Whole Genome Resequencing Reveals Selection Signals Related to Wool Color in Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection and Sequencing
2.3. Alignments and Quality Control
2.4. Population Structure Analysis
2.5. Analysis of Selection Signals
2.6. Detection and Annotation of Candidate Genes
2.7. Candidate Gene Enrichment Analysis
3. Results
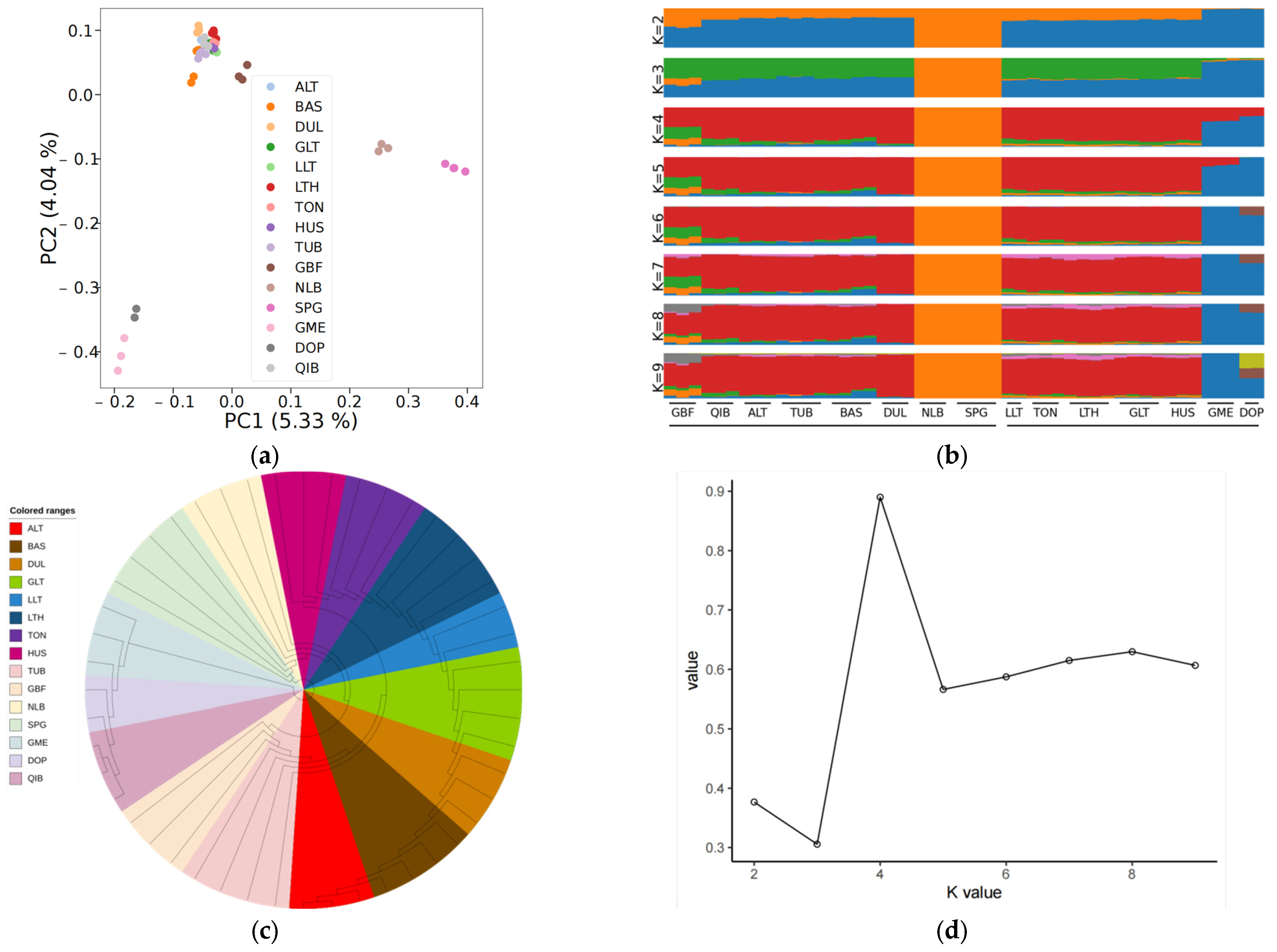
3.1. Genetic Variation and Population Genetic Analysis
3.2. Analysis of Selection Signals
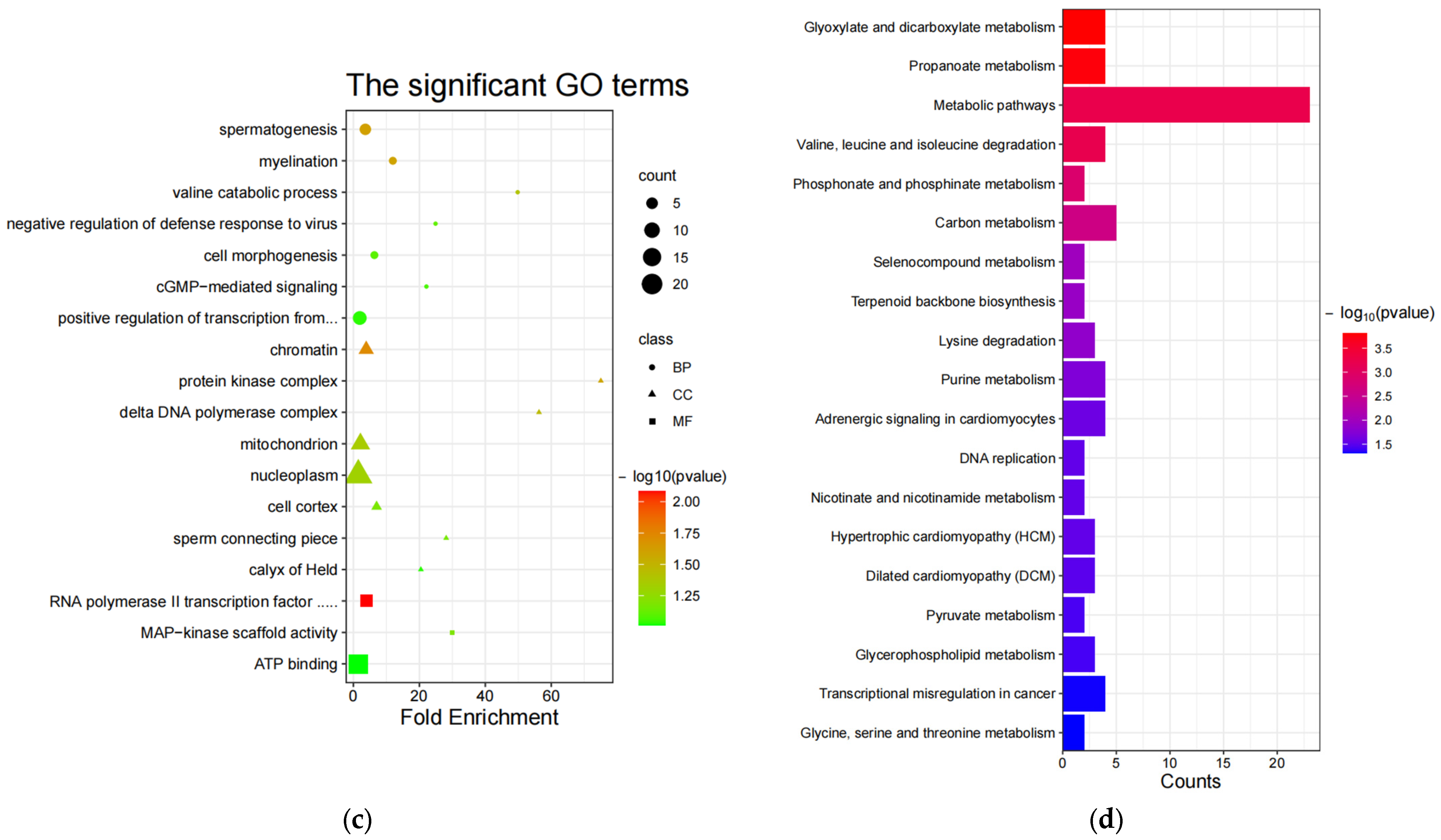
3.3. Enrichment Analysis
4. Discussion
4.1. Sample Control and Population Genetic Analysis
4.2. Selective Signal Analysis
4.2.1. GO Terms and Pathways Associated with Non-White Wool
4.2.2. GO terms and Pathways Associated with White Wool
4.2.3. Genes Associated with Wool Color
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chevis, H. Why early modern english clothiers started using spanish wool. Text. Hist. 2021, 52, 122–143. [Google Scholar] [CrossRef]
- Liu, J.; Jin, J. Dyes and colours of textiles in europe and asia from the seventeenth to the nineteenth century. Text. Cloth. Along Silk Roads Themat. Collect. Cult. Exch. Along Silk Roads 2022, 1, 347. [Google Scholar]
- Jenkins, D.T. The western wool textile industry in the nineteenth century. Camb. Hist. West. Text. 2003, 2, 761–789. [Google Scholar]
- Roberts, J.F.; White, R. Colour inheritance in sheep: V. Dominant black. J. Genet. 1930, 22, 181–190. [Google Scholar] [CrossRef]
- Ryder, M.L. The natural pigmentation of animal textile fibres. Text. Hist. 1990, 21, 135–148. [Google Scholar] [CrossRef]
- Smail, J. Merchants, Markets and Manufacture: The English Wool Textile Industry in the Eighteenth Century; Palgrave Macmillan: London, UK, 1999. [Google Scholar]
- Kalds, P.; Zhou, S.; Gao, Y.; Cai, B.; Huang, S.; Chen, Y.; Wang, X. Genetics of the phenotypic evolution in sheep: A molecular look at diversity-driving genes. Genet. Sel. Evol. 2022, 54, 61. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, M.; Chen, W.; Talbot, R.; Maddox, J.F.; Faraut, T.; Wu, C.; Muzny, D.M.; Li, Y.; Zhang, W.; et al. The sheep genome illuminates biology of the rumen and lipid metabolism. Science 2014, 344, 1168–1173. [Google Scholar] [CrossRef]
- Davenport, K.M.; Bickhart, D.M.; Worley, K.; Murali, S.C.; Salavati, M. An improved ovine reference genome assembly to facilitate in-depth functional annotation of the sheep genome. GigaScience 2022, 11, giab096. [Google Scholar] [CrossRef]
- Türkoğlu, G.C.; Avcı, B.B.; Erkan, G.; Özen, C.; Tozan Rüzgar, Ş.; Akkaya, A. Eco-friendly approach on wool pretreatment and effect on the wool structure and dyeability. Color. Technol. 2023, 139, 136–146. [Google Scholar] [CrossRef]
- Parisi, M.L.; Fatarella, E.; Spinelli, D.; Pogni, R.; Basosi, R. Environmental impact assessment of an eco-efficient production for coloured textiles. J. Clean. Prod. 2015, 108, 514–524. [Google Scholar] [CrossRef]
- Patel, M.; Sahu, A.; Rajak, R. Solid waste management in textile industry. In Handbook of Solid Waste Management: Sustainability through Circular Economy; Springer: Singapore, 2021; pp. 1–32. [Google Scholar]
- Bulbach, S. The importance of wool. Orient. Rug Rev. 1988, 8, 3. [Google Scholar]
