Partial Substitution of Whey Protein Concentrate with Spray–Dried Porcine Plasma or Soy Protein Isolate in Milk Replacer Differentially Modulates Ileal Morphology, Nutrient Digestion, Immunity and Intestinal Microbiota of Neonatal Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals and Growth Performance
2.2. Milk Replacer
2.3. Sample Collection
2.4. Histomorphology
2.5. Digestive Enzyme Activity
2.6. Gene Expressions
2.7. 16S rRNA Amplicon Sequencing
2.8. SCFA Analysis
2.9. Statistical Analysis
3. Results
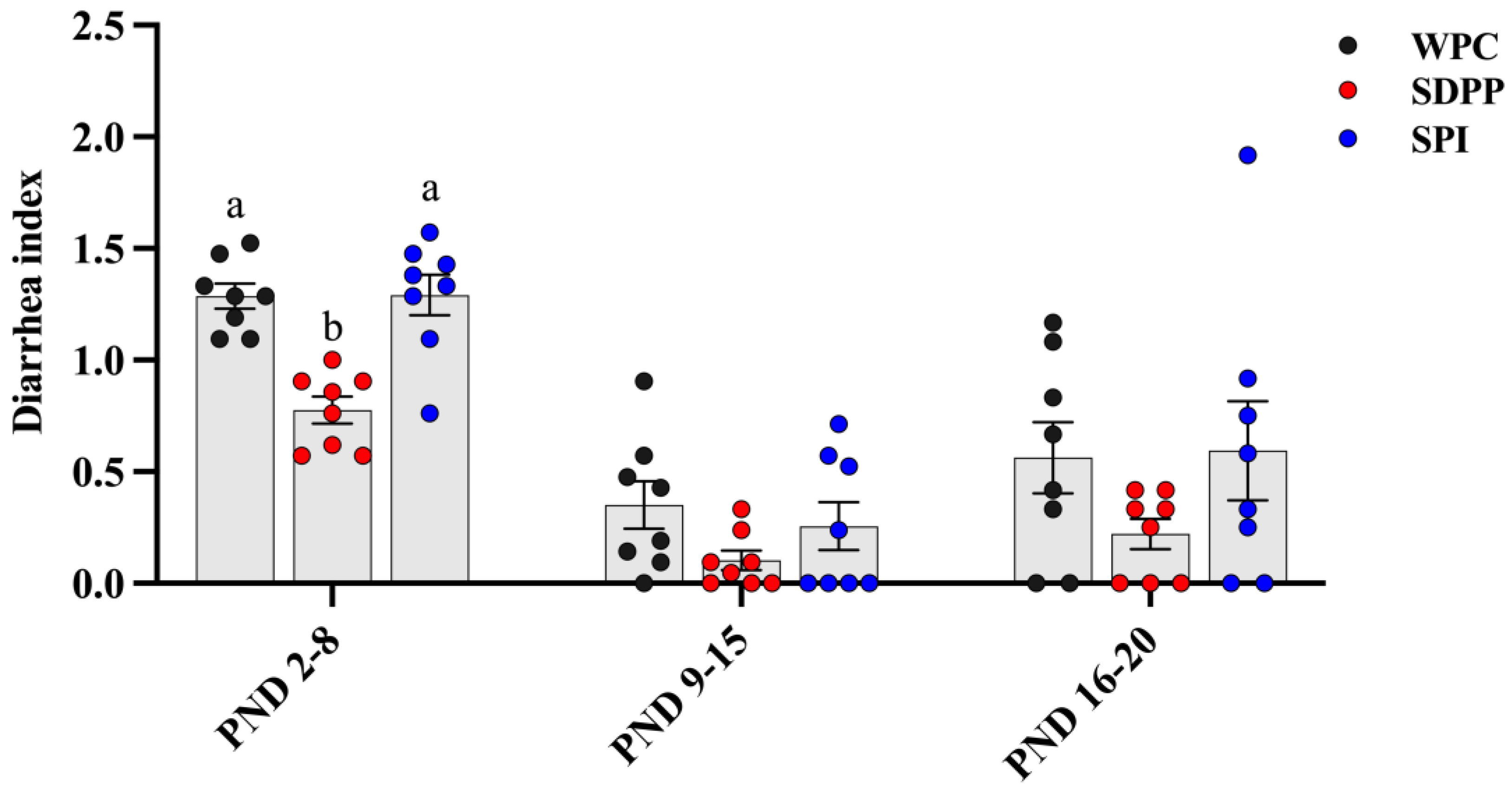
3.1. Growth Performance
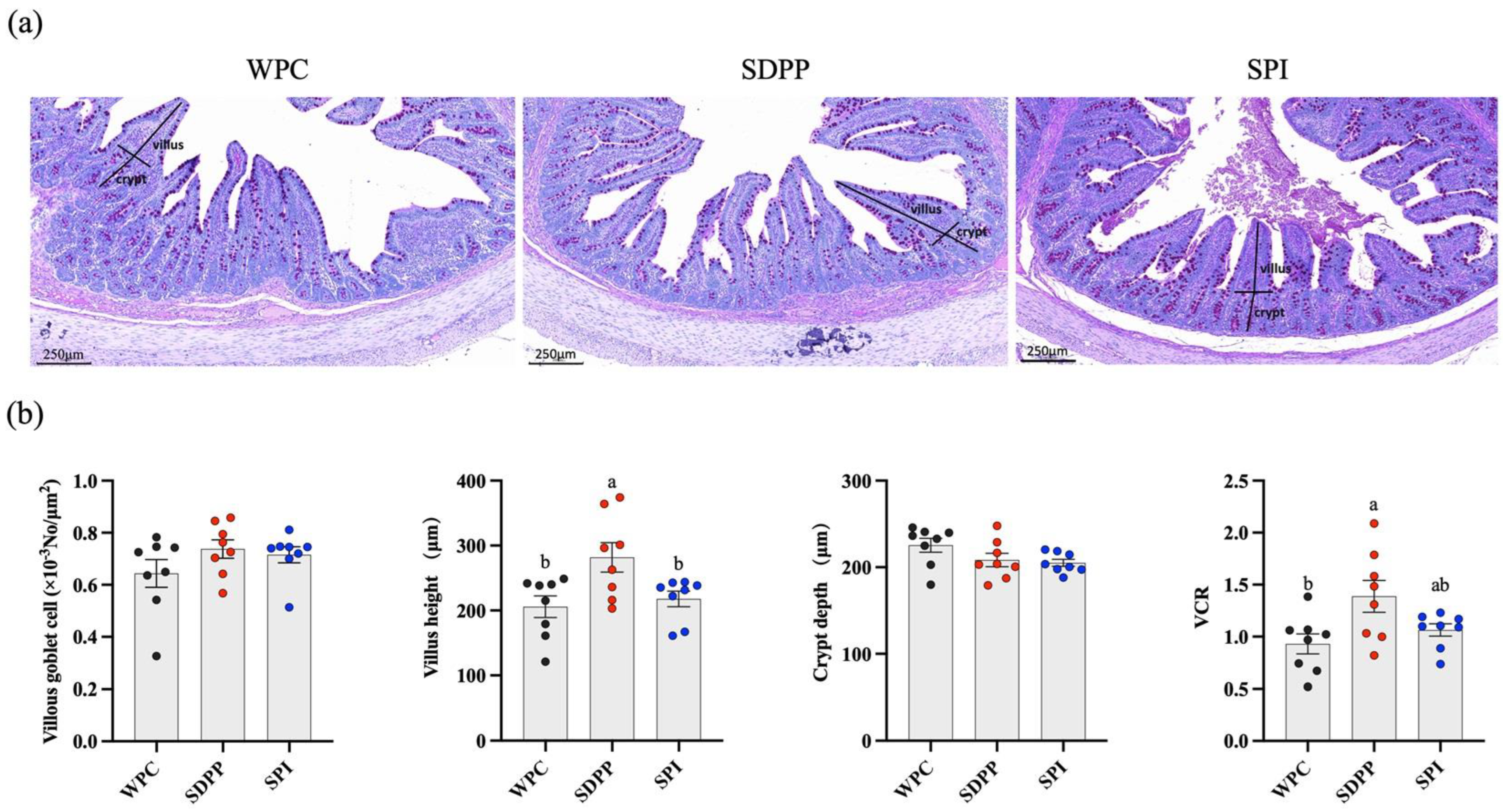
3.2. Ileal Morphology
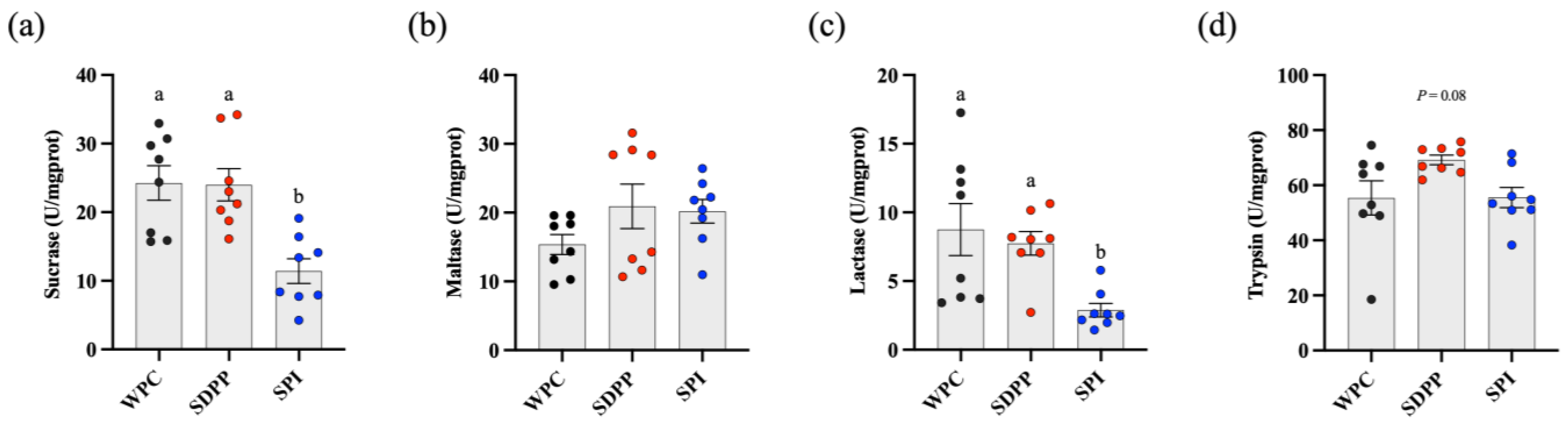
3.3. Activities of Enzymes
3.4. Relative mRNA Expressions of Barrier-Function- and Inflammation-Related Genes
3.5. Plasma Biochemical Parameters
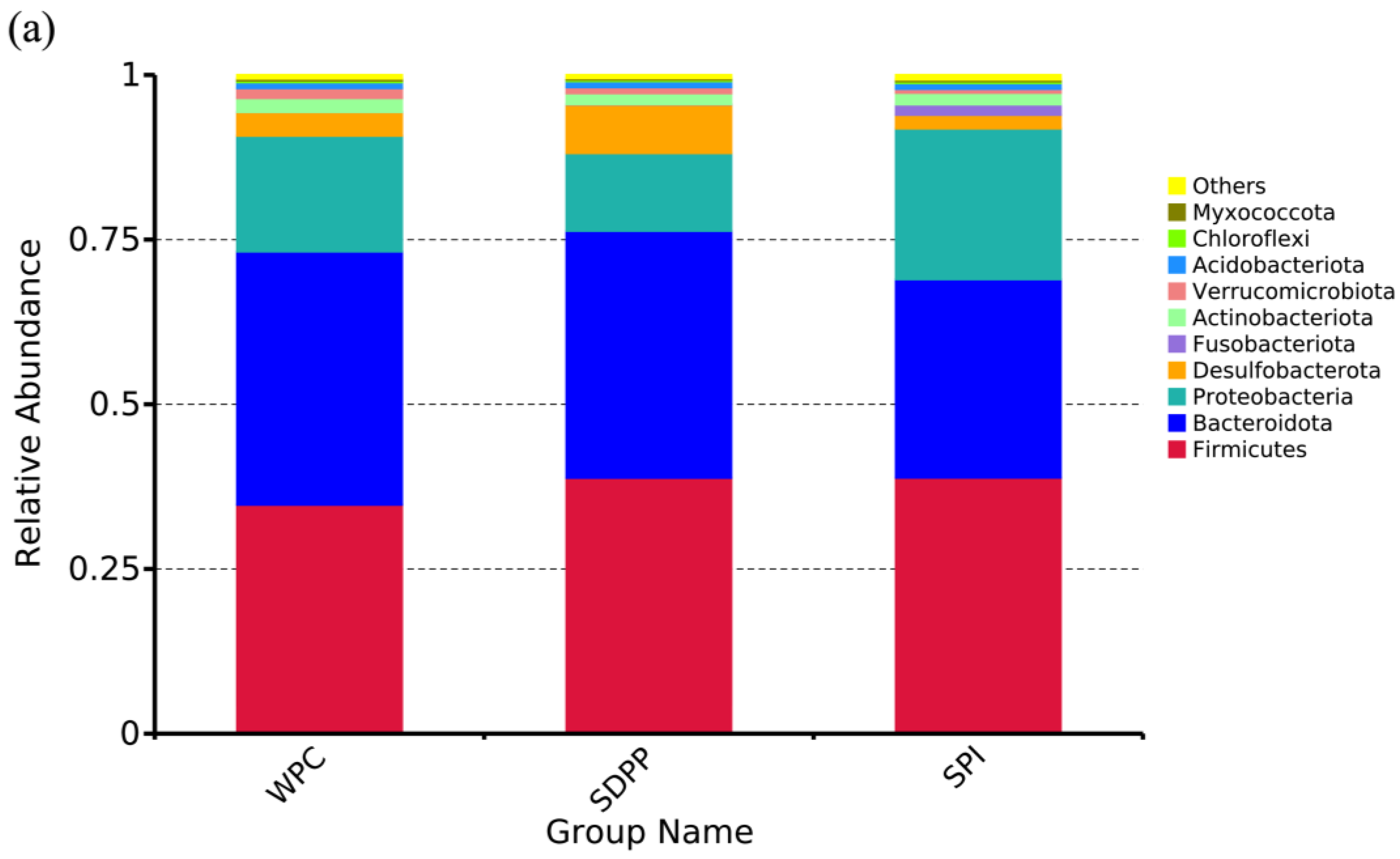
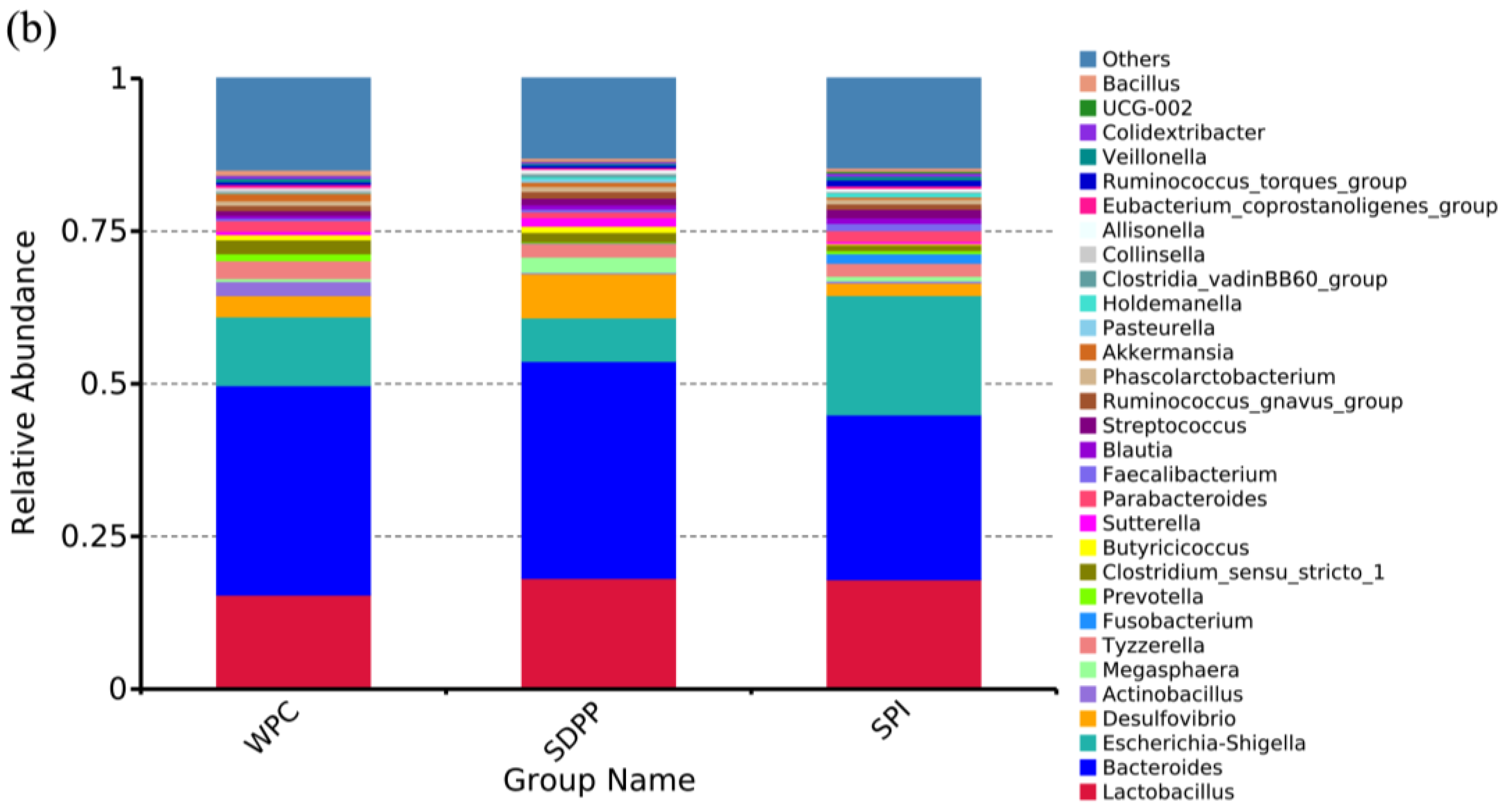
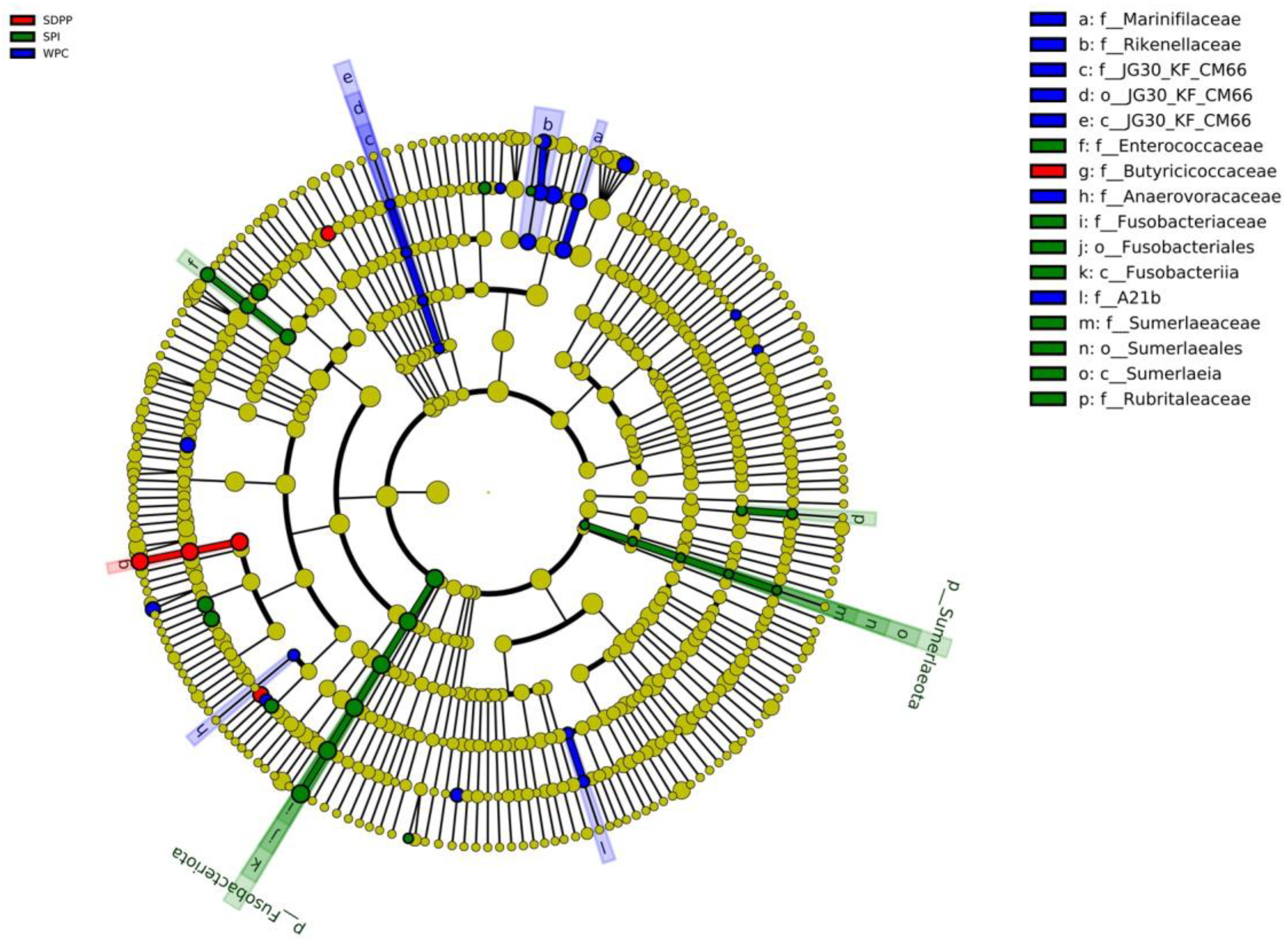
3.6. Colonic Microbiome
