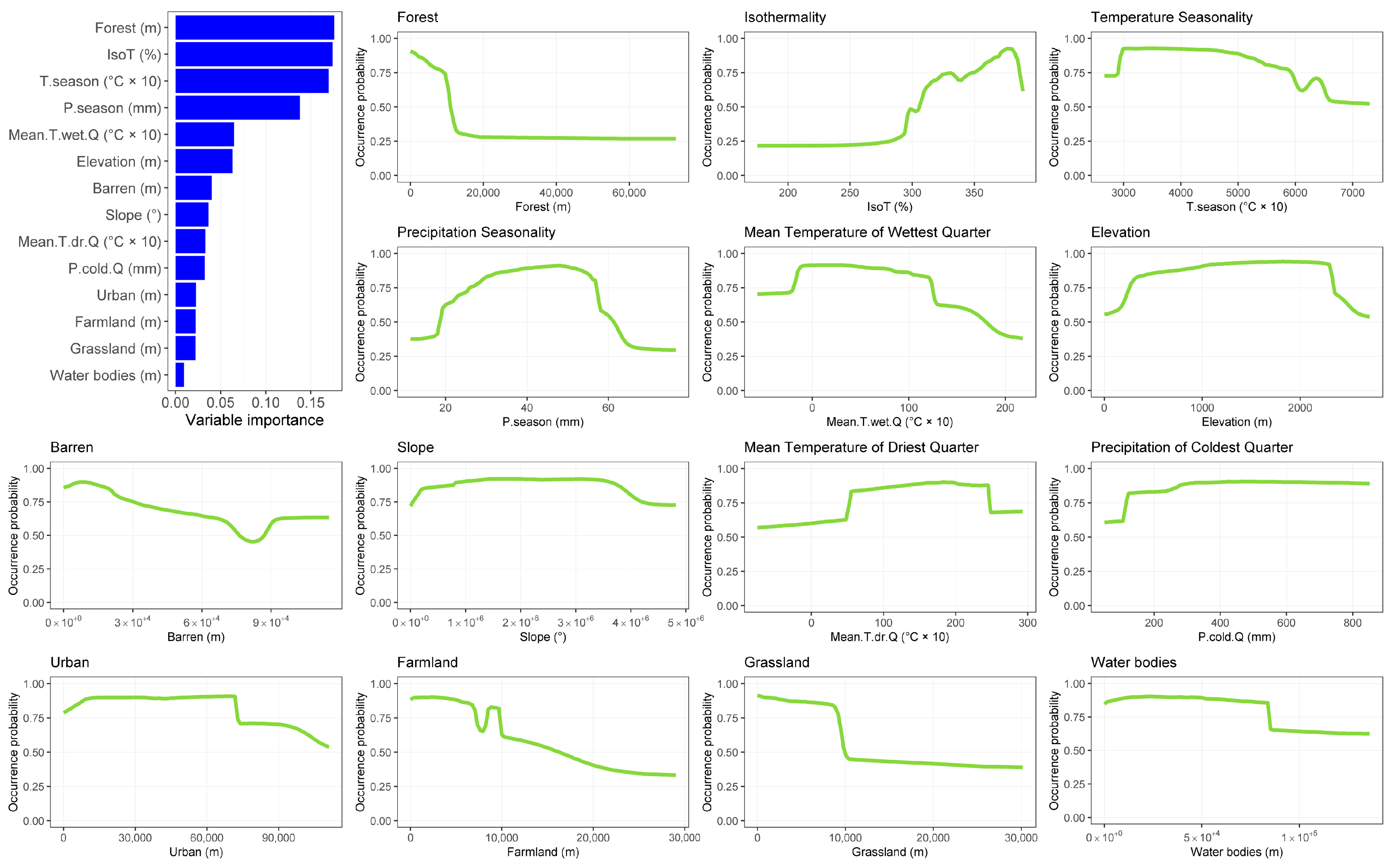
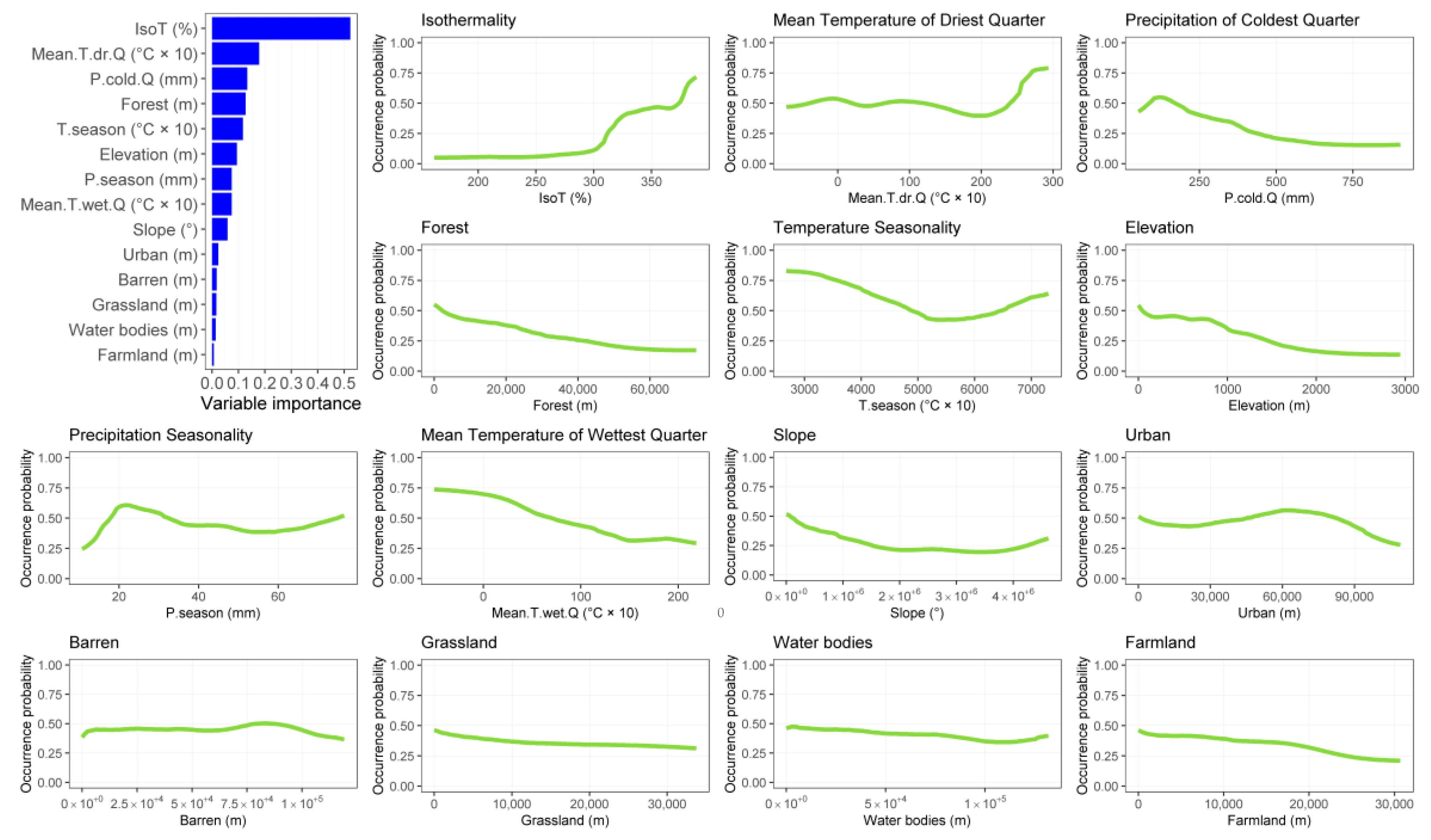
3.1. Species Distribution Models
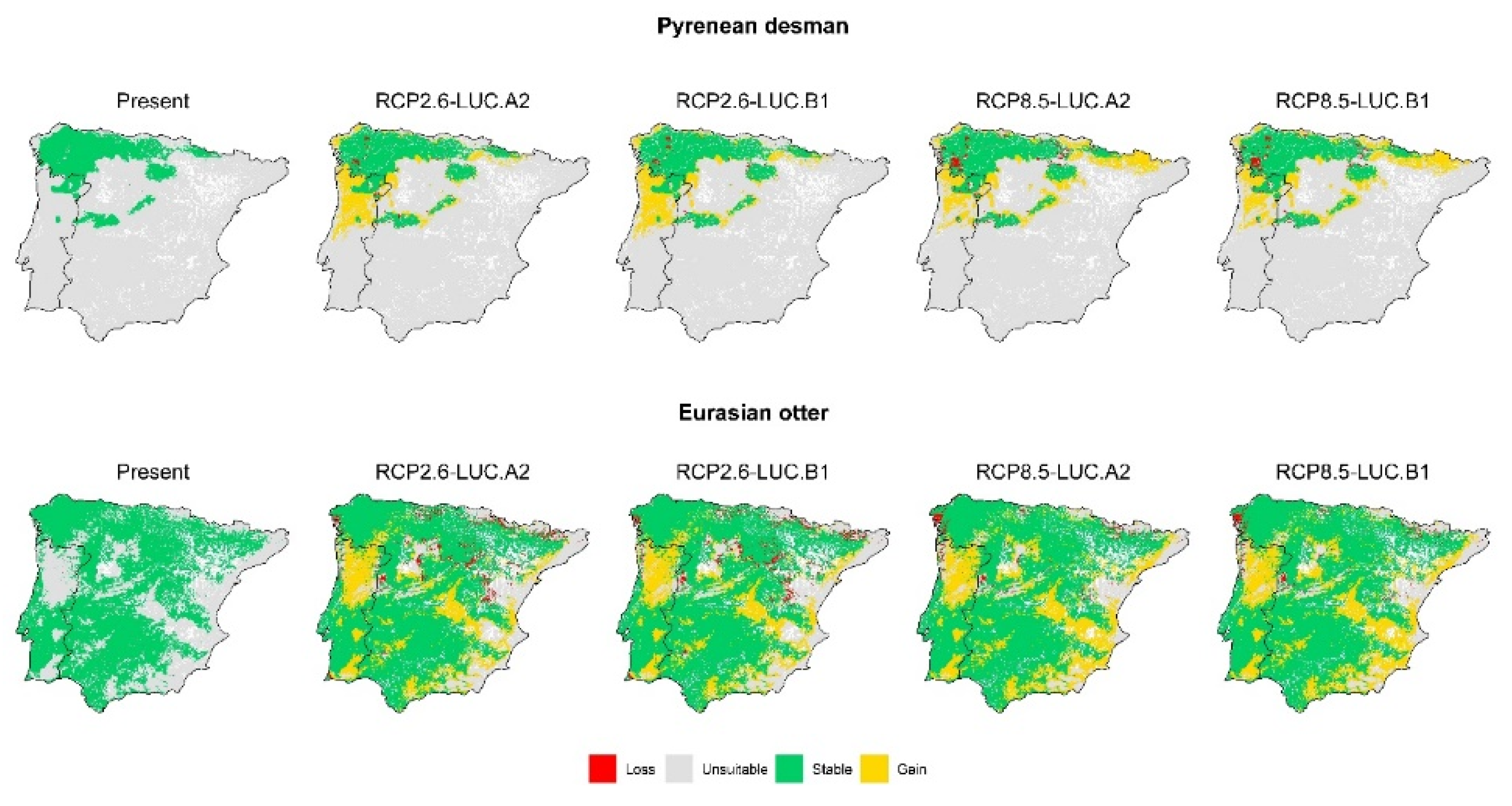
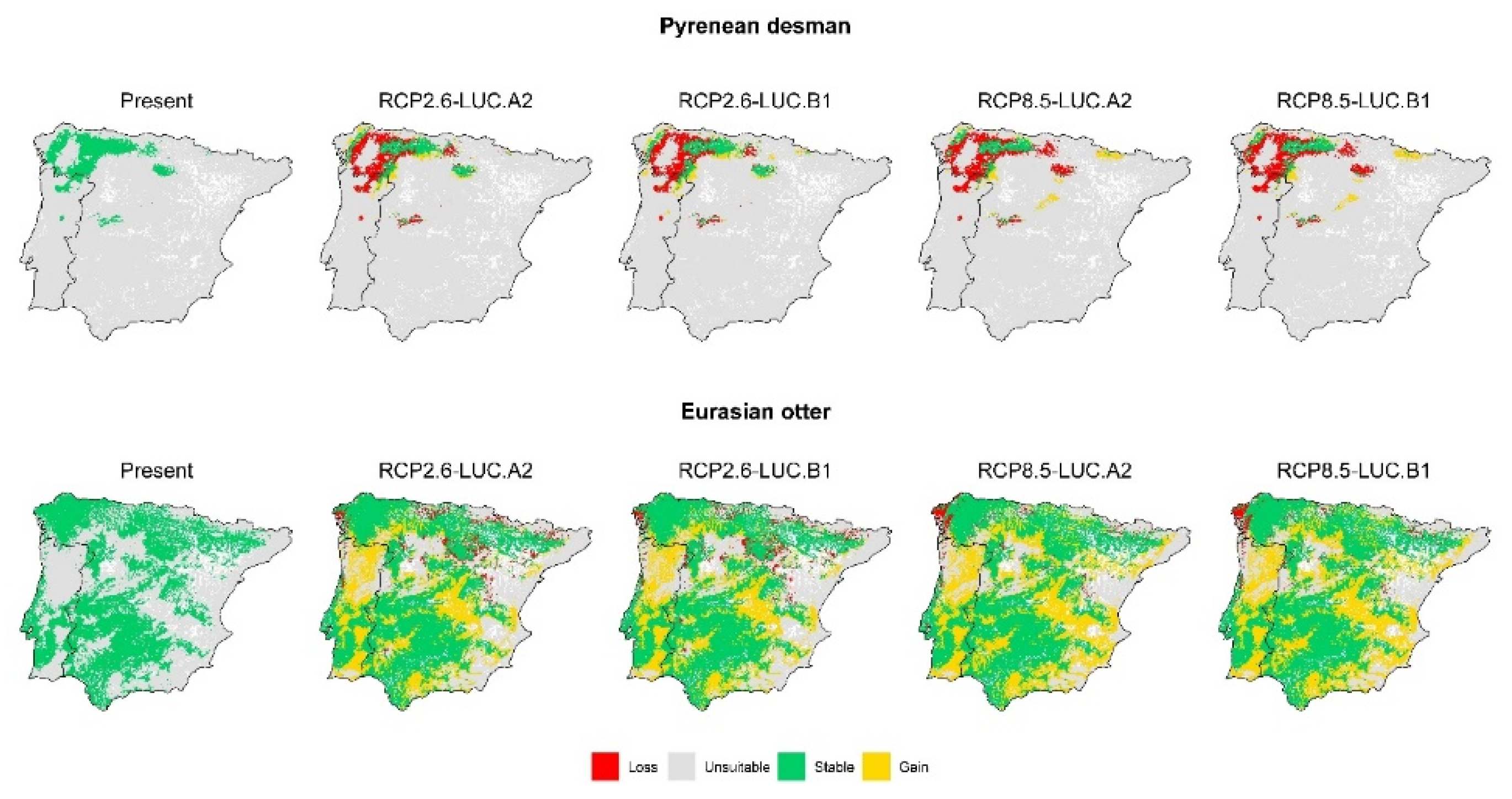
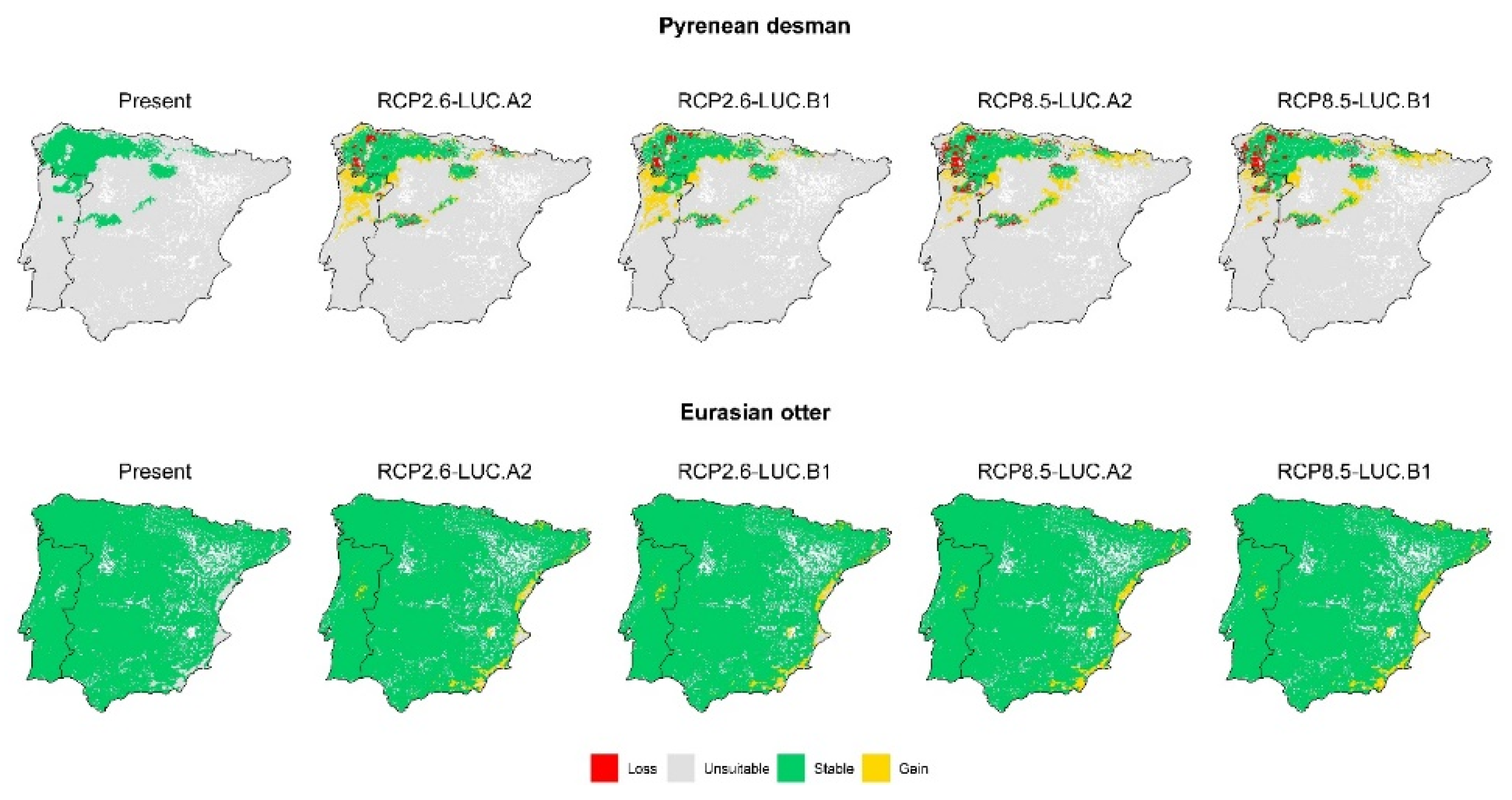
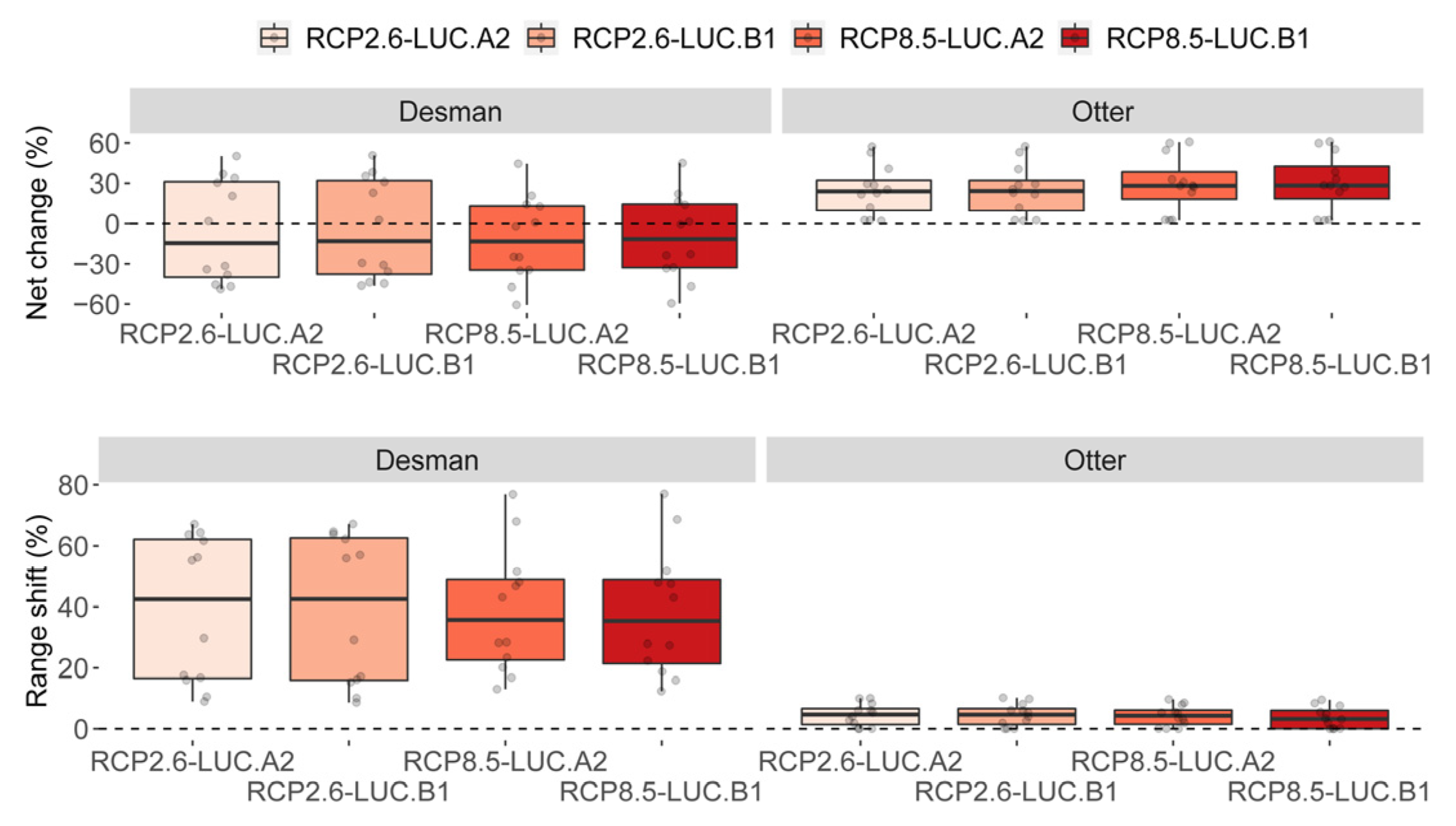
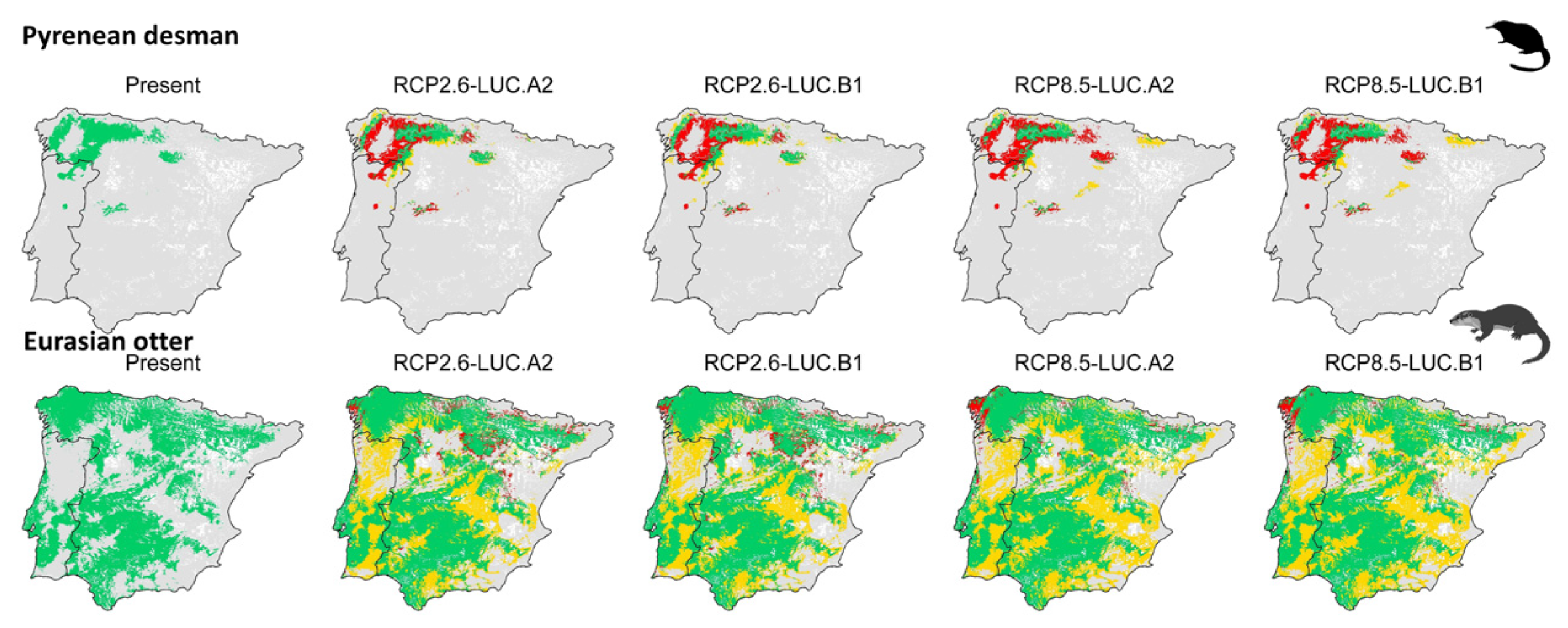
3.2. Effect of Global Change Drivers on Species Distribution
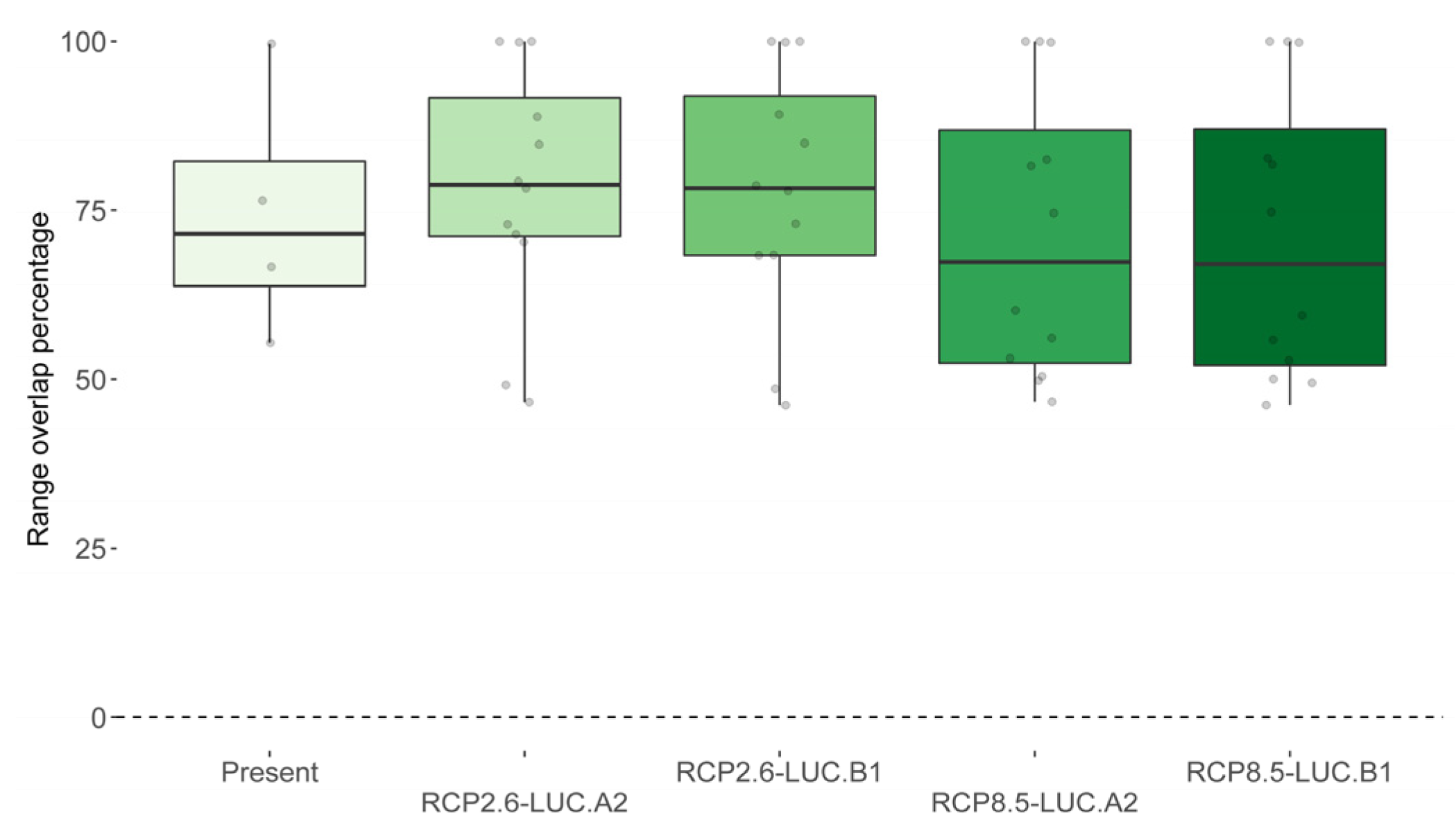
3.3. Spatial Overlap
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Codes | Names |
|---|---|
| BIO1 | Annual Mean Temperature |
| BIO2 | Mean Diurnal Range (Mean of monthly (max temp–min temp)) |
| BIO3 | Isothermality (BIO2/BIO7) (×100) |
| BIO4 | Temperature Seasonality (standard deviation × 100) |
| BIO5 | Max Temperature of Warmest Month |
| BIO6 | Min Temperature of Coldest Month |
| BIO7 | Temperature Annual Range (BIO5-BIO6) |
| BIO8 | Mean Temperature of Wettest Quarter |
| BIO9 | Mean Temperature of Driest Quarter |
| BIO10 | Mean Temperature of Warmest Quarter |
| BIO11 | Mean Temperature of Coldest Quarter |
| BIO12 | Annual Precipitation |
| Scenario | Main Characteristics |
|---|---|
| RCP.26 | The scenario assumes that global annual CO2 emissions will peak between 2010–2020, declining substantially thereafter. A +1 °C global warming is forecasted [65] |
| RCP.85 | The scenario assumes CO2 emissions keep rising throughout the 21th century. A +2 °C global warming is forecasted [65]. |
| LUC.A2 | The scenario assumes a high population growth, a sprawling urban expansion and a medium economic growth. As a result, the scenario predicts an increase in farmlands and a decrease in grasslands and forests [44]. |
| LUC.B1 | The scenario assumes a low population growth, a compact urban expansion and a high economic growth. As a result, the scenario predicts a decrease in farmlands and an increase in grasslands and forests [44]. |

References
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated Modern Human-Induced Species Losses: Entering the Sixth Mass Extinction. Sci. Adv. 2015, 1, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Sirami, C.; Caplat, P.; Popy, S.; Clamens, A.; Arlettaz, R.; Jiguet, F.; Brotons, L.; Martin, J.L. Impacts of Global Change on Species Distributions: Obstacles and Solutions to Integrate Climate and Land Use. Glob. Ecol. Biogeogr. 2017, 26, 385–394. [Google Scholar] [CrossRef]
- Ohlemüller, R.; Anderson, B.J.; Araújo, M.B.; Butchart, S.H.M.; Kudrna, O.; Ridgely, R.S.; Thomas, C.D. The Coincidence of Climatic and Species Rarity: High Risk to Small-Range Species from Climate Change. Biol. Lett. 2008, 4, 568–572. [Google Scholar] [CrossRef]
- Hof, A.R.; Jansson, R.; Nilsson, C. Future Climate Change Will Favour Non-Specialist Mammals in the (Sub)Arctics. PLoS ONE 2012, 7, 52574. [Google Scholar] [CrossRef]