- Li, M.; Tiirikka, T.; Kantanen, J. A genome-wide scan study identifies a single nucleotide substitution in asip associated with white versus non-white coat-colour variation in sheep (Ovis aries). Heredity 2014, 112, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Renieri, C.; Valbonesi, A.; La Manna, V.; Antonini, M.; Lauvergne, J. Inheritance of coat colour in merino sheep. Small Rumin. Res. 2008, 74, 23–29. [Google Scholar] [CrossRef]
- Kawęcka, A.; Gurgul, A.; Miksza-Cybulska, A. The use of snp microarrays for biodiversity studies of sheep—A review. Ann. Anim. Sci. 2016, 16, 975–987. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Liu, C.; Peng, X.; Lin, J.; He, S.; Li, X.; Han, B.; Zhang, N.; Wu, Y.; et al. Alteration of sheep coat color pattern by disruption of asip gene via crispr cas9. Sci. Rep. 2017, 7, 8149. [Google Scholar] [CrossRef] [PubMed]
- Kijas, J.W.; Lenstra, J.A.; Hayes, B.; Boitard, S.; Porto Neto, L.R.; San Cristobal, M.; Servin, B.; McCulloch, R.; Whan, V.; Gietzen, K.; et al. Genome-wide analysis of the world’s sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 2012, 10, e1001258. [Google Scholar] [CrossRef]
- Koseniuk, A.; Ropka-Molik, K.; Rubiś, D.; Smołucha, G. Genetic background of coat colour in sheep. Arch. Anim. Breed. 2018, 61, 173–178. [Google Scholar] [CrossRef]
- Kalds, P.; Zhou, S.; Cai, B.; Liu, J.; Wang, Y.; Petersen, B.; Sonstegard, T.; Wang, X.; Chen, Y. Sheep and goat genome engineering: From random transgenesis to the crispr era. Front. Genet. 2019, 10, 750. [Google Scholar] [CrossRef]
- Dalrymple, B.P.; Kirkness, E.F.; Nefedov, M.; McWilliam, S.; Ratnakumar, A.; Barris, W.; Zhao, S.; Shetty, J.; Maddox, J.F.; O’Grady, M.; et al. Using comparative genomics to reorder the human genome sequence into a virtual sheep genome. Genome Biol. 2007, 8, R152. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Shen, M.; Xie, X.-L.; Liu, G.-J.; Xu, Y.-X.; Lv, F.-H.; Yang, H.; Yang, Y.-L.; Liu, C.-B. Whole-genome resequencing of wild and domestic sheep identifies genes associated with morphological and agronomic traits. Nat. Commun. 2020, 11, 2815. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Liang, L.; He, S. Understanding key genetic make-up of different coat colour in bayinbuluke sheep through a comparative transcriptome profiling analysis. Small Rumin. Res. 2023, 226, 107028. [Google Scholar] [CrossRef]
- Cieslak, M.; Reissmann, M.; Hofreiter, M.; Ludwig, A. Colours of domestication. Biol. Rev. 2011, 86, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wu, J.; Lang, X.; Wang, C.; Bai, Y.; Riley, D.G.; Liu, L.; Ma, X. Comparative transcriptome and histological analyses provide insights into the skin pigmentation in minxian black fur sheep (Ovis aries). PeerJ 2021, 9, e11122. [Google Scholar] [CrossRef]
- Fan, R.; Xie, J.; Bai, J.; Wang, H.; Tian, X.; Bai, R.; Jia, X.; Yang, L.; Song, Y.; Herrid, M.; et al. Skin transcriptome profiles associated with coat color in sheep. BMC Genom. 2013, 14, 389. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Bao, A.; Hong, W.; Hou, C.; Zhang, Z.; Liang, X.; Aniwashi, J. Transcriptome profiling analysis reveals key genes of different coat color in sheep skin. PeerJ 2019, 7, e8077. [Google Scholar] [CrossRef]
- Kobayashi, T.; Imokawa, G.; Bennett, D.C.; Hearing, V.J. Tyrosinase stabilization by tyrp1 (the brown locus protein). J. Biol. Chem. 1998, 273, 31801–31805. [Google Scholar] [CrossRef] [PubMed]
- Oetting, W.; Austin, L.; Bennett, D. Color Genes: European Society for Pigment Cell Research. World Wide Web 2009. Available online: http://www.espcr.org/micemut/ (accessed on 29 June 2023).