3.7. SCFA Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobek-Kjeldager, C.; Moustsen, C.; Theil, V.A.; Pedersen, P.K.; Lene, J. Effect of litter size, milk replacer and housing on behaviour and welfare related to sibling competition in litters from hyper-prolific sows. Appl. Anim. Behav. Sci. 2020, 230, 105032. [Google Scholar] [CrossRef]
- Kobek-Kjeldager, C.; Moustsen, V.A.; Pedersen, L.J.; Theil, P.K. Impact of litter size, supplementary milk replacer and housing on the body composition of piglets from hyper-prolific sows at weaning. Animal 2020, 15, 100007–100008. [Google Scholar] [CrossRef]
- Roggero, P.; Liotto, N.; Pozzi, C.; Braga, D.; Troisi, J.; Menis, C.; Giannì, M.L.; Canani, R.B.; Paparo, L.; Nocerino, R.; et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei CBA L74-fermented formula. Nat. Commun. 2020, 11, 2703–2704. [Google Scholar] [CrossRef]
- Herfel, T.M.; Jacobi, S.K.; Lin, X.; Walker, D.C.; Jouni, Z.E.; Odle, J. Safety evaluation of polydextrose in infant formula using a suckling piglet model. Food Chem. Toxicol. 2009, 47, 1530–1537. [Google Scholar] [CrossRef]
- Li, M.; Bauer, L.L.; Chen, X.; Wang, M.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Fahey, G.C., Jr.; Donovan, S.M. Microbial Composition and In Vitro Fermentation Patterns of Human Milk Oligosaccharides and Prebiotics Differ between Formula-Fed and Sow-Reared Piglets. J. Nutr. 2012, 142, 681–689. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Z.; Wang, X.; An, Q.; Huang, K.; Dai, Y.; Meng, Q.; Zhang, Y. Lactoferrin, a Critical Player in Neonate Intestinal Development: RHLF may be a Good Choice in Formula. J. Agric. Food Chem. 2021, 69, 8726–8736. [Google Scholar] [CrossRef]
- Li, F.; Jin, X.; Liu, B.; Zhuang, W.; Scalabrin, D. Follow-up formula consumption in 3- to 4-year-olds and respiratory infections: An RCT. Pediatrics 2014, 133, 1533–1540. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Zhao, Q.; Tang, C.; Zhang, J.; Qin, Y. Fermented Soy and Fish Protein Dietary Sources Shape Ileal and Colonic Microbiota, Improving Nutrient Digestibility and Host Health in a Piglet Model. Front. Microbiol. 2022, 13, 911500–911501. [Google Scholar] [CrossRef]
- Gan, J.; Bornhorst, G.M.; Henrick, B.M.; German, J.B. Protein Digestion of Baby Foods: Study Approaches and Implications for Infant Health. Mol. Nutr. Food Res. 2018, 62, 10–11. [Google Scholar] [CrossRef]
- Xu, T.; Chen, J.; Yang, K.; Qiao, W.; Zhao, J.; Chen, L. Quantitative Determination of Whey Protein to Casein Ratio in Infant Formula Milk Powder. Front. Chem. 2022, 10, 872251–872252. [Google Scholar] [CrossRef]
- Castenmiller, J.; Hirsch-Ernst, K.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; Pentieva, K.; et al. Efficacy of an infant formula manufactured from a specific protein hydrolysate derived from whey protein isolate and concentrate produced by Société des Produits Nestlé S.A. in reducing the risk of developing atopic dermatitis. EFSA J. 2021, 19, e06603–e06604. [Google Scholar] [PubMed]
