Insect Peptide CopA3 Mitigates the Effects of Heat Stress on Porcine Muscle Satellite Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Peptide Synthesis
2.3. PMSC Isolation
2.4. Immunocytochemistry
2.5. Experimental Design and Cell Culture Conditions
2.6. Cell Viability Assay
2.7. Flow Cytometry Analysis
2.8. Protein Extraction and Western Blotting Analysis
2.9. Statistical Analyses
3. Results
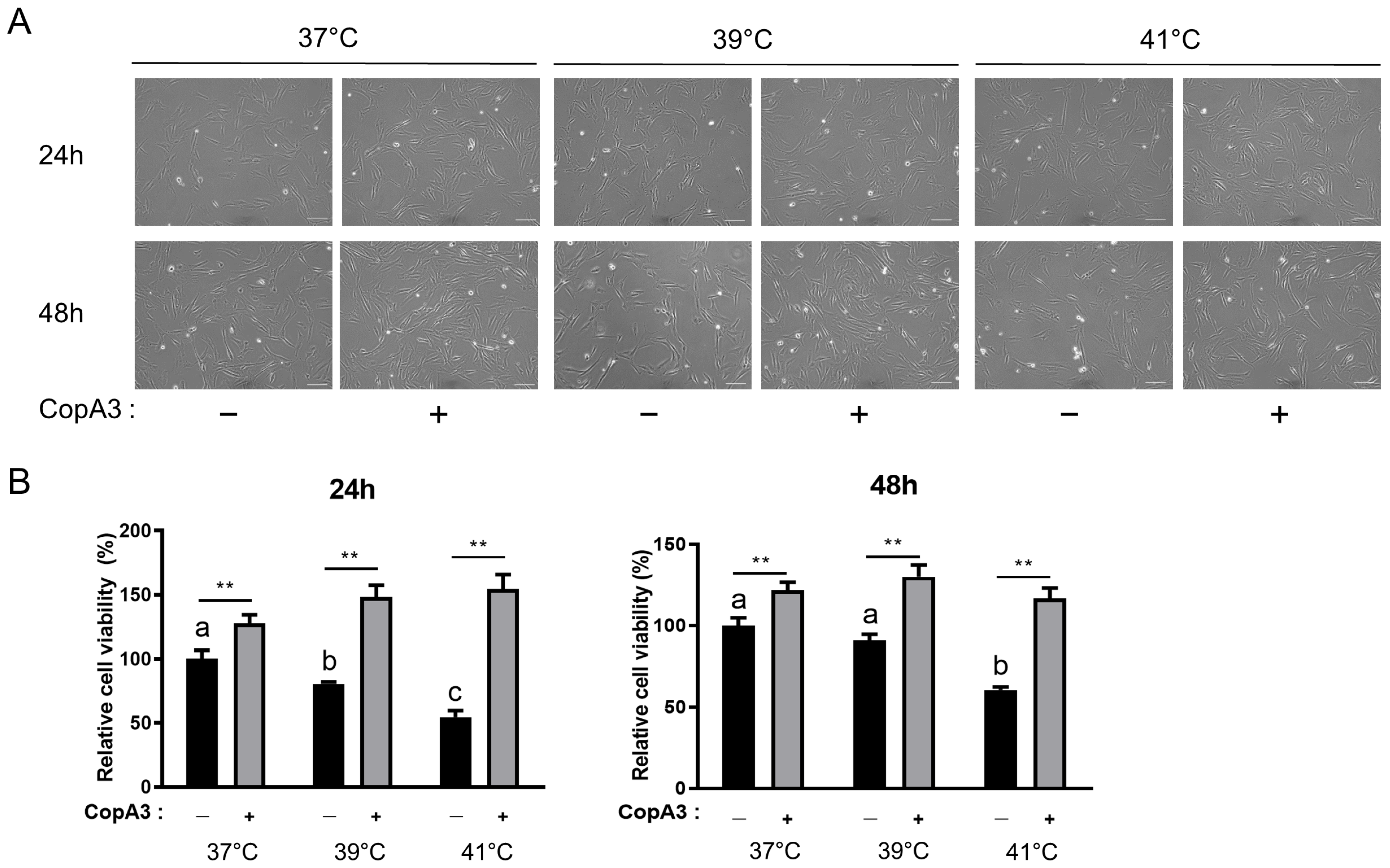
3.1. CopA3 Cytotoxicity on PMSCs
3.2. Effect of CopA3 on PMSC Viability
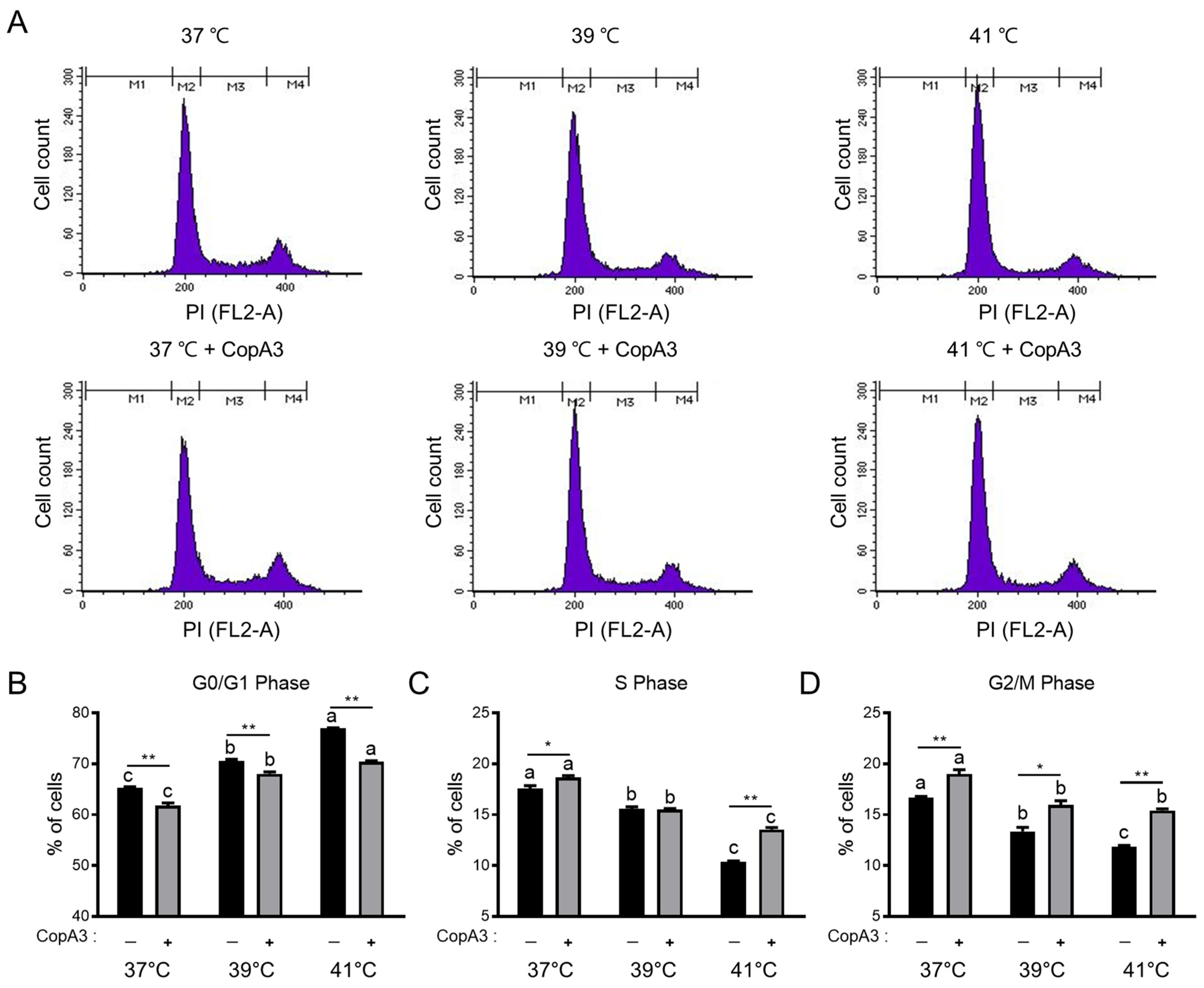
3.3. Effect of CopA3 on the Cell Cycle Distribution of PMSCs
3.4. Effect of CopA3 on PMSC Apoptosis
3.5. Changes in the Expression Levels of HSPs in PMSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Kang, D.; Park, J.; Khan, M.; Belal, S.A.; Shin, D.; Shim, K. Altered relationship between gluconeogenesis and immunity in broilers exposed to heat stress for different durations. Poult. Sci. 2021, 100, 101274. [Google Scholar] [CrossRef]
- Kurop, M.K.; Huyen, C.M.; Kelly, J.H.; Blagg, B.S. The heat shock response and small molecule regulators. Eur. J. Med. Chem. 2021, 226, 113846. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.T.; Wang, H.; Li, L.; Liu, Y.; Deng, X.; Huo, S.; Yuan, F.; Liu, Z.; Tong, H.; Su, L. Heat stress induces apoptosis through transcription-independent p53-mediated mitochondrial pathways in human umbilical vein endothelial cell. Sci. Rep. 2014, 4, 4469. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional physiology and biochemistry of dairy cattle under the influence of heat stress: Consequences and opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Reynolds, C.; Hollinger, K.; Pearce, S.C.; Gabler, N.K.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1288–R1296. [Google Scholar] [CrossRef]
- Ganesan, S.; Volodina, O.; Pearce, S.C.; Gabler, N.K.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Acute heat stress activated inflammatory signaling in porcine oxidative skeletal muscle. Physiol. Rep. 2017, 5, e13397. [Google Scholar] [CrossRef]
- Montilla, S.I.R.; Johnson, T.P.; Pearce, S.C.; Gardan-Salmon, D.; Gabler, N.K.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Lonergan, S.M.; Selsby, J.T. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature 2014, 1, 42–50. [Google Scholar] [CrossRef]
- Tsan, M.-F.; Gao, B. Heat shock protein and innate immunity. Cell. Mol. Immunol. 2004, 1, 274–279. [Google Scholar]
- Yu, J.; Yin, P.; Liu, F.; Cheng, G.; Guo, K.; Lu, A.; Zhu, X.; Luan, W.; Xu, J. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 119–128. [Google Scholar] [CrossRef]