- Pacifici, M.; Rondinini, C.; Rhodes, J.R.; Burbidge, A.A.; Cristiano, A.; Watson, J.E.M.; Woinarski, J.C.Z.; Di Marco, M. Global Correlates of Range Contractions and Expansions in Terrestrial Mammals. Nat. Commun. 2020, 11, 2840. [Google Scholar] [CrossRef] [PubMed]
- Baisero, D.; Visconti, P.; Pacifici, M.; Cimatti, M.; Rondinini, C. Projected Global Loss of Mammal Habitat Due to Land-Use and Climate Change. One Earth 2020, 2, 578–585. [Google Scholar] [CrossRef]
- Boutin, S.; Lane, J.E.; Stan Boutin, C. Climate Change and Mammals: Evolutionary versus Plastic Responses. Evol. Appl. 2013, 7, 29–41. [Google Scholar] [CrossRef]
- Réale, D.; McAdam, A.G.; Boutin, S.; Berteaux, D. Genetic and Plastic Responses of a Northern Mammal to Climate Change. Proc. R. Soc. London Ser. B Biol. Sci. 2003, 270, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Hetem, R.S.; Fuller, A.; Maloney, S.K.; Mitchell, D. Responses of Large Mammals to Climate Change. Temperature 2014, 1, 115–127. [Google Scholar] [CrossRef]
- Williams, J.E.; Blois, J.L. Range Shifts in Response to Past and Future Climate Change: Can Climate Velocities and Species’ Dispersal Capabilities Explain Variation in Mammalian Range Shifts? J. Biogeogr. 2018, 45, 2175–2189. [Google Scholar] [CrossRef]
- Blois, J.L.; Zarnetske, P.L.; Fitzpatrick, M.C.; Finnegan, S. Climate Change and the Past, Present, and Future of Biotic Interactions. Science 2013, 341, 499–504. [Google Scholar] [CrossRef]
- Vucic-Pestic, O.; Ehnes, R.B.; Rall, B.C.; Brose, U. Warming up the System: Higher Predator Feeding Rates but Lower Energetic Efficiencies. Glob. Change Biol. 2011, 17, 1301–1310. [Google Scholar] [CrossRef]
- Öhlund, G.; Hedström, P.; Norman, S.; Hein, C.L.; Englund, G. Temperature Dependence of Predation Depends on the Relative Performance of Predators and Prey. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142254. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.D.; Kovacs, K.M.; Ims, R.A.; Aars, J.; Lydersen, C. An Arctic Predator-Prey System in Flux: Climate Change Impacts on Coastal Space Use by Polar Bears and Ringed Seals. J. Anim. Ecol. 2017, 86, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Aryal, A.; Shrestha, U.B.; Ji, W.; Ale, S.B.; Shrestha, S.; Ingty, T.; Maraseni, T.; Cockfield, G.; Raubenheimer, D. Predicting the Distributions of Predator (Snow leopard) and Prey (Blue sheep) under Climate Change in the Himalaya. Ecol. Evol. 2016, 6, 4065–4075. [Google Scholar] [CrossRef]