- Li, Y.; Nguyen, D.N.; Obelitz-Ryom, K.; Andersen, A.D.; Thymann, T.; Chatterton, D.E.W.; Purup, S.; Heckmann, A.B.; Bering, S.B.; Sangild, P.T. Bioactive Whey Protein Concentrate and Lactose Stimulate Gut Function in Formula-Fed Preterm Pigs. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Tu, Y.; Bingwen, S.; Guishan, X.; Diao, Q. Effects of protein sources for milk replacers on growth performance and serum biochemical indexes of suckling calves. Anim. Nutr. J. 2015, 1, 349–355. [Google Scholar] [CrossRef]
- Lasekan, J.B.; Ostrom, K.M.; Jacobs, J.R.; Blatter, M.M.; Ndife, L.I.; Gooch, W.M., 3rd; Cho, S. Growth of newborn, term infants fed soy formulas for 1 year. Clin. Pediatr. 1999, 38, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.; Ekhard Ziegler, E.; Mitmesser, S.H.; Harris, C.L.; Diersen-Schade, D.A. Soy-based infant formula supplemented with DHA and ARA supports growth and increases circulating levels of these fatty acids in infants. Lipids 2008, 43, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, J.B.; Baggs, G.E. Efficacy of Soy-Based Formulas in Alleviating Gastrointestinal Symptoms in Infants with Milk-Based Formula Intolerance: A Randomized Clinical Trial. Clin. Pediatr. 2021, 60, 184–192. [Google Scholar] [CrossRef]
- Bhatia, J.; Greer, F. Use of Soy Protein-Based in Infant Feeding. Pediatrics 2008, 121, 1062–1068. [Google Scholar] [CrossRef]
- Andres, A.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Body Fat and Bone Mineral Content of Infants Fed Breast Milk, Cow’s Milk Formula, or Soy Formula during the First Year of Life. J. Pediatr. 2013, 163, 49–54. [Google Scholar] [CrossRef]
- Wood, D. Milk Replacer Ingredients: What and Why? Vet. Clin. North Am.-Food Anim. Pract. 2022, 38, 133–152. [Google Scholar] [CrossRef]