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of mitf. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Guo, J.; Tao, H.; Li, P.; Li, L.; Zhong, T.; Wang, L.; Ma, J.; Chen, X.; Song, T.; Zhang, H. Whole-genome sequencing reveals selection signatures associated with important traits in six goat breeds. Sci. Rep. 2018, 8, 10405. [Google Scholar] [CrossRef]
- Praetorius, C.; Grill, C.; Stacey, S.N.; Metcalf, A.M.; Gorkin, D.U.; Robinson, K.C.; Van Otterloo, E.; Kim, R.S.; Bergsteinsdottir, K.; Ogmundsdottir, M.H.; et al. A polymorphism in irf4 affects human pigmentation through a tyrosinase-dependent mitf/tfap2a pathway. Cell 2013, 155, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.-E.; Valencia, J.C.; Wakamatsu, K.; Ito, S.; Solano, F.; Milac, A.L.; Vieira, W.D.; Yamaguchi, Y.; Rouzaud, F.; Petrescu, A.-J. Mutations in dopachrome tautomerase (dct) affect eumelanin/pheomelanin synthesis, but do not affect intracellular trafficking of the mutant protein. Biochem. J. 2005, 391, 249–259. [Google Scholar] [CrossRef]
- Guyonneau, L.; Murisier, F.; Rossier, A.; Moulin, A.; Beermann, F. Melanocytes and pigmentation are affected in dopachrome tautomerase knockout mice. Mol. Cell Biol. 2004, 24, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Chaubal, V.A.; Shioda, T.; Coser, K.R.; Mojamdar, M. Over-expression of msg1 transcriptional co-activator increases melanin in b16 melanoma cells: A possible role for msg1 in melanogenesis. Pigment Cell Res. 2001, 14, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Cockerham, C.C. Estimating f-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [PubMed]
- Lin, T.; Zhu, G.; Zhang, J.; Xu, X.; Yu, Q.; Zheng, Z.; Zhang, Z.; Lun, Y.; Li, S.; Wang, X. Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 2014, 46, 1220–1226. [Google Scholar] [CrossRef]
- Nei, M.; Li, W.-H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.-R.; Lv, F.-H.; He, S.-G.; Tian, S.-L.; Peng, W.-F.; Sun, Y.-W.; Zhao, Y.-X.; Tu, X.-L.; Zhang, M.; et al. Whole-genome sequencing of native sheep provides insights into rapid adaptations to extreme environments. Mol. Biol. Evol. 2016, 33, 2576–2592. [Google Scholar] [CrossRef]
- Sabeti, P.C.; Varilly, P.; Fry, B.; Lohmueller, J.; Hostetter, E.; Cotsapas, C.; Xie, X.; Byrne, E.H.; McCarroll, S.A.; Gaudet, R.; et al. Genome-wide detection and characterization of positive selection in human populations. Nature 2007, 449, 913–918. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and samtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Sabeti, P.C.; Schaffner, S.F.; Fry, B.; Lohmueller, J.; Varilly, P.; Shamovsky, O.; Palma, A.; Mikkelsen, T.S.; Altshuler, D.; Lander, E.S. Positive natural selection in the human lineage. Science 2006, 312, 1614–1620. [Google Scholar] [CrossRef]
- Jin, M.; Lu, J.; Fei, X.; Lu, Z.; Quan, K.; Liu, Y.; Chu, M.; Di, R.; Wei, C.; Wang, H. Selection signatures analysis reveals genes associated with high-altitude adaptation in tibetan goats from Nagqu, Tibet. Animals 2020, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, H.; Liu, G.; Zhao, F.; Kijas, J.W.; Ma, Y.; Lu, J.; Zhang, L.; Cao, J.; Wu, M.; et al. Genome-wide analysis reveals adaptation to high altitudes in tibetan sheep. Sci. Rep. 2016, 6, 26770. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. Plink: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Bruno, W.J.; Socci, N.D.; Halpern, A.L. Weighted neighbor joining: A likelihood-based approach to distance-based phylogeny reconstruction. Mol. Biol. Evol. 2000, 17, 189–197. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (itol) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome. Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and vcftools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Scheet, P.; Stephens, M. A fast and flexible statistical model for large-scale population genotype data: Applications to inferring missing genotypes and haplotypic phase. Am. J. Hum. Genet. 2006, 78, 629–644. [Google Scholar] [CrossRef]
- Utsunomiya, Y.T.; Pérez O’Brien, A.M.; Sonstegard, T.S.; Van Tassell, C.P.; do Carmo, A.S.; Meszaros, G.; Sölkner, J.; Garcia, J.F. Detecting loci under recent positive selection in dairy and beef cattle by combining different genome-wide scan methods. PLoS ONE 2013, 8, e64280. [Google Scholar] [CrossRef]
- Foll, M.; Gaggiotti, O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A bayesian perspective. Genetics 2008, 180, 977–993. [Google Scholar] [CrossRef]
- Wright, S. Genetical structure of populations. Nature 1950, 166, 247–249. [Google Scholar] [CrossRef]
- Li, X.; Su, R.; Wan, W.; Zhang, W.; Jiang, H.; Qiao, X.; Fan, Y.; Zhang, Y.; Wang, R.; Liu, Z. Identification of selection signals by large-scale whole-genome resequencing of cashmere goats. Sci. Rep. 2017, 7, 15142. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Tian, Y.; Huang, Y.; Zhang, Y.; Wang, J.; Sun, X.; Zhou, H.; Zhang, D.; Pan, W. Genome-wide analysis of genetic diversity in plasmodium falciparum isolates from china–myanmar border. Front. Genet. 2019, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Kudaravalli, S.; Wen, X.; Pritchard, J.K. A map of recent positive selection in the human genome. PLoS Biol. 2006, 4, e72. [Google Scholar]
- Wang, F.; Zha, Z.; He, Y.; Li, J.; Zhong, Z.; Xiao, Q.; Tan, Z. Genome-wide re-sequencing data reveals the population structure and selection signature analysis of tunchang pigs in china. Animals 2023, 13, 1835. [Google Scholar] [CrossRef]
- Gratten, J.; Wilson, A.; McRae, A.; Beraldi, D.; Visscher, P.; Pemberton, J.; Slate, J. A localized negative genetic correlation constrains microevolution of coat color in wild sheep. Science 2008, 319, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Tiesnamurti, B.; Destomo, A.; Febresiana, A. Coat cover characteristics of sheep in north sumatera, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 788, 012009. [Google Scholar] [CrossRef]
- Mortimer, S.; Hatcher, S.; Fogarty, N.; Van Der Werf, J.; Brown, D.; Swan, A.; Greeff, J.; Refshauge, G.; Edwards, J.H.; Gaunt, G. Genetic parameters for wool traits, live weight, and ultrasound carcass traits in merino sheep. J. Anim. Sci. 2017, 95, 1879–1891. [Google Scholar] [CrossRef]
- Dowling, M.; Schlink, A.; Greeff, J. Breeding Merino Wool for Colour Stability is Achievable. Assoc. Adv. Anim. Breed. Genet. 2007, 17, 328–331. [Google Scholar]
- Adalsteinsson, S. Inheritance of colours, fur characteristics and skin quality traits in north european sheep breeds: A review. Livest. Prod. Sci. 1983, 10, 555–567. [Google Scholar] [CrossRef]
- Wu, C.; Ma, S.; Zhao, B.; Qin, C.; Wu, Y.; Di, J.; Suo, L.; Fu, X. Drivers of plateau adaptability in cashmere goats revealed by genomic and transcriptomic analyses. BMC Genom. 2023, 24, 428. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. Annovar: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. The gene ontology resource: Enriching a gold mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. Kegg: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. Kobas-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.; Venny, C. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007–2015. 2018. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 29 June 2023).
- Ernfors, P. Cellular origin and developmental mechanisms during the formation of skin melanocytes. Exp. Cell Res. 2010, 316, 1397–1407. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Adv. Dermatol. Allergol. /Postępy Dermatol. I Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Abdel-Malek, Z.A.; Boissy, R.E.; Rheins, L.A. Pigment cell biology: An historical review. J. Investig. Dermatol. 1989, 92, S53–S60. [Google Scholar] [CrossRef]
- Harland, D.P.; Plowman, J.E. Development of hair fibres. Hair Fibre Proteins Struct. Dev. 2018, 1054, 109–154. [Google Scholar]
- Tuma, M.C.; Gelfand, V.I. Molecular mechanisms of pigment transport in melanophores. Pigment Cell Res. 1999, 12, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Higley, M.J.; Sabatini, B.L. Calcium signaling in dendritic spines. Cold Spring Harb. Perspect. Biol. 2012, 4, a005686. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Oancea, E.V. Ion transport in pigmentation. Arch. Biochem. Biophys. 2014, 563, 35–41. [Google Scholar] [CrossRef]
- Bang, J.; Zippin, J.H. Cyclic adenosine monophosphate (camp) signaling in melanocyte pigmentation and melanomagenesis. Pigment. Cell Melanoma Res. 2021, 34, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Kashina, A.S.; Semenova, I.V.; Ivanov, P.A.; Potekhina, E.S.; Zaliapin, I.; Rodionov, V.I. Protein kinase a, which regulates intracellular transport, forms complexes with molecular motors on organelles. Curr. Biol. 2004, 14, 1877–1881. [Google Scholar] [CrossRef]
- DePina, A.S.; Langford, G.M. Vesicle transport: The role of actin filaments and myosin motors. Microsc. Res. Tech. 1999, 47, 93–106. [Google Scholar] [CrossRef]
- Milograna, S.R.; Ribeiro, M.R.; Baqui, M.M.A.; McNamara, J.C. Pigment granule translocation in red ovarian chromatophores from the palaemonid shrimp macrobrachium olfersi (weigmann, 1836): Functional roles for the cytoskeleton and its molecular motors. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 178, 90–101. [Google Scholar] [CrossRef]
- DePina, A.S.; Wöllert, T.; Langford, G.M. Membrane associated nonmuscle myosin ii functions as a motor for actin-based vesicle transport in clam oocyte extracts. Cell Motil. Cytoskelet. 2007, 64, 739–755. [Google Scholar] [CrossRef]
- Ivanov, A.I.; McCall, I.C.; Parkos, C.A.; Nusrat, A. Role for actin filament turnover and a myosin ii motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol. Biol. Cell 2004, 15, 2639–2651. [Google Scholar] [CrossRef]
- Mermall, V.; Post, P.L.; Mooseker, M.S. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science 1998, 279, 527–533. [Google Scholar] [CrossRef]
- Boyle, R.T.; McNamara, J.C. Association of kinesin and myosin with pigment granules in crustacean chromatophores. Pigment Cell Res. 2006, 19, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Heissler, S.M.; Sellers, J.R. Kinetic adaptations of myosins for their diverse cellular functions. Traffic 2016, 17, 839–859. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Bowers, B.; Rao, K.; Wei, Q.; Hammer, J.A. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin v function in vivo. J. Cell Biol. 1998, 143, 1899–1918. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, V.; Yi, J.; Kashina, A.; Oladipo, A.; Gross, S.P. Switching between microtubule- and actin-based transport systems in melanophores is controlled by camp levels. Curr. Biol. 2003, 13, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.L.; Karcher, R.L.; Roland, J.T.; Minin, A.A.; Steffen, W.; Gelfand, V.I. Regulation of melanosome movement in the cell cycle by reversible association with myosin v. J. Cell Biol. 1999, 146, 1265–1276. [Google Scholar] [CrossRef]
- Lo, C.-M.; Buxton, D.B.; Chua, G.C.; Dembo, M.; Adelstein, R.S.; Wang, Y.-L. Nonmuscle myosin iib is involved in the guidance of fibroblast migration. Mol. Biol. Cell 2004, 15, 982–989. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Djurdjevič, I.; Furmanek, T.; Miyazawa, S.; Sušnik Bajec, S. Comparative transcriptome analysis of trout skin pigment cells. BMC Genom. 2019, 20, 359. [Google Scholar] [CrossRef]
- Enkhtaivan, E.; Lee, C.H. Role of amine neurotransmitters and their receptors in skin pigmentation: Therapeutic implication. Int. J. Mol. Sci. 2021, 22, 8071. [Google Scholar] [CrossRef]
- Fuziwara, S.; Inoue, K.; Denda, M. Nmda-type glutamate receptor is associated with cutaneous barrier homeostasis. J. Investig. Dermatol. 2003, 120, 1023–1029. [Google Scholar] [CrossRef]
- González-Burgos, I. From synaptic transmission to cognition: An intermediary role for dendritic spines. Brain Cogn. 2012, 80, 177–183. [Google Scholar] [CrossRef]
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002, 64, 313–353. [Google Scholar] [CrossRef]
- Chiu, S.; Kriegler, S. Neurotransmitter-mediated signaling between axons and glial cells. Glia 1994, 11, 191–200. [Google Scholar] [CrossRef]
- Choquet, D.; Triller, A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat. Rev. Neurosci. 2003, 4, 251–265. [Google Scholar] [CrossRef]
- Kneussel, M.; Hausrat, T.J. Postsynaptic neurotransmitter receptor reserve pools for synaptic potentiation. Trends Neurosci. 2016, 39, 170–182. [Google Scholar] [CrossRef]
- Khanam, S.; Siddique, Y.H. Dopamine: Agonists and neurodegenerative disorders. Curr. Drug Targets 2018, 19, 1599–1611. [Google Scholar] [CrossRef]
- Lee, E.; Choi, S.Y.; Bin, B.H.; Kim, N.H.; Kim, K.; Choi, D.H.; Han, J.; Choi, H.; Lee, A.Y.; Lee, T. Interferon-inducible t-cell alpha chemoattractant (itac) induces the melanocytic migration and hypopigmentation through destabilizing p53 via histone deacetylase 5: A possible role of itac in pigment-related disorders. Br. J. Dermatol. 2017, 176, 127–137. [Google Scholar] [CrossRef]
- Rakyan, V.K.; Preis, J.; Morgan, H.D.; Whitelaw, E. The marks, mechanisms and memory of epigenetic states in mammals. Biochem. J. 2001, 356, 1–10. [Google Scholar] [CrossRef]
- Zamudio, N.M.; Chong, S.; O’Bryan, M.K. Epigenetic regulation in male germ cells. Reproduction 2008, 136, 131–146. [Google Scholar] [CrossRef]
- Henikoff, S.; Greally, J.M. Epigenetics, cellular memory and gene regulation. Curr. Biol. 2016, 26, R644–R648. [Google Scholar] [CrossRef] [PubMed]
- Blewitt, M.; Whitelaw, E. The use of mouse models to study epigenetics. Cold Spring Harb. Perspect. Biol. 2013, 5, a017939. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Escobar, I.E.; Lefkovith, A.J.; Marks, M.S.; Oancea, E. An intracellular anion channel critical for pigmentation. eLife 2014, 3, e04543. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.F.; Liang, Y.; Gerwin, J.; Franchini, P.; Meyer, A. Comparative ontogenetic and transcriptomic analyses shed light on color pattern divergence in cichlid fishes. Evol. Dev. 2022, 24, 158–170. [Google Scholar] [CrossRef]
- Borovanský, J. Zinc in pigmented cells and structures, interactions and possible roles. Sb. Lek. 1994, 95, 309–320. [Google Scholar]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of human tyrosinase related protein 1 reveals a binuclear zinc active site important for melanogenesis. Angew. Chem. Int. Ed. 2017, 56, 9812–9815. [Google Scholar] [CrossRef]
- Hemesath, T.J.; Price, E.R.; Takemoto, C.; Badalian, T.; Fisher, D.E. Map kinase links the transcription factor microphthalmia to c-kit signalling in melanocytes. Nature 1998, 391, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Kunene, L.M.; Muchadeyi, F.C.; Hadebe, K.; Mészáros, G.; Sölkner, J.; Dugmore, T.; Dzomba, E.F. Genetics of base coat colour variations and coat colour-patterns of the south african nguni cattle investigated using high-density snp genotypes. Front. Genet. 2022, 13, 832702. [Google Scholar] [CrossRef]
- Saburina, I.N.; Zurina, I.M.; Kosheleva, N.V.; Gorkun, A.A.; Volkova, E.N.; Grinakovskaya, O.S.; Rybakov, A.S.; Kaysheva, A.L.; Kopylov, A.T.; Morozov, S.G. Mapk and notch-mediated effects of meso-xanthin f199 compounds on proliferative activity and apoptosis of human melanocytes in three-dimensional culture. BioMed Res. Int. 2021, 2021, 8463161. [Google Scholar] [CrossRef]
- Yan, J.; Roy, S.; Apolloni, A.; Lane, A.; Hancock, J.F. Ras isoforms vary in their ability to activate raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 1998, 273, 24052–24056. [Google Scholar] [CrossRef]
- Buscà, R.; Abbe, P.; Mantoux, F.; Aberdam, E.; Peyssonnaux, C.; Eychène, A.; Ortonne, J.P.; Ballotti, R. Ras mediates the camp-dependent activation of extracellular signal-regulated kinases (erks) in melanocytes. EMBO J. 2000, 19, 2900–2910. [Google Scholar] [CrossRef]
- Dumaz, N.; Marais, R. Integrating signals between camp and the ras/raf/mek/erk signalling pathways: Based on the anniversary prize of the gesellschaft für biochemie und molekularbiologie lecture delivered on 5 July 2003 at the special febs meeting in brussels. FEBS J. 2005, 272, 3491–3504. [Google Scholar] [CrossRef]
- Lim, X.; Nusse, R. Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008029. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xing, Y.; Liu, Y.; Luo, Y.; Deng, F.; Yang, T.; Yang, K.; Li, Y. Wnt/β-catenin signaling pathway activates melanocyte stem cells in vitro and in vivo. J. Dermatol. Sci. 2016, 83, 45–51. [Google Scholar] [CrossRef]
- Vibert, L.; Aquino, G.; Gehring, I.; Subkankulova, T.; Schilling, T.F.; Rocco, A.; Kelsh, R.N. An ongoing role for wnt signaling in differentiating melanocytes in vivo. Pigment Cell Melanoma Res. 2017, 30, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Blume-Jensen, P.; Janknecht, R.; Hunter, T. The kit receptor promotes cell survival via activation of pi 3-kinase and subsequent akt-mediated phosphorylation of bad on ser136. Curr. Biol. 1998, 8, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of bad couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Vredeveld, L.C.; Possik, P.A.; Smit, M.A.; Meissl, K.; Michaloglou, C.; Horlings, H.M.; Ajouaou, A.; Kortman, P.C.; Dankort, D.; McMahon, M.; et al. Abrogation of brafv600e-induced senescence by pi3k pathway activation contributes to melanomagenesis. Genes Dev. 2012, 26, 1055–1069. [Google Scholar] [CrossRef]
- Tarafder, A.K.; Bolasco, G.; Correia, M.S.; Pereira, F.J.; Iannone, L.; Hume, A.N.; Kirkpatrick, N.; Picardo, M.; Torrisi, M.R.; Rodrigues, I.P. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J. Investig. Dermatol. 2014, 134, 1056–1066. [Google Scholar] [CrossRef]
- Wamelen, D.J.v.; Aziz, N.A.; Zhao, J.; Balesar, R.; Unmehopa, U.; Roos, R.A.; Swaab, D.F. Decreased hypothalamic prohormone convertase expression in huntington disease patients. J. Neuropathol. Exp. Neurol. 2013, 72, 1126–1134. [Google Scholar] [CrossRef]
- Tell-Marti, G.; Puig-Butille, J.A.; Gimenez-Xavier, P.; Segu-Roig, A.; Potrony, M.; Badenas, C.; Alvarez, V.; Millán, J.M.; Trujillo-Tiebas, M.J.; Ramos-Arroyo, M.A. The p. R151c polymorphism in mc1r gene modifies the age of onset in spanish huntington’s disease patients. Mol. Neurobiol. 2017, 54, 3906–3910. [Google Scholar] [CrossRef]
- Reddy, B.; Jow, T.; Hantash, B.M. Bioactive oligopeptides in dermatology: Part i. Exp. Dermatol. 2012, 21, 563–568. [Google Scholar] [CrossRef]
- Delijewski, M.; Wrześniok, D.; Otręba, M.; Beberok, A.; Rok, J.; Buszman, E. Nicotine impact on melanogenesis and antioxidant defense system in hemn-dp melanocytes. Mol. Cell. Biochem. 2014, 395, 109–116. [Google Scholar] [CrossRef]
- Delijewski, M.; Beberok, A.; Otręba, M.; Wrześniok, D.; Rok, J.; Buszman, E. Effect of nicotine on melanogenesis and antioxidant status in hemn-lp melanocytes. Environ. Res. 2014, 134, 309–314. [Google Scholar] [CrossRef]
- Yerger, V.B.; Malone, R.E. Melanin and nicotine: A review of the literature. Nicotine Tob. Res. 2006, 8, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Pissios, P.; Trombly, D.J.; Tzameli, I.; Maratos-Flier, E. Melanin-concentrating hormone receptor 1 activates extracellular signal-regulated kinase and synergizes with gs-coupled pathways. Endocrinology 2003, 144, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- York, R.D.; Yao, H.; Dillon, T.; Ellig, C.L.; Eckert, S.P.; McCleskey, E.W.; Stork, P.J. Rap1 mediates sustained map kinase activation induced by nerve growth factor. Nature 1998, 392, 622–626. [Google Scholar] [CrossRef]
- Chen, Q.; Chai, Y.; Zhang, W.; Cheng, Y.; Zhang, Z.; An, Q.; Chen, S.; Man, C.; Du, L.; Zhang, W. Whole-genome sequencing reveals the genomic characteristics and selection signatures of hainan black goat. Genes 2022, 13, 1539. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Zhang, R.; Li, H.; Zhu, H. Cell junction and vesicle trafficking-mediated melanosome/melanin transfer are involved in the dynamic transformation of goldfish carassius auratus skin color. Int. J. Mol. Sci. 2022, 23, 12214. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, X.; Fu, Y.; Zhang, C.; Cao, Y.; Wang, J.; Zhang, Y.; Li, Y.; Chen, Y.; Li, Z. Transcriptome analysis of the breast muscle of xichuan black-bone chickens under tyrosine supplementation revealed the mechanism of tyrosine-induced melanin deposition. Front. Genet. 2019, 10, 457. [Google Scholar] [CrossRef]
- Zhou, J.; An, X.; Dong, J.; Wang, Y.; Zhong, H.; Duan, L.; Ling, J.; Ping, F.; Shang, J. Il-17 induces cellular stress microenvironment of melanocytes to promote autophagic cell apoptosis in vitiligo. FASEB J. 2018, 32, 4899–4916. [Google Scholar] [CrossRef]
- Stenger, P.-L.; Ky, C.-L.; Reisser, C.; Duboisset, J.; Dicko, H.; Durand, P.; Quintric, L.; Planes, S.; Vidal-Dupiol, J. Molecular pathways and pigments underlying the colors of the pearl oyster pinctada margaritifera var. Cumingii (linnaeus 1758). Genes 2021, 12, 421. [Google Scholar] [CrossRef]
- Lan, L.; Cheng, A.; Dunman, P.M.; Missiakas, D.; He, C. Golden pigment production and virulence gene expression are affected by metabolisms in staphylococcus aureus. J. Bacteriol. 2010, 192, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.A.; Lutz, A.; Rankin, K.J.; Stuart-Fox, D.; Moussalli, A. Revealing the biochemical and genetic basis of color variation in a polymorphic lizard. Mol. Biol. Evol. 2017, 34, 1924–1935. [Google Scholar] [CrossRef]
- Yang, B.-T.; Wen, B.; Ji, Y.; Wang, Q.; Zhang, H.-R.; Zhang, Y.; Gao, J.-Z.; Chen, Z.-Z. Comparative metabolomics analysis of pigmentary and structural coloration in discus fish (symphysodon haraldi). J. Proteom. 2021, 233, 104085. [Google Scholar] [CrossRef]
- Tian, H.; Liu, S.Q.; Jing, W.H.; Hao, Z.H.; Li, Y.H.; Lu, Z.H.; Ding, Z.K.; Huang, S.L.; Xu, Y.S.; Wang, H.B. Imaginal disc growth factor is involved in melanin synthesis and energy metabolism in bombyx mori. Arch. Insect Biochem. Physiol. 2023, 112, e21995. [Google Scholar] [CrossRef]
- Bian, F.; Yang, X.; Ou, Z.; Luo, J.; Tan, B.; Yuan, M.; Chen, T.; Yang, R. Morphological characteristics and comparative transcriptome analysis of three different phenotypes of pristella maxillaris. Front. Genet. 2019, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jiang, H.; Zhang, L.; Gu, Q.; Wang, W.; Wen, Y.; Luo, F.; Jin, W.; Cao, X. Integrated proteomic and transcriptomic analysis reveals that polymorphic shell colors vary with melanin synthesis in bellamya purificata snail. J. Proteom. 2021, 230, 103950. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.-P.; Lin, X.-Y.; Wu, Y.; Zhou, S.-Y.; Huang, Z.-b.; Huang, Y.-F.; Li, A.; Huang, X.-H. Isolation of blue-green eggshell pigmentation-related genes from putian duck through rna-seq. BMC Genom. 2019, 20, 66. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Li, Y.; Zhao, L.; Liu, Z.; Kang, Y.; Wang, J. Integrative mrna-mirna interaction analysis reveals the molecular mechanism of skin color variation between wild-type and yellow mutant rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100914. [Google Scholar] [CrossRef]
- Liao, X.; Shi, X.; Hu, H.; Han, X.; Jiang, K.; Liu, Y.; Xiong, G. Comparative metabolomics analysis reveals the unique nutritional characteristics of breed and feed on muscles in chinese taihe black-bone silky fowl. Metabolites 2022, 12, 914. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, H.; Jiang, L.; Fu, C.; Zhang, Y.; Hu, Y.; Zhang, X.; Zhu, L.; Zhang, F.; Huang, J. Heat promotes melanogenesis by increasing the paracrine effects in keratinocytes via the trpv3/ca2+/hh signaling pathway. iScience 2023, 26, 106749. [Google Scholar] [CrossRef]
- Guang-Qi, G.; Li-Shuang, S.; Bin, T.; Guang-Peng, L. Expression levels of gsta2 and apod genes might be associated with carotenoid coloration in golden pheasant (Chrysolophus pictus) plumage. Zool. Res. 2016, 37, 144. [Google Scholar]
- Marzabani, R.; Rezadoost, H.; Choopanian, P.; Kolahdooz, S.; Mozafari, N.; Mirzaie, M.; Karimi, M.; Nieminen, A.I.; Jafari, M. Metabolomic signature of amino acids in plasma of patients with non-segmental vitiligo. Metabolomics 2021, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Palstra, R.-J.; Kayser, M. Human skin color is influenced by an intergenic DNA polymorphism regulating transcription of the nearby bnc2 pigmentation gene. Hum. Mol. Genet. 2014, 23, 5750–5762. [Google Scholar] [CrossRef]
- Lyon, M.F. Sex chromatin and gene action in the mammalian x-chromosome. Am. J. Hum. Genet. 1962, 14, 135. [Google Scholar]
- Visser, M.; Kayser, M.; Palstra, R.-J. Herc2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the oca2 promoter. Genome Res. 2012, 22, 446–455. [Google Scholar] [CrossRef]
- Liu, F.; Visser, M.; Duffy, D.L.; Hysi, P.G.; Jacobs, L.C.; Lao, O.; Zhong, K.; Walsh, S.; Chaitanya, L.; Wollstein, A. Genetics of skin color variation in europeans: Genome-wide association studies with functional follow-up. Hum. Genet. 2015, 134, 823–835. [Google Scholar] [CrossRef]
- Busca, R.; Ballotti, R. Cyclic amp a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef]
- Wang, Y.; Viennet, C.; Robin, S.; Berthon, J.Y.; He, L.; Humbert, P. Precise role of dermal fibroblasts on melanocyte pigmentation. J. Dermatol. Sci. 2017, 88, 159–166. [Google Scholar] [CrossRef]
- Kim, E.S.; Park, S.J.; Goh, M.J.; Na, Y.J.; Jo, D.S.; Jo, Y.K.; Shin, J.H.; Choi, E.S.; Lee, H.K.; Kim, J.Y.; et al. Mitochondrial dynamics regulate melanogenesis through proteasomal degradation of mitf via ros-erk activation. Pigment Cell Melanoma Res. 2014, 27, 1051–1062. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, J.; Sviderskaya, E.V.; Wei, A.; Li, W. Mitochondrial nckx5 regulates melanosomal biogenesis and pigment production. J. Cell Sci. 2019, 132, jcs232009. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.L.; Smith, A.G.; Smit, D.J.; Leonard, J.H.; Sturm, R.A. Co-expression of sox9 and sox10 during melanocytic differentiation in vitro. Exp. Cell Res. 2005, 308, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Yang, H.J.; Moon, H.I.; Cho, Y.S. Branched-chain amino acids complex inhibits melanogenesis in b16f0 melanoma cells. Immunopharmacol. Immunotoxicol. 2012, 34, 256–264. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Branch chain amino acid and melanogenesis. Immunopharmacol. Immunotoxicol. 2012, 34, 539. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, X.; Sun, S.; Zhang, L.; Shan, S.; Zhu, H. Production of natural melanin by auricularia auricula and study on its molecular structure. Food Chem. 2016, 190, 801–807. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A. Health benefits of carotenoids and potential application in poultry industry: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Andrade, P.; Carneiro, M. Pterin-based pigmentation in animals. Biol. Lett. 2021, 17, 20210221. [Google Scholar] [CrossRef]
- Li, G.; Xu, H.; Xu, L.; Qu, M.; You, J.; Yi, Z.; Pan, K. Effects of dietary selenium supplementation on growth performance and melanin content in tissues of taihe silky fowls. Sci. Agric. Sin. 2011, 44, 2777–2786. [Google Scholar]
- Ahn, S.; Lee, S.; Ha, S.; Lee, K.; Kang, T.; Kim, S. Regulation of melanin synthesis by selenium containing compounds. J. Investig. Dermatol. 2005, 124, A147. [Google Scholar]
- Owings, W.J.; Balloun, S.L. Relation of arginine and lysine to feather tyrosinase activity. Poult. Sci. 1959, 38, 1285–1289. [Google Scholar] [CrossRef]
- Brito, S.; Baek, J.M.; Cha, B.; Heo, H.; Lee, S.H.; Lei, L.; Jung, S.Y.; Lee, S.M.; Lee, S.H.; Kwak, B.M.; et al. Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting camp/wnt signaling. J. Dermatol. Sci. 2022, 106, 159–169. [Google Scholar] [CrossRef]
- Choi, S.-G.; Kim, J.-H.; Hong, S.-H.; Lee, O.Y.; Kang, N.-G. Exogenous pyruvate alleviates uv-induced hyperpigmentation via restraining dendrite outgrowth and rac1 gtpase activity. J. Dermatol. Sci. 2021, 101, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wen, B.; Micah, A.D.; Gao, J.-Z.; Chen, Z.-Z. Integration of transcriptomics and metabolomics reveals amelanism mechanism of oscar astronotus ocellatus (agassiz, 1831). Hydrobiologia 2022, 850, 2275–2298. [Google Scholar] [CrossRef]
- Gupta, R.; Forloni, M.; Bisserier, M.; Dogra, S.K.; Yang, Q.; Wajapeyee, N. Interferon alpha-inducible protein 6 regulates nrasq61k-induced melanomagenesis and growth. eLife 2016, 5, e16432. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, J.; Song, X.; An, Q. M6a mrna methylation analysis provides novel insights into pigmentation in sheep skin. Epigenetics 2023, 18, 2230662. [Google Scholar] [CrossRef]
- Pavan, W.J.; Sturm, R.A. The genetics of human skin and hair pigmentation. Annu. Rev. Genom. Hum. Genet. 2019, 20, 41–72. [Google Scholar] [CrossRef]
- Nie, C.; Zhang, Z.; Zheng, J.; Sun, H.; Ning, Z.; Xu, G.; Yang, N.; Qu, L. Genome-wide association study revealed genomic regions related to white/red earlobe color trait in the rhode island red chickens. BMC Genet. 2016, 17, 115. [Google Scholar] [CrossRef]
- Fatoki, T.H.; Ibraheem, O.; Adeseko, C.J.; Afolabi, B.L.; Momodu, D.U.; Sanni, D.M.; Enibukun, J.M.; Ogunyemi, I.O.; Adeoye, A.O.; Ugboko, H.U. Melanogenesis, its regulatory process, and insights on biomedical, biotechnological, and pharmacological potentials of melanin as antiviral biochemical. Biointerface Res. Appl. Chem. 2020, 11, 11969–11984. [Google Scholar]
- Nicolaï, M.P.; D’Alba, L.; Goldenberg, J.; Gansemans, Y.; Van Nieuwerburgh, F.; Clusella-Trullas, S.; Shawkey, M.D. Untangling the structural and molecular mechanisms underlying colour and rapid colour change in a lizard, Agama atra. Mol. Ecol. 2021, 30, 2262–2284. [Google Scholar] [CrossRef]
- McLean, C.A.; Lutz, A.; Rankin, K.J.; Elliott, A.; Moussalli, A.; Stuart-Fox, D. Red carotenoids and associated gene expression explain colour variation in frillneck lizards. Proc. R. Soc. B 2019, 286, 20191172. [Google Scholar] [CrossRef]
- Shi, J.; Lu, D.; Gu, R.; Xu, Y.; Pan, R.; Bo, F.; Zhang, Y. Identification of key biomarkers and immune infiltration in sporadic vestibular schwannoma basing transcriptome-wide profiling. World Neurosurg. 2022, 160, e591–e600. [Google Scholar] [CrossRef]
- Cook, D.; Brooks, S.; Bellone, R.; Bailey, E. Missense mutation in exon 2 of slc36a1 responsible for champagne dilution in horses. PLoS Genet. 2008, 4, e1000195. [Google Scholar] [CrossRef]
- Holl, H.; Pflug, K.; Yates, K.; Hoefs-Martin, K.; Shepard, C.; Cook, D.; Lafayette, C.; Brooks, S. A candidate gene approach identifies variants in slc 45a2 that explain dilute phenotypes, pearl and sunshine, in compound heterozygote horses. Anim. Genet. 2019, 50, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Reimann, E.; Kingo, K.; Karelson, M.; Reemann, P.; Loite, U.; Keermann, M.; Abram, K.; Vasar, E.; Silm, H.; Kõks, S. Expression profile of genes associated with the dopamine pathway in vitiligo skin biopsies and blood sera. Dermatology 2012, 224, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, D.; Popik, M.; Salwinski, A.; Plonka, P.M. Extradermal melanin transfer? Lack of macroscopic spleen melanization in old c57bl/6 mice with de-synchronized hair cycle. Acta Biochim. Pol. 2009, 56, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.I.; Setaluri, V. Cyclic amp (camp) signaling in melanocytes and melanoma. Arch. Biochem. Biophys. 2014, 563, 22–27. [Google Scholar] [CrossRef]
- Visconti, A.; Duffy, D.L.; Liu, F.; Zhu, G.; Wu, W.; Chen, Y.; Hysi, P.G.; Zeng, C.; Sanna, M.; Iles, M.M. Genome-wide association study in 176,678 europeans reveals genetic loci for tanning response to sun exposure. Nat. Commun. 2018, 9, 1684. [Google Scholar] [CrossRef]
- Allen, J.R.; Skeath, J.B.; Johnson, S.L. Maintenance of melanocyte stem cell quiescence by gaba-a signaling in larval zebrafish. Genetics 2019, 213, 555–566. [Google Scholar] [CrossRef]
- Wang, H.; Wen, J.; Li, H.; Zhu, T.; Zhao, X.; Zhang, J.; Zhang, X.; Tang, C.; Qu, L.; Gemingguli, M. Candidate pigmentation genes related to feather color variation in an indigenous chicken breed revealed by whole genome data. Front. Genet. 2022, 13, 985228. [Google Scholar] [CrossRef]
- Lona-Durazo, F.; Hernandez-Pacheco, N.; Fan, S.; Zhang, T.; Choi, J.; Kovacs, M.A.; Loftus, S.K.; Le, P.; Edwards, M.; Fortes-Lima, C.A. Meta-analysis of gwa studies provides new insights on the genetic architecture of skin pigmentation in recently admixed populations. BMC Genet. 2019, 20, 59. [Google Scholar] [CrossRef]
- Yan, X.; Wei, L.; Huang, J.; Wang, J.; Yang, Z.; Gan, B.; Liu, K.; Teng, Z.; Zhang, S.; Ye, X. Comparative skin transcriptome between common carp and the variety jinbian carp (Cyprinus carpio v. Jinbian). Aquac. Res. 2020, 51, 187–196. [Google Scholar] [CrossRef]
- Mazar, J.; Khaitan, D.; DeBlasio, D.; Zhong, C.; Govindarajan, S.S.; Kopanathi, S.; Zhang, S.; Ray, A.; Perera, R.J. Epigenetic regulation of microrna genes and the role of mir-34b in cell invasion and motility in human melanoma. PLoS ONE 2011, 6, e24922. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, T.; Hsu, C.K.; Yang, H.S.; Chen, H.Y.; Wong, T.W.; Tsai, W.L.; Chao, S.C.; Lee, J.Y.; Akiyama, M.; Simpson, M. Progressive hyperpigmentation in a taiwanese child due to an inborn error of vitamin b12 metabolism (cblj). Br. J. Dermatol. 2015, 172, 1111–1115. [Google Scholar] [CrossRef]
- Braz, S.; Benicio, R.; Tonelli, G.; Báo, S.; Moretti, P.; Pic-Taylor, A.; Oliveira, S.; Acevedo, A.; Costa, I.; Mazzeu, J. Cobalamin f deficiency in a girl with severe skin hyperpigmentation and a homozygous lmbrd1 variant. Clin. Exp. Dermatol. 2022, 47, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Bai, C.; Pu, Y.; Kong, Q.; Guo, Y.; Ouzhuluobu; Gengdeng; Liu, X.; Zhao, Q.; Qiu, Z. Genetic adaptation of skin pigmentation in highland tibetans. Proc. Natl. Acad. Sci. USA 2022, 119, e2200421119. [Google Scholar] [CrossRef]
- Leung, A.M. Identifying Functional Interactors of the Retinal Transcription Factor, vsx2, during Early Retinal Development. 2023. Available online: https://ir.vanderbilt.edu/handle/1803/18320 (accessed on 21 June 2023).
- Bharti, K.; Gasper, M.; Ou, J.; Brucato, M.; Clore-Gronenborn, K.; Pickel, J.; Arnheiter, H. A regulatory loop involving pax6, mitf, and wnt signaling controls retinal pigment epithelium development. PLoS Genet. 2012, 8, e1002757. [Google Scholar] [CrossRef]
- Norris, B.J.; Whan, V.A. A gene duplication affecting expression of the ovine asip gene is responsible for white and black sheep. Genome Res. 2008, 18, 1282–1293. [Google Scholar] [CrossRef]
- Kim, B.; Lee, S.-H.; Choi, K.-Y.; Kim, H.-S. N-nicotinoyl tyramine, a novel niacinamide derivative, inhibits melanogenesis by suppressing mitf gene expression. Eur. J. Pharmacol. 2015, 764, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Allouche, J.; Rachmin, I.; Adhikari, K.; Pardo, L.M.; Lee, J.H.; McConnell, A.M.; Kato, S.; Fan, S.; Kawakami, A.; Suita, Y. Nnt mediates redox-dependent pigmentation via a uvb-and mitf-independent mechanism. Cell 2021, 184, 4268–4283. [Google Scholar] [CrossRef]
- Allouche, J.; Rachmin, I.; Fisher, D.E.; Roider, E. Commentary on nnt mediates redox-dependent pigmentation via a uvb-and mitf-independent mechanism. J. Cell Sci. Ther. 2021, 12, 316. [Google Scholar] [PubMed]
- Sebaratnam, D.F.; Bandera, A.I.R.; Lowe, P.M. Hair repigmentation with anti–pd-1 and anti–pd-l1 immunotherapy: A novel hypothesis. JAMA Dermatol. 2018, 154, 112–113. [Google Scholar] [CrossRef]
- Kong, G.; Lee, H.; Vo, T.-T.T.; Juang, U.; Kwon, S.H.; Park, J.; Park, J.; Kim, S.-H. Functional characteristics and research trends of pde11a in human diseases. Mol. Med. Rep. 2022, 26, 298. [Google Scholar] [CrossRef]
- Reemann, P.; Reimann, E.; Ilmjarv, S.; Porosaar, O.; Silm, H.; Jaks, V.; Vasar, E.; Kingo, K.; Koks, S. Melanocytes in the skin-comparative whole transcriptome analysis of main skin cell types. PLoS ONE 2014, 9, e115717, Erratum in PLoS ONE 2017, 12, e0173792. [Google Scholar] [CrossRef]
- Pausch, H.; Wang, X.; Jung, S.; Krogmeier, D.; Edel, C.; Emmerling, R.; Götz, K.-U.; Fries, R. Identification of qtl for uv-protective eye area pigmentation in cattle by progeny phenotyping and genome-wide association analysis. PLoS ONE 2012, 7, e36346. [Google Scholar] [CrossRef]
- Gupta, I.; Shankrit, S.; Narta, K.; Ghazi, M.; Grover, R.; Pandey, R.; Kar, H.K.; Menon, S.M.; Gupta, A.; Yenamandra, V.K. Whole exome-sequencing of vitiligo lesions indicate lower burden of somatic variations: Implications in risk for non-melanoma skin cancers. bioRxiv 2022, 143, 1111–1114. [Google Scholar]
- Kiszner, G.; Wichmann, B.; Nemeth, I.B.; Varga, E.; Meggyeshazi, N.; Teleki, I.; Balla, P.; Maros, M.E.; Penksza, K.; Krenacs, T. Cell cycle analysis can differentiate thin melanomas from dysplastic nevi and reveals accelerated replication in thick melanomas. Virchows Arch. 2014, 464, 603–612. [Google Scholar] [CrossRef]
- Gupta, R.; Misri, R.; Gupta, A.; Chowdhary, M.; Singh, A. Genome-wide profiling reveals pervasive transcriptional alterations in fibroblasts derived from lesional skin in vitiligo including a reduced potential to proliferate. Exp. Dermatol. 2023, 32, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Rennison, D.; Tapanes, E. The genetic basis of divergent melanic pigmentation in benthic and limnetic threespine stickleback. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Schnabel, V.; Hermasch, M.A.; Wolf, S.; Schön, M.P.; Betz, R.C.; Frank, J. A woman with hyperpigmented macules and papules. J. Dtsch. Dermatol. Ges. 2021, 19, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, K.; Steder, M.; Logotheti, S.; Angerilli, A.; Spitschak, A.; Marquardt, S.; Schumacher, T.; Engelmann, D.; Herchenroeder, O.; Rupp, R.A. Dnp73-induced degradation of tyrosinase links depigmentation with emt-driven melanoma progression. Cancer Lett. 2019, 442, 299–309. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Hu, S.; Qi, S.; Jia, Q.; Yang, W.; Yang, S.; Ji, K.; Liu, X.; Dong, C. Microrna-379 mediates pigmentation, migration and proliferation of melanocytes by targeting the insulin-like growth factor 1 receptor. Exp. Dermatol. 2020, 29, 467–476. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Park, J.-K.; Lee, M.-H. Effect of igf-1 on the proliferation of cultured normal human melanocytes. Korean J. Dermatol. 2000, 38, 1315–1324. [Google Scholar]
- Moon, H.; Donahue, L.R.; Choi, E.; Scumpia, P.O.; Lowry, W.E.; Grenier, J.K.; Zhu, J.; White, A.C. Melanocyte stem cell activation and translocation initiate cutaneous melanoma in response to ultraviolet exposure. Cell Stem Cell 2017, 21, 665–678.e6. [Google Scholar] [CrossRef]
- Li, M.-Y.; Flora, P.; Pu, H.; Bar, C.; Silva, J.; Cohen, I.; Galbo, P.M.; Liu, H.; Yu, X.; Jin, J. Uv-induced reduction in polycomb repression promotes epidermal pigmentation. Dev. Cell 2021, 56, 2547–2561. [Google Scholar] [CrossRef]
- Maeda, R.; Mood, K.; Jones, T.L.; Aruga, J.; Buchberg, A.M.; Daar, I.O. Xmeis1, a protooncogene involved in specifying neural crest cell fate in xenopus embryos. Oncogene 2001, 20, 1329–1342. [Google Scholar] [CrossRef]
- Apopo, S.; Liu, H.; Jing, L.; Du, X.; Xie, S.; Gong, Y.; Xu, R.; Li, S. Identification and profiling of micro rna s associated with white and black plumage pigmentation in the white and black feather bulbs of ducks by rna sequencing. Anim. Genet. 2015, 46, 627–635. [Google Scholar] [CrossRef]
- Wu, Z.; Fu, Y.; Cao, J.; Yu, M.; Tang, X.; Zhao, S. Identification of differentially expressed mirnas between white and black hair follicles by rna-sequencing in the goat (Capra hircus). Int. J. Mol. Sci. 2014, 15, 9531–9545. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K. Chemistry and function of polypeptide hormones. Annu. Rev. Biochem. 1962, 31, 213–246. [Google Scholar] [CrossRef] [PubMed]
- Kandil, E. Treatment of localized vitiligo with intradermal injections of triamcinolone acetonide. Dermatology 1970, 140, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.C.Q.; Tawata, S. Mimosine dipeptide enantiomsers: Improved inhibitors against melanogenesis and cyclooxygenase. Molecules 2015, 20, 14334–14347. [Google Scholar] [CrossRef] [PubMed]



| NO. | Breed | Abbr. | Photo | Category | Size | Color |
|---|---|---|---|---|---|---|
| 1 | Bashbay sheep | BAS |  | Domestic_East Asia_Kazakh | 4 | Brown wool with white face |
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| 5 | Duolang sheep | DUL |  | Domestic_East Asia_Kazakh | 3 | Gray white wool with dark gray head and limbs, tawny neck |
| 6 | ||||||
| 7 | ||||||
| 8 | Altay sheep | ALT |  | Domestic_East Asia_Kazakh | 3 | Brown red wool with white head |
| 9 | ||||||
| 10 | ||||||
| 11 | Qira Black sheep | QIB |  | Domestic_East Asia_Kazakh | 3 | Black brown wool |
| 12 | ||||||
| 13 | ||||||
| 14 | Turfan Black sheep | TUB |  | Domestic_East Asia_Kazakh | 4 | Black wool |
| 15 | ||||||
| 16 | ||||||
| 17 | ||||||
| 18 | Guide Black Fur sheep | GBF |  | Domestic_East Asia_Tibet | 3 | Black red wool |
| 19 | ||||||
| 20 | ||||||
| 21 | Ninglang Black sheep | NLB |  | Domestic_East Asia_Yunnan | 3 | Black wool |
| 22 | ||||||
| 23 | ||||||
| 24 | Shiping Gray sheep | SPG |  | Domestic_East Asia_Yunnan | 4 | Cyan wool with black limbs |
| 25 | ||||||
| 26 | ||||||
| 27 | ||||||
| 28 | German Mutton Merino | GME |  | Domestic_Europe | 3 | White wool |
| 29 | ||||||
| 30 | ||||||
| 31 | Poll Dorset | DOP |  | Domestic_Europe | 2 | White wool |
| 32 | ||||||
| 33 | Large-tailed Han sheep | LTH |  | Domestic_East Asia_Mongolia | 4 | White wool |
| 34 | ||||||
| 35 | ||||||
| 36 | ||||||
| 37 | Guangling large-tailed sheep | GLT |  | Domestic_East Asia_Mongolia | 4 | White wool |
| 38 | ||||||
| 39 | ||||||
| 40 | ||||||
| 41 | Hu Sheep | HUS |  | Domestic_East Asia_Mongolia | 3 | White wool |
| 42 | ||||||
| 43 | ||||||
| 44 | Tong Sheep | TON |  | Domestic_East Asia_Mongolia | 3 | White wool |
| 45 | ||||||
| 46 | ||||||
| 47 | Lanzhou Large-tailed sheep | LLT |  | Domestic_East Asia_Mongolia | 2 | White wool |
| 48 |
| Catalogue | SNP Numbers |
|---|---|
| Upstream | 93,281 |
| Exonic | 151,411 |
| Intronic | 8,123,336 |
| Splicing | 4227 |
| Downstream | 125,137 |
| upstream/downstream | 2550 |
| Intergenic | 13,414,800 |
| ts | 14,501,815 |
| tv | 7,631,392 |
| ts/tv | 1.9 |
| Total | 22,133,207 |
| Category | Gene | Number of Relevant Pathways |
|---|---|---|
| Non-White | PPP1CB | 7 |
| CALML4 | 9 | |
| PPP3CA | 10 | |
| GRM5 | 6 | |
| GRIN1 | 8 | |
| MYLK | 6 | |
| FGF18 | 5 | |
| FGFR2 | 6 | |
| FGF2 | 5 | |
| MAPK10 | 8 | |
| NOS3 | 6 | |
| White | ACAT2 | 5 |
| PCCB | 2 | |
| ALDH6A1 | 2 | |
| ACSS2 | 2 | |
| PAPSS2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Jin, M.; Lu, Z.; Li, T.; Wang, H.; Yuan, Z.; Wei, C. Whole Genome Resequencing Reveals Selection Signals Related to Wool Color in Sheep. Animals 2023, 13, 3265. https://doi.org/10.3390/ani13203265
Zhang W, Jin M, Lu Z, Li T, Wang H, Yuan Z, Wei C. Whole Genome Resequencing Reveals Selection Signals Related to Wool Color in Sheep. Animals. 2023; 13(20):3265. https://doi.org/10.3390/ani13203265
Chicago/Turabian StyleZhang, Wentao, Meilin Jin, Zengkui Lu, Taotao Li, Huihua Wang, Zehu Yuan, and Caihong Wei. 2023. "Whole Genome Resequencing Reveals Selection Signals Related to Wool Color in Sheep" Animals 13, no. 20: 3265. https://doi.org/10.3390/ani13203265
APA StyleZhang, W., Jin, M., Lu, Z., Li, T., Wang, H., Yuan, Z., & Wei, C. (2023). Whole Genome Resequencing Reveals Selection Signals Related to Wool Color in Sheep. Animals, 13(20), 3265. https://doi.org/10.3390/ani13203265