- Bastille-Rousseau, G.; Schaefer, J.A.; Peers, M.J.L.; Ellington, E.H.; Mumma, M.A.; Rayl, N.D.; Mahoney, S.P.; Murray, D.L. Climate Change Can Alter Predator–Prey Dynamics and Population Viability of Prey. Oecologia 2018, 186, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Penteriani, V.; Zarzo-Arias, A.; Novo-Fernández, A.; Bombieri, G.; López-Sánchez, C.A. Responses of an Endangered Brown Bear Population to Climate Change Based on Predictable Food Resource and Shelter Alterations. Glob. Change Biol. 2019, 25, 1133–1151. [Google Scholar] [CrossRef]
- Quaglietta, L. Galemys Pyrenaicus. IUCN Red List Threat. Species 2021, e.T8826A20. [Google Scholar] [CrossRef]
- Morueta-Holme, N.; Fløjgaard, C.; Svenning, J.-C. Climate Change Risks and Conservation Implications for a Threatened Small-Range Mammal Species. PLoS ONE 2010, 5, e10360. [Google Scholar] [CrossRef]
- Charbonnel, A.; Laffaille, P.; Biffi, M.; Blanc, F.; Maire, A.; Némoz, M.; Sanchez-Perez, J.M.; Sauvage, S.; Buisson, L. Can Recent Global Changes Explain the Dramatic Range Contraction of an Endangered Semi-Aquatic Mammal Species in the French Pyrenees? PLoS ONE 2016, 11, e0159941. [Google Scholar] [CrossRef]
- Roos, A.; Loy, A.; de Silva, P.; Hajkova, P.; Zemanová, B. Lutra Lutra. IUCN Red List Threat. Species 2015, e.T12419A21935287. [Google Scholar]
- Hung, N.; Law, C.J. Lutra Lutra (Carnivora: Mustelidae). Mamm. Species 2016, 48, 109–122. [Google Scholar] [CrossRef]
- Clavero, M.; Prenda, J.; Delibes, M. Trophic Diversity of the Otter (Lutra lutra L.) in Temperate and Mediterranean Freshwater Habitats. J. Biogeogr. 2003, 30, 761–769. [Google Scholar] [CrossRef]
- Melero, Y.; Palazón, S.; Bonesi, L.; Gosàlbez, J. Feeding Habits of Three Sympatric Mammals in NE Spain: The American Mink, the Spotted Genet, and the Eurasian Otter. Acta Theriol. 2008, 53, 263–273. [Google Scholar] [CrossRef]
- Remonti, L.; Prigioni, C.; Balestrieri, A.; Sgrosso, S.; Priore, G. Eurasian Otter (Lutra lutra) Prey Selection in Response to a Variation of Fish Abundance. Ital. J. Zool. 2010, 77, 331–338. [Google Scholar] [CrossRef]
- Gillet, F.; Mouton, A.; Vanutryve, S.; Blanc, F.; Némoz, M.; Fournier, P.; Fournier Chambrillon, C.; Marc, D.; Michaux, J. Evidence of Predation and Possible Competition among Three Semi Aquatic Species, the Endangered Pyrenean Desman (Galemys pyrenaicus), the Aquatic Shrew (Neomys fodiens) and the European Otter (Lutra lutra) Using next-Generation Sequencing Methods from Fae. In Proceedings of the Congenomics Conference, Porto, Portugal, 3–6 May 2016. [Google Scholar]
- Fernández-López, J.; Fernández-González, Á.; Fernández-Menéndez, D. Confirmación de La Depredación de Nutria Paleártica Lutra Lutra (Linnaeus, 1758) Sobre Desmán Ibérico Galemys Pyrenaicus (E. Geoffroy Saint-Hilaire, 1811) Mediante El Empleo de Técnicas Moleculares. Galemys Span. J. Mammal. 2014, 22, 96–99. [Google Scholar] [CrossRef]