- Rosell-Cardona, C.; Grian-Ferré, C.; Pérez-Bosque, A.; Polo, J.; Pallàs, M.; Amat, C.; Moretó, M.; Miró, L. Dietary Spray-Dried Porcine Plasma Reduces Neuropathological Alzheimer’s Disease Hallmarks in SAMP8 Mice. Nutrients 2021, 13, 2369. [Google Scholar] [CrossRef]
- Pérez-Bosque, A.; Miró, L.; Amat, C.; Polo, J.; Moretó, M. The Anti-Inflammatory Effect of Spray-Dried Plasma Is Mediated by a Reduction in Mucosal Lymphocyte Activation and Infiltration in a Mouse Model of Intestinal Inflammation. Nutrients 2016, 8, 657. [Google Scholar] [CrossRef]
- Pierce, J.L.; Cromwell, G.L.; Lindemann, M.D.; Russell, L.E.; Weaver, E.M. Effects of spray-dried animal plasma and immunoglobulins on performance of early weaned pigs. J. Anim. Sci. 2005, 83, 2876–2885. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Wu, J.; Chen, Q.; Zhou, Q.; Wu, F.; Zhang, R.; Fang, Z.; Lin, Y.; Xu, S.; et al. Comparative effects of enzymatic soybean, fish meal and milk powder in diets on growth performance, immunological parameters, SCFAs production and gut microbiome of weaned piglets. J. Anim. Sci. Biotechnol. 2021, 12, 106. [Google Scholar] [CrossRef]
- Nuntapaitoon, M.; Juthamanee, P.; Theil, P.K.; Tummaruk, P. Impact of sow parity on yield and composition of colostrum and milk in Danish Landrace × Yorkshire crossbred sows. Prev. Vet. Med. 2020, 181, 105085–105086. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Tsukahara, T. Composition and physiological functions of the porcine colostrum. Anim. Sci. J. 2021, 92, e13618–e13619. [Google Scholar] [CrossRef] [PubMed]
- Krogh, U.; Bruun, T.S.; Poulsen, J.; Theil, P.K. Impact of fat source and dietary fibers on feed intake, plasma metabolites, litter gain and the yield and composition of milk in sows. Animal 2017, 11, 975–983. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Navis, M.; Muncan, V.; Sangild, P.T.; Willumsen, L.M.; Koelink, P.J.; Wildenberg, M.E.; Abrahamse, E.; Thymann, T.; van Elburg, R.M.; Renes, I.B. Beneficial Effect of Mildly Pasteurized Whey Protein on Intestinal Integrity and Innate Defense in Preterm and Near-Term Piