1. Introduction
The behaviour of pigs is highly influenced by the environment in the building [
1], although it is also affected by other parameters, such as gas concentration, illuminance, noise, or health problems [
2,
3,
4,
5,
6,
7]. Animal activity is an important indicator of welfare that is especially relevant in intensive systems [
8] because it is an early gauge for behavioural changes [
9] that are caused by poor environmental conditions in the building or by health issues, among others [
1,
5,
9,
10,
11]. Therefore, measuring animal activity can provide key data to reduce deficiencies in animal welfare, thus improving production efficiency and enabling sustainable pig farming [
9].
The activity of pigs during their different growth stages is influenced by various factors such as breed, health condition, population density, housing system or lighting, among others. Also, the time of day in which observations are conducted is highly relevant [
12]. In fact, there are substantial differences in the amount of activity of pigs between daytime and night-time, and their patterns of activity show variations depending on the time of day and the stage of growth [
13]. Daily activity generally fits models with two peaks [
8,
12,
14,
15], although single-peak models have also been found during the first weeks postweaning [
14] or in buildings with natural ventilation [
12]. In other studies, daily activity has shown four daily peaks [
13].
Unlike other environmental parameters, such as room temperature, light has not received enough attention within pig farming. Although inadequate lighting is known to have a negative impact on the welfare of many animals in captivity [
2,
16,
17,
18], knowledge of the consequences of different types of illuminance on pig housing is limited. In fact, the ideal illuminance level is not yet clear [
19].
Preferences of growing pigs for different illuminances were found to depend on the activities undertaken [
3,
4,
10]. However, a number of studies concerned with illuminance in the building have reported contradictory results in terms of the preference for light or darkness [
20,
21,
22]. Whereas [
20] suggests that piglets prefer illuminated compartments, [
21] claims the opposite for young pigs, and [
22] does not find any preference for illuminated or non-illuminated pens. Furthermore, it has been proven that high levels of illuminance increase tolerance to other stressors (elevated concentrations of ammonia and noise) [
3], which contradicts the findings reported by [
23], who found less aggressive behaviours in darker pens.
Photoperiod research has determined that an increase in photoperiod length causes an increase in feed intake [
24,
25]. However, no positive effects on growth performance were found for long photoperiods [
26,
27], which may be caused by the increase in activity that occurs when the photoperiod increases [
16,
26].
Using natural lighting in weaned piglet buildings has proved more beneficial than using artificial lighting [
28] because it reduces competition over feed and the potential startling effects when lights are turned on or off [
27]. Based on the literature review conducted, although the influence of illuminance on productivity and general animal behaviour is known [
4,
29], the ideal illuminance level is not clear, and further research is needed on this topic [
16,
19].
Noise level is an expression of the behaviour of the pigs and, for this reason, it conveys information regarding their current health and welfare situation [
30,
31]. Currently, there is a need to establish indicators of animal welfare that may be relevant for professionals, and also quick and easy to implement. A number of authors have claimed that the level of noise could be one of those indicators [
32,
33]. There is hardly any information in the scientific literature regarding noise levels in pig farming. Accordingly, further research is needed to determine whether noise level is a suitable variable to draw conclusions about animal welfare, although it may be one of the indicators of welfare [
34].
Some authors have not found evidence that continuous exposure to a noise level of ~80 dB (A) has negative effects on pigs, which may be due to their quick adaptation to that noise [
3]. In fact, the hypothesis of a relationship between high levels of noise and animal welfare is not confirmed, which has led to the conclusion that noise levels under 85 dB do not seem to affect animal welfare [
34]. However, repeated exposure to 90 dB may cause stress to growing pigs, affecting productivity and animal welfare [
35,
36]. Moreover, a correct interpretation of their vocalisations may determine the causes for a lower level of welfare [
37]. The vocalisations and other noises performed by the animals contain information concerning their emotional, physiological and individual state, which can prove useful in assessing their health condition and welfare [
30,
38], as well as the environment of the stable [
39]. This may contribute to the early detection of several problems such as the outbreak of illnesses, or the prevention of escalations in aggressiveness. Various authors estimated pig welfare by analysing the sound waves they produced [
33,
40] and even drew a distinction for noises that were caused by pain [
41,
42]. The time of day was also proven to have an influence, particularly during feeding [
43], with higher levels of noise detected for pigs that were fed automatically [
24].
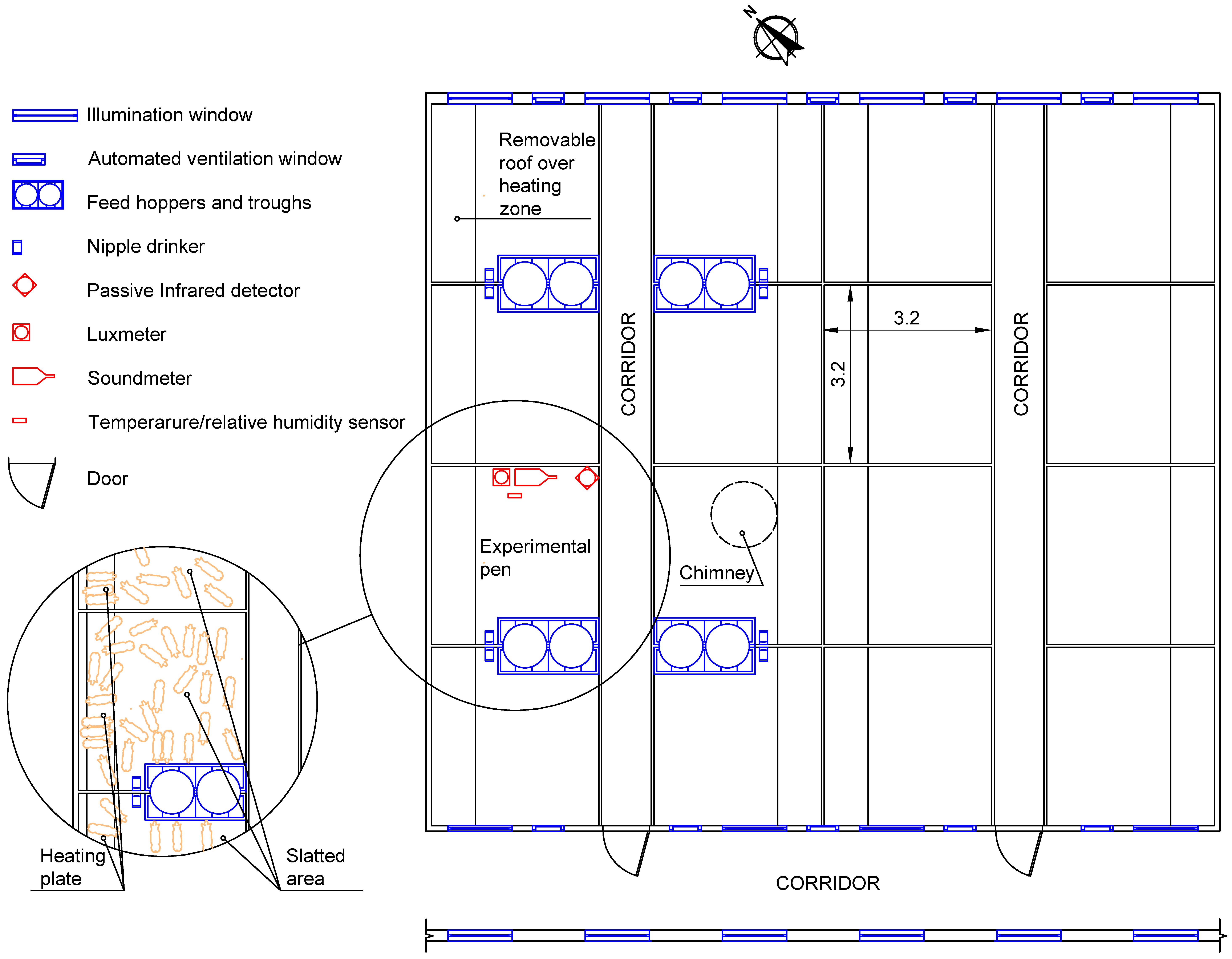
Various methods have been used to measure animal activity, among which are microphones [
39,
44], sensors attached to the animals [
45,
46], analysis of video footage [
47] and other low-cost practices like passive infrared detectors (PID) [
8,
14]. These methods for monitoring and analysing animal behaviour are widely used to quantify levels of animal activity, albeit with the disadvantage of not allowing for individual observation of animals housed in groups. In general, these methods are used exclusively for research and require a higher level of development if they are to be used on commercial livestock farms [
14] to improve health and animal welfare or to improve efficiency [
48]. Passive infrared detectors have proved useful and reliable for measuring pig activity, regardless of the number of animals or the structure of the pen [
11], showing strong correlations with video footage [
11,
14,
15]. Nevertheless, passive infrared detectors pose several problems, such as placement, physical barriers on farms, area of coverage or the need to normalize data. On the other hand, even though it is technologically simple, continuous monitoring of illuminance and noise levels is not a part of current animal welfare monitoring systems. However, a deeper knowledge of the relationships between these variables and animal activity could provide an interesting, reliable and affordable tool that is easily implemented on conventional farms, and that can supply accurate and comparable data for different types of buildings.
Illuminance and noise level are easily measured variables, which, unlike PID, are not affected by the physical barriers that divide the farm. Therefore, the aim of this study is to define linear models that relate animal activity, illuminance, and noise level, and also to model and validate the daily evolution of those variables based on cosine models that were previously designed for animal activity in weaned piglets. These models are intended to be effectively implemented on conventional farms.
3. Results and Discussion
Table 1 shows statistical data for the sample composed of 1152 observations during the modelling period of the following variables: AA, scaled between the values 0 and 1, L (lx), N (dB), T (°C) and RH (%).
Animal activity is scaled to 1, but it ranges from 0.00 to 0.81 (
Table 1), such that there are no 10 min intervals with continuous activity (
Figure 2a). The evolution of animal activity shows lower values during the night and higher values during the day, with several daily peaks and a high oscillation between measurements. Illuminance shows a value between 20.32 and 124.22 lx. The lowest value occurs during the night and increases abruptly (
Figure 2b). The level of noise is in the range between 55.70 and 83.00 dB. Temperature oscillates between 20.01 and 27.28 °C, with an average value of 25.05 °C and a standard deviation of 0.87 °C. Relative humidity shows values in the range of 38.80% to 93.60%, with an average value of 61.31% and a standard deviation of 5.67.
Illuminance values fall within the range reported in other studies [
3,
28]. The noise level only surpassed 73.87 dB for 4 h and 80.18 dB for 30′, which are the values established by [
51] to identify that pigs are thirsty and hungry or stressed due to heat. Average values of illuminance and noise level are above 40 lx and under 85 dB (A), the values established by Council Directive 2008/120/EC of 18 December 2008. Temperature values are within the intervals set by [
52], between 32 °C and 19 °C, for weaned piglets from 5 to 20 kg. The temperature exceeds the values found by [
53] for 19 h and 10′ between 24 and 26 °C for the studied age. The relative humidity is stable and falls within the recommended values of 50 and 75% [
54], with the exception of a period of 3 h and 10′, when the maximum value was reached. Therefore, the conditions on the farm are suitable for the variables of illuminance, noise level, temperature and relative humidity and, accordingly, for animal welfare.
The evolution of animal activity, illuminance and noise during the modelling period is shown in
Figure 2.
The evolution of the variables (
Figure 2) shows that there is daily periodicity. In line with most of the behaviours of growing pigs described in [
12], the three studied variables show a periodic pattern. Nevertheless, the behaviour of the variables during the day shows differences, because illuminance has one daily peak, while activity and noise level have two peaks (morning and afternoon), responding to a light–dark pattern of lighting regime [
2,
11]. The evolution of animal activity shows lower values during the night and greater values during the day, but the large variations in measurements hinder the visualization of a clear daily pattern (
Figure 2a). The daily variation of noise level shows a certain parallel with animal activity, although the wave is more stable (
Figure 2a,c).
3.1. Regression Models
Positive correlations were obtained between all the pairs of variables (
Table 2). Despite the parallelism found between the evolution of animal activity and noise level, the strongest linear relation was found between illuminance and noise level (R = 0.50), while lower values were obtained between illuminance and animal activity (R = 0.40). In all cases, correlations were significant. This analysis confirms that the three variables are related, although linear models explain their evolution only in part due to their great complexity.
3.2. Cosine Models
Cosine model fitting yielded valid time patterns, according to Equation (1).
Table 3 shows the coefficients and angles for each of the variables.
Figure 3 shows the measured wave, the wave fitting, and the three harmonics that form the wave. Applying a cosine model draws clear patterns of daily animal activity, illuminance and noise level with two peaks, one in the morning and one in the afternoon. In the case of illuminance, the second peak is much softer.
Cosine model fitting resulted in the independent term A
0 matching the average value. The amplitude of the first harmonic (A
1) always had the highest value, which suggests that the light–dark pattern has a greater influence on the piglets than other variables (
Table 3). The third harmonic is more relevant for every variable than the second one (A
3 > A
2), but amplitude is almost identical for illuminance and much higher for animal activity and noise (
Figure 3b,d,f).
The analysis of the first harmonic reveals that the wave of illuminance is forward, as compared to the waves of activity and noise level, because its angle is greater (
Table 3). Therefore, light can be considered as a generating factor of activity that causes a response in piglets that is delayed by 1 h 20′ for activity and 2 h 50′ for noise levels (
Figure 3b,d,f). In contrast, in the second harmonic, the wave of lighting is delayed in comparison to the waves for animal activity (5 h 30′) and noise level (4 h 30′). The analysis of the third harmonic shows that illuminance is delayed by 40′ and that the noise level is delayed by 1 h 20′ as compared to animal activity. In the three harmonics, activity precedes noise level, possibly due to habits regarding feeding, water intake or games. Therefore, the time of day is essential because of the sharp differences in animal behaviour.
Table 4 summarizes the results of the cosine model fitting to the studied variables. The low R
2 values obtained in regression analysis improved notably when using cosine models, with high R
2 for the three variables. Thus, cosine models can be a useful tool to study and predict these variables.
Whereas other authors presented models with up to four daily peaks [
13], in this research, animal behaviour shows a pattern with two daily peaks [
8,
12,
14,
15], coinciding with human activity inside the building, with a preference for inactivity when there is less light [
2,
55] and around midday [
12,
14]. These two peaks occur after the distribution of feed (08:00 h, 12:00 h and 16:00 h), which may be the cause for such peaks, in agreement with the evolution of the third harmonic. However, there is not a similar response to the distribution of feed during night-time (01:00 h and 20:00 h). Like other models that have been developed for six different behaviours, with an approximate period of 22 h [
12], the generated models can be applied to animal activity, illuminance and noise level with a fundamental period of 24 h and two harmonics (12 h and 8 h of a period,
Figure 3).
In this study, conducted in conditions of natural lighting indoors, the arrival of the day involves an abrupt increase in illuminance, which is not perceived immediately by the animals, insofar as activity levels increase afterwards and in a more gradual manner (
Figure 3a,c). Two levels of diurnal lighting are defined, around 80 lx in the morning and 50 lx in the afternoon (
Figure 3c), when activity mostly exceeds the average value (0.3,
Figure 3a). Under 40 lx, the minimum prescribed by Council Directive 2008/120/EC of 18 December 2008, activity is always below the average. After the first peak of activity during the morning, piglets are able to rest even with high values of illuminance, which is in contrast to the findings reported by [
19], who found a higher level of activity for several hours in the middle of the day that were associated to a higher level of illuminance. Lighting levels in both studies were quite different (124.22 vs. 600 lx).
Noise level and illuminance are easily measured variables that could provide reliable information about animal activity by implementing a single sensor. When direct measurements of the activity of a batch housed in a room are required, PIDs and sonometers provide very similar data, but the noise level is a bit delayed in comparison to the response of the PID (between 1 h and 1 h 30′).
3.3. Validation of the Models
The validation of the linear model shows very similar results to the results obtained during the modelling period (
Table 5).
The application of cosine models showed a good fit during the validation period, as suggested by the statistics in
Table 6.
The good results obtained during the development of the model are confirmed by the validation of the model in another period, where R values range between 0.91 and 0.92 and average errors are higher than in the model development phase.