- Querejeta, M.; Fernández-González, A.; Romero, R.; Castresana, J. Postglacial Dispersal Patterns and Mitochondrial Genetic Structure of the Pyrenean Desman (Galemys pyrenaicus) in the Northwestern Region of the Iberian Peninsula. Ecol. Evol. 2017, 7, 4486–4495. [Google Scholar] [CrossRef]
- Cianfrani, C.; Broennimann, O.; Loy, A.; Guisan, A. More than Range Exposure: Global Otter Vulnerability to Climate Change. Biol. Conserv. 2018, 221, 103–113. [Google Scholar] [CrossRef]
- Cianfrani, C.; Lay, G.L.; Maiorano, L.; Satizábal, H.F.; Loy, A.; Guisan, A. Adapting Global Conservation Strategies to Climate Change at the European Scale: The Otter as a Flagship Species. Biol. Conserv. 2011, 144, 2068–2080. [Google Scholar] [CrossRef]
- Melero, Y.; Aymerich, P.; Santulli, G.; Gosàlbez, J. Activity and Space Patterns of Pyrenean Desman (Galemys pyrenaicus) Suggest Non-Aggressive and Non-Territorial Behaviour. Eur. J. Wildl. Res. 2014, 60, 707–715. [Google Scholar] [CrossRef]
- Melero, Y.; Aymerich, P.; Luque-Larena, J.J.; Gosàlbez, J. New Insights into Social and Space Use Behaviour of the Endangered Pyrenean Desman (Galemys pyrenaicus). Eur. J. Wildl. Res. 2012, 58, 185–193. [Google Scholar] [CrossRef]
- Gonzalez-Esteban, J.; Esnaola, A.; Aihartza, J. A New Sampling Method to Detect the Pyrenean Desman (Galemys pyrenaicus). Hystrix Ital. J. Mammal. 2018, 29, 190–194. [Google Scholar] [CrossRef]
- Grilo, C.; Afonso, B.C.; Afonso, F.; Alexandre, M.; Aliácar, S.; Almeida, A.; Alonso, I.P.; Álvares, F.; Alves, P.; Alves, P.C.; et al. MAMMALS IN PORTUGAL: A Data Set of Terrestrial, Volant, and Marine Mammal Occurrences in Portugal. Ecology 2022, 103, ecy.3654. [Google Scholar] [CrossRef] [PubMed]
- Palazón, S. La Nutria En España. Treinta Años de Seguimiento y Recuperación de Un Mamífero Amenazado; Sociedad Española para la Conservación y Estudio de los Mamíferos (SECEM), Grupo Nutria: Seville, Spain, 2021; pp. 279–303. [Google Scholar]
- Fabrizio, M.; Di Febbraro, M.; Loy, A. Where Will It Cross next? Optimal Management of Road Collision Risk for Otters in Italy. J. Environ. Manag. 2019, 251, 109609. [Google Scholar] [CrossRef]
- Russo, L.F.; Barrientos, R.; Fabrizio, M.; Di Febbraro, M.; Loy, A. Prioritizing Road-Kill Mitigation Areas: A Spatially Explicit National-Scale Model for an Elusive Carnivore. Divers. Distrib. 2020, 26, 1093–1103. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Escoda, L.; Fernández-González, Á.; Castresana, J. Quantitative Analysis of Connectivity in Populations of a Semi-Aquatic Mammal Using Kinship Categories and Network Assortativity. Mol. Ecol. Resour. 2019, 19, 310–326. [Google Scholar] [CrossRef]
- Gillet, F.; Le Roux, B.; Blanc, F.; Bodo, A.; Fournier-Chambrillon, C.; Fournier, P.; Jacob, F.; Lacaze, V.; Némoz, M.; Aulagnier, S.; et al. Genetic Monitoring of the Endangered Pyrenean Desman (Galemys pyrenaicus) in the Aude River, France. Belg. J. Zool. 2016, 146, 44–52. [Google Scholar] [CrossRef]