- Li, B.-J.; Li, P.-H.; Huang, R.-H.; Sun, W.-X.; Wang, H.; Li, Q.-F.; Chen, J.; Wu, W.-J.; Liu, H.L. Isolation, culture and identification of porcine skeletal muscle satellite cells. Asian-Australas. J. Anim. Sci. 2015, 28, 1171. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, J.; Shim, K. Effects of heat stress exposure on porcine muscle satellite cells. J. Therm. Biol. 2023, 114, 103569. [Google Scholar] [CrossRef]
- Nam, S.T.; Kim, D.H.; Lee, M.B.; Nam, H.J.; Kang, J.K.; Park, M.J.; Lee, I.H.; Seok, H.; Lee, D.G.; Hwang, J.S. Insect peptide CopA3-induced protein degradation of p27Kip1 stimulates proliferation and protects neuronal cells from apoptosis. Biochem. Biophys. Res. Commun. 2013, 437, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hwang, J.S.; Lee, I.H.; Nam, S.T.; Hong, J.; Zhang, P.; Lu, L.F.; Lee, J.; Seok, H.; Pothoulakis, C.; et al. The insect peptide CopA3 increases colonic epithelial cell proliferation and mucosal barrier function to prevent inflammatory responses in the gut. J. Biochem. 2016, 291, 3209–3223. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, I.W.; Kim, S.H.; Yun, E.Y.; Nam, S.-H.; Ahn, M.Y.; Kang, D.C.; Hwang, J.S. Anticancer activity of CopA3 dimer peptide in human gastric cancer cells. BMB Rep. 2015, 48, 324. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, M.; Lee, H.J.; Baek, M.; Kim, I.W.; Kim, S.Y.; Kim, M.A.; Kim, S.H.; Hwang, J.S. Anti-inflammatory activity of antimicrobial peptide allomyrinasin derived from the dynastid beetle, allomyrina dichotoma. J. Microbiol. Biotechnol. 2019, 29, 687–695. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Khan, M.; Park, J.; Lee, J.; Choe, H.; Shim, K.; Kang, D. COPA3 peptide supplementation alleviates the heat stress of chicken fibroblasts. Front. Vet. Sci. 2023, 10, 985040. [Google Scholar] [CrossRef]
- Cottrell, J.; Liu, F.; Hung, A.; DiGiacomo, K.; Chauhan, S.; Leury, B.; Furness, J.; Celi, P.; Dunshea, F.R. Nutritional strategies to alleviate heat stress in pigs. Anim. Prod. Sci. 2015, 55, 1391–1402. [Google Scholar] [CrossRef]
- Renaudeau, D.; Collin, A.; Yahav, S.; De Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728. [Google Scholar] [CrossRef]
- Hu, F.; Gao, X.; She, R.; Chen, J.; Mao, J.; Xiao, P.; Shi, R.J. Effects of antimicrobial peptides on growth performance and small intestinal function in broilers under chronic heat stress. Animal 2017, 96, 798–806. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, T.; Li, S.; Wang, W.; Wang, Y.; Cao, Z.; Yang, H. Effects of selenium as a dietary source on performance, inflammation, cell damage, and reproduction of livestock induced by heat stress: A review. Front. Immunol. 2022, 12, 820853. [Google Scholar] [CrossRef] [PubMed]
- Harding, R.L.; Halevy, O.; Yahav, S.; Velleman, S.G. The effect of temperature on proliferation and differentiation of chicken skeletal muscle satellite cells isolated from different muscle types. Physiol. Rep. 2016, 4, e12770. [Google Scholar] [CrossRef] [PubMed]
- Tumaneng, K.; Russell, R.C.; Guan, K.L. Organ size control by Hippo and TOR pathways. Curr. Biol. 2012, 22, R368–R379. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Iizumi, T.; Fujiwara, Y.; Zhao, Q.L.; Tabuchi, Y.; Nomura, T.; Kondo, T. Inhibition of checkpoint kinase 1 abrogates G2/M checkpoint activation and promotes apoptosis under heat stress. Apoptosis 2012, 17, 102–112. [Google Scholar] [CrossRef]
- Kühl, N.; Rensing, L. Heat shock effects on cell cycle progression. Cell. Mol. Life Sci. CMLS 2000, 57, 450–463. [Google Scholar] [CrossRef]
- Schafer, K.A. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, J.S.; Yoon, I.N.; Lee, J.H.; Lee, J.; Park, K.C.; Seok, H.; Kim, H. The insect peptide CopA3 blocks programmed cell death by directly binding caspases and inhibiting their proteolytic activation. Biochem. Biophys. Res. Commun. 2021, 547, 82–88. [Google Scholar] [CrossRef]
- Gu, Z.; Li, L.; Wu, F.; Zhao, P.; Yang, H.; Liu, Y.; Geng, Y.; Zhao, M.; Su, L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015, 5, 11497. [Google Scholar] [CrossRef]
- Vogel, S.; Raulf, N.; Bregenhorn, S.; Biniossek, M.L.; Maurer, U.; Czabotar, P.; Borner, C. Cytosolic Bax: Does it require binding proteins to keep its pro-apoptotic activity in check? J. Biol. Chem. 2012, 287, 9112–9127. [Google Scholar] [CrossRef]
- Wang, X. development. The expanding role of mitochondria in apoptosis. Genes Dev. 2001, 15, 2922–2933. [Google Scholar]
- Gabai, V.L.; Sherman, M.Y. Invited review: Interplay between molecular chaperones and signaling pathways in survival of heat shock. J. Appl. Physiol. 2002, 92, 1743–1748. [Google Scholar] [CrossRef]
- Al-Zuhaeri, A.; Al-Shakour, A.; Ali Mansour, A. Serum level of heat shock protein 70 in patients with type 2 diabetes mellitus in Basrah, Iraq. Arch. Razi Inst. 2022, 77, 1837–1844. [Google Scholar]
- Amirkavei, M.; Plastino, F.; Kvanta, A.; Kaarniranta, K.; André, H.; Koskelainen, A. Hormetic heat shock enhances autophagy through HSF1 in retinal pigment epithelium cells. Cells 2022, 11, 1778. [Google Scholar] [CrossRef]
- Thakur, S.S.; James, J.L.; Cranna, N.J.; Chhen, V.L.; Swiderski, K.; Ryall, J.G.; Lynch, G.S. Expression and localization of heat-shock proteins during skeletal muscle cell proliferation and differentiation and the impact of heat stress. Cell Stress Chaperones 2019, 24, 749–761. [Google Scholar] [CrossRef]
- Mayer, M.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Ferry, A.L.; Dupont-Versteegden, E.E.J.A. Cell death-resistance of differentiated myotubes is associated with enhanced anti-apoptotic mechanisms compared to myoblasts. Apoptosis 2011, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.K.; Singh, A.; Yadav, N.K.; Cheruvu, S.H.; Hossain, Z.; Meena, S.; Maheshwari, S.; Singh, A.K.; Shahab, U.; Sharma, C.; et al. Anti-breast tumor activity of Eclipta extract in-vitro and in-vivo: Novel evidence of endoplasmic reticulum specific localization of Hsp60 during apoptosis. Sci. Rep. 2015, 5, 18457. [Google Scholar] [CrossRef] [PubMed]
- Veereshwarayya, V.; Kumar, P.; Rosen, K.M.; Mestril, R.; Querfurth, H.W. Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular β-amyloid-induced inhibition of complex IV and limit apoptosis. J. Biol. Chem. 2006, 281, 29468–29478. [Google Scholar] [CrossRef]
- Deocaris, C.C.; Kaul, S.C.; Wadhwa, R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 2006, 11, 116. [Google Scholar] [CrossRef]
- Taguchi, T.; Razzaque, M.S. The collagen-specific molecular chaperone HSP47: Is there a role in fibrosis? Trends Mol. Med. 2007, 13, 45–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Cao, Y.; Zhang, S.; Li, H.; Huang, Y.; Ding, Y.-Q.; Liu, X. The Hsp40 family chaperone protein DnaJB6 enhances Schlafen1 nuclear localization which is critical for promotion of cell-cycle arrest in T-cells. Biochem. J. 2008, 413, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tsai, B.; Li, N.; Gao, N. Structural remodeling of ribosome associated Hsp40-Hsp70 chaperones during co-translational folding. Nat. Commun. 2022, 13, 3410. [Google Scholar] [CrossRef] [PubMed]
- Sahu, W.; Bai, T.; Panda, P.K.; Mazumder, A.; Das, A.; Ojha, D.K.; Verma, S.K.; Elangovan, S.; Reddy, K.S. Plasmodium falciparum HSP40 protein eCiJp traffics to the erythrocyte cytoskeleton and interacts with the human HSP70 chaperone HSPA1. FEBS Lett. 2022, 596, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.; Bursać, D.; Law, Y.C.; Cyr, D.; Lithgow, T. The J-protein family: Modulating protein assembly, disassembly and translocation. EMBO Rep. 2004, 5, 567–571. [Google Scholar] [CrossRef]
- Xiao, Y.; Rungruang, S.; Hall, L.; Collier, J.; Dunshea, F.; Collier, R.J. Effects of niacin and betaine on bovine mammary and uterine cells exposed to thermal shock in vitro. J. Dairy Sci. 2017, 100, 4025–4037. [Google Scholar] [CrossRef]
- Willingham, B.D.; Ragland, T.J.; Ormsbee, M.J. Betaine supplementation may improve heat tolerance: Potential mechanisms in humans. Nutrients 2020, 12, 2939. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, C.; Xuan, Y.; Zhou, P.; Fang, Z.; Che, L.; Xu, S.; Feng, B.; Li, J.; Jiang, X.; et al. Effect of maternal or post-weaning methyl donor supplementation on growth performance, carcass traits, and meat quality of pig offspring. J. Sci. Food Agric. 2019, 99, 2096–2107. [Google Scholar] [CrossRef]
- Tang, J.; Cao, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; Shang, H.; Zhao, H. The protective effect of selenium from heat stress-induced porcine small intestinal epithelial cell line (IPEC-J2) injury is associated with regulation expression of selenoproteins. Br. J. Nutr. 2019, 122, 1081–1090. [Google Scholar] [CrossRef]
- Liu, Y.; He, A.; Tang, J.; Shah, A.M.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; Kang, B.; et al. Selenium alleviates the negative effect of heat stress on myogenic differentiation of C2C12 cells with the response of selenogenome. J. Therm. Biol. 2021, 97, 102874. [Google Scholar] [CrossRef]
- Bathaie, S.; Bahmani, F.; Farajzadeh, A. Handbook of Nutrition, Diet and the Eye; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Zhou, L.; Lu, R.; Huang, C.; Lin, D. Taurine protects C2C12 myoblasts from impaired cell proliferation and myotube differentiation under cisplatin-induced ROS exposure. Front. Mol. Biosci. 2021, 8, 685362. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, T.; Yu, Y.; Zhou, N.; Kou, H.; Guo, Y.; Yang, L.; Yan, P. Cytoprotective effects of taurine on heat-induced bovine mammary epithelial cells in vitro. Cells 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Belal, S.A.; Lin, X.; Park, J.; Shim, K. Insect Peptide CopA3 Mitigates the Effects of Heat Stress on Porcine Muscle Satellite Cells. Animals 2023, 13, 3209. https://doi.org/10.3390/ani13203209
Lee J, Belal SA, Lin X, Park J, Shim K. Insect Peptide CopA3 Mitigates the Effects of Heat Stress on Porcine Muscle Satellite Cells. Animals. 2023; 13(20):3209. https://doi.org/10.3390/ani13203209
Chicago/Turabian StyleLee, Jeongeun, Shah Ahmed Belal, Xi Lin, Jinryong Park, and Kwanseob Shim. 2023. "Insect Peptide CopA3 Mitigates the Effects of Heat Stress on Porcine Muscle Satellite Cells" Animals 13, no. 20: 3209. https://doi.org/10.3390/ani13203209