- Quaglietta, L.; Fonseca, V.C.; Mira, A.; Boitani, L. Sociospatial Organization of a Solitary Carnivore, the Eurasian Otter (Lutra lutra). J. Mammal. 2014, 95, 140–150. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Liu, X.; Liang, X.; Wang, S.; Chen, Y.; Pei, F.; Xu, X. A New Global Land-Use and Land-Cover Change Product at a 1-Km Resolution for 2010 to 2100 Based on Human–Environment Interactions. Ann. Am. Assoc. Geogr. 2017, 107, 1040–1059. [Google Scholar] [CrossRef]
- Jamwal, P.S.; Di Febbraro, M.; Carranza, M.L.; Savage, M.; Loy, A. Global Change on the Roof of the World: Vulnerability of Himalayan Otter Species to Land Use and Climate Alterations. Divers. Distrib. 2022, 28, 1635–1649. [Google Scholar] [CrossRef]
- Sala, O.E.; Stuart Chapin, F., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Schneider, A.; Jost, A.; Coulon, C.; Silvestre, M.; Théry, S.; Ducharne, A. Global-Scale River Network Extraction Based on High-Resolution Topography and Constrained by Lithology, Climate, Slope, and Observed Drainage Density. Geophys. Res. Lett. 2017, 44, 2773–2781. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bauer, S.; Shamoun-Baranes, J.; Nilsson, C.; Farnsworth, A.; Kelly, J.F.; Reynolds, D.R.; Dokter, A.M.; Krauel, J.F.; Petterson, L.B.; Horton, K.G.; et al. The Grand Challenges of Migration Ecology That Radar Aeroecology Can Help Answer. Ecography 2019, 42, 861–875. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The Crucial Role of the Accessible Area in Ecological Niche Modeling and Species Distribution Modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-Validation Strategies for Data with Temporal, Spatial, Hierarchical, or Phylogenetic Structure. Ecography (Cop.) 2017, 40, 913–929. [Google Scholar] [CrossRef]
- Fourcade, Y.; Besnard, A.G.; Secondi, J. Paintings Predict the Distribution of Species, or the Challenge of Selecting Environmental Predictors and Evaluation Statistics. Glob. Ecol. Biogeogr. 2018, 27, 245–256. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for Customizable and Reproducible Modeling of Species’ Niches and Distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Pio, D.V.; Engler, R.; Linder, H.P.; Monadjem, A.; Cotterill, F.P.D.; Taylor, P.J.; Schoeman, M.C.; Price, B.W.; Villet, M.H.; Eick, G.; et al. Climate Change Effects on Animal and Plant Phylogenetic Diversity in Southern Africa. Glob. Change Biol. 2014, 20, 1538–1549. [Google Scholar] [CrossRef]
- Jørgensen, S.E. Model Selection and Multimodel Inference. Ecol. Model. 2004, 172, 96–97. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Sci. Sci. 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Martinoli, A.; Russo, D.; Preatoni, D.; Bertolino, S. Modelling the Effects of Climate Change on the Risk of Invasion by Alien Squirrels. Hystrix 2016, 27, 1–8. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of Consensus Methods in Predictive Species Distribution Modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.M.B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013 the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridg, UK, 2013. [Google Scholar] [CrossRef]
- Buisson, L.; Thuiller, W.; Casajus, N.; Lek, S.; Grenouillet, G. Uncertainty in Ensemble Forecasting of Species Distribution. Glob. Change Biol. 2010, 16, 1145–1157. [Google Scholar] [CrossRef]
- Sanderson, B.M.; Knutti, R.; Caldwell, P. A Representative Democracy to Reduce Interdependency in a Multimodel Ensemble. J. Clim. 2015, 28, 5171–5194. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Menchetti, M.; Russo, D.; Ancillotto, L.; Aloise, G.; Roscioni, F.; Preatoni, D.G.; Loy, A.; Martinoli, A.; Bertolino, S.; et al. Integrating Climate and Land-use Change Scenarios in Modelling the Future Spread of Invasive Squirrels in Italy. Divers. Distrib. 2019, 25, 644–659. [Google Scholar] [CrossRef]
- Engler, R.; Hordijk, W.; Guisan, A. The MIGCLIM R Package—Seamless Integration of Dispersal Constraints into Projections of Species Distribution Models. Ecography (Cop.) 2012, 35, 872–878. [Google Scholar] [CrossRef]
- Sales, L.P.; Ribeiro, B.R.; Pires, M.M.; Chapman, C.A.; Loyola, R. Recalculating Route: Dispersal Constraints Will Drive the Redistribution of Amazon Primates in the Anthropocene. Ecography 2019, 42, 1789–1801. [Google Scholar] [CrossRef]
- Franklin, J.; Davis, F.W.; Ikegami, M.; Syphard, A.D.; Flint, L.E.; Flint, A.L.; Hannah, L. Modeling Plant Species Distributions under Future Climates: How Fine Scale Do Climate Projections Need to Be? Glob. Change Biol. 2013, 19, 473–483. [Google Scholar] [CrossRef]
- Jo, Y.S.; Won, C.M.; Fritts, S.R.; Wallace, M.C.; Baccus, J.T. Distribution and Habitat Models of the Eurasian Otter, Lutra Lutra, in South Korea. J. Mammal. 2017, 98, 1105–1117. [Google Scholar] [CrossRef]
- Biffi, M.; Charbonnel, A.; Buisson, L.; Blanc, F.; Némoz, M.; Laffaille, P. Spatial Differences across the French Pyrenees in the Use of Local Habitat by the Endangered Semi-Aquatic Pyrenean Desman (Galemys pyrenaicus). Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 761–774. [Google Scholar] [CrossRef]
- Prenda, J.; Granado-Lorencio, C. The Relative Influence of Riparian Habitat Structure and Fish Availability on Otter Lutra lutra L. Sprainting Activity in a Small Mediterranean Catchment. Biol. Conserv. 1996, 76, 9–15. [Google Scholar] [CrossRef]
- Quaglietta, L.; Paupério, J.; Martins, F.M.S.; Alves, P.C.; Beja, P. Recent Range Contractions in the Globally Threatened Pyrenean Desman Highlight the Importance of Stream Headwater Refugia. Anim. Conserv. 2018, 21, 515–525. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J.; Olmo-Vidal, J.M.; Mañas, S.; Batet, A. The Influence of Resource Seasonality on the Breeding Patterns of the Eurasian Otter (Lutra Lutra) in Mediterranean Habitats. Can. J. Zool. 2002, 80, 2178–2189. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Real, R.; Olivero, J.; Vargas, J.M. Otter (Lutra lutra) Distribution Modeling at Two Resolution Scales Suited to Conservation Planning in the Iberian Peninsula. Biol. Conserv. 2003, 114, 377–387. [Google Scholar] [CrossRef]
- Mason, C.F.; Macdonald, S.M. Growth in Otter (Lutra lutra) Populations in the UK as Shown by Long-Term Monitoring. Ambio 2004, 33, 148–152. [Google Scholar] [CrossRef]
- Elmeros, M.; Hammershøj, M.; Madsen, A.; Søgaard, B. Recovery of the Otter Lutra Lutra in Denmark Monitored by Field Surveys and Collection of Carcasses. Hystrix Ital. J. Mammal. 2006, 17, 17–28. [Google Scholar] [CrossRef]
- McDonald, R.A.; O’Hara, K.; Morrish, D.J. Decline of Invasive Alien Mink (Mustela Vison) Is Concurrent with Recovery of Native Otters (Lutra lutra). Divers. Distrib. 2007, 13, 92–98. [Google Scholar] [CrossRef]
- Russo, L.F.; Meloro, C.; De Silvestri, M.; Chadwick, E.A.; Loy, A. Better Sturdy or Slender? Eurasian Otter Skull Plasticity in Response to Feeding Ecology. PLoS ONE 2022, 17, e0274893. [Google Scholar] [CrossRef]
- Lanszki, J.; Lehoczky, I.; Kotze, A.; Somers, M.J. Diet of Otters (Lutra lutra) in Various Habitat Types in the Pannonian Biogeographical Region Compared to Other Regions of Europe. PeerJ 2016, 4, e2266. [Google Scholar] [CrossRef]
- Martínez-Abraín, A.; Marí-Mena, N.; Vizcaíno, A.; Vierna, J.; Veloy, C.; Amboage, M.; Guitián-Caamaño, A.; Key, C.; Vila, M. Determinants of Eurasian Otter (Lutra lutra) Diet in a Seasonally Changing Reservoir. Hydrobiologia 2020, 847, 1803–1816. [Google Scholar] [CrossRef]
- Moorhouse-Gann, R.J.; Kean, E.F.; Parry, G.; Valladares, S.; Chadwick, E.A. Dietary Complexity and Hidden Costs of Prey Switching in a Generalist Top Predator. Ecol. Evol. 2020, 10, 6395–6408. [Google Scholar] [CrossRef]
- Remonti, L.; Prigioni, C.; Balestrieri, A.; Sgrosso, S.; Priore, G. Trophic Flexibility of the Otter (Lutra lutra) in Southern Italy. Mamm. Biol. 2008, 73, 293–302. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J.; Jiménez, J. Diet Diversity and Breeding of Top Predators Are Determined by Habitat Stability and Structure: A Case Study with the Eurasian Otter (Lutra lutra L.). Eur. J. Wildl. Res. 2009, 55, 133–144. [Google Scholar] [CrossRef]
- Esnaola, A.; González-Esteban, J.; Elosegi, A.; Arrizabalaga-Escudero, A.; Aihartza, J. Need for Speed: Preference for Fast-Flowing Water by the Endangered Semi-Aquatic Pyrenean Desman (Galemys pyrenaicus) in Two Contrasting Streams. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 600–609. [Google Scholar] [CrossRef]
- Suraci, J.P.; Smith, J.A.; Chamaillé-Jammes, S.; Gaynor, K.M.; Jones, M.; Luttbeg, B.; Ritchie, E.G.; Sheriff, M.J.; Sih, A. Beyond Spatial Overlap: Harnessing New Technologies to Resolve the Complexities of Predator–Prey Interactions. Oikos 2022, 2022, e09004. [Google Scholar] [CrossRef]
- Sims, D.W.; Witt, M.J.; Richardson, A.J.; Southall, E.J.; Metcalfe, J.D. Encounter Success of Free-Ranging Marine Predator Movements across a Dynamic Prey Landscape. Proc. R. Soc. B Biol. Sci. 2006, 273, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Turesson, H.; Brönmark, C. Predator-Prey Encounter Rates in Freshwater Piscivores: Effects of Prey Density and Water Transparency. Oecologia 2007, 153, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.T.; Stahler, D.R.; Metz, M.C.; Forester, J.D.; Kauffman, M.J.; Varley, N.; White, P.J.; Smith, D.W.; MacNulty, D.R. Diel Predator Activity Drives a Dynamic Landscape of Fear. Ecol. Monogr. 2018, 88, 638–652. [Google Scholar] [CrossRef]
- Caravaggi, A.; Gatta, M.; Vallely, M.C.; Hogg, K.; Freeman, M.; Fadaei, E.; Dick, J.T.A.; Montgomery, W.I.; Reid, N.; Tosh, D.G. Seasonal and Predator-Prey Effects on Circadian Activity of Free-Ranging Mammals Revealed by Camera Traps. PeerJ 2018, 2018, 5827. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, L.F.; Fernández-González, Á.; Penteriani, V.; Delgado, M.d.M.; Palazón, S.; Loy, A.; Di Febbraro, M. The Different Fate of the Pyrenean Desman (Galemys pyrenaicus) and the Eurasian Otter (Lutra lutra) under Climate and Land Use Changes. Animals 2023, 13, 274. https://doi.org/10.3390/ani13020274
Russo LF, Fernández-González Á, Penteriani V, Delgado MdM, Palazón S, Loy A, Di Febbraro M. The Different Fate of the Pyrenean Desman (Galemys pyrenaicus) and the Eurasian Otter (Lutra lutra) under Climate and Land Use Changes. Animals. 2023; 13(2):274. https://doi.org/10.3390/ani13020274
Chicago/Turabian StyleRusso, Luca Francesco, Ángel Fernández-González, Vincenzo Penteriani, María del Mar Delgado, Santiago Palazón, Anna Loy, and Mirko Di Febbraro. 2023. "The Different Fate of the Pyrenean Desman (Galemys pyrenaicus) and the Eurasian Otter (Lutra lutra) under Climate and Land Use Changes" Animals 13, no. 2: 274. https://doi.org/10.3390/ani13020274
APA StyleRusso, L. F., Fernández-González, Á., Penteriani, V., Delgado, M. d. M., Palazón, S., Loy, A., & Di Febbraro, M. (2023). The Different Fate of the Pyrenean Desman (Galemys pyrenaicus) and the Eurasian Otter (Lutra lutra) under Climate and Land Use Changes. Animals, 13(2), 274. https://doi.org/10.3390/ani13020274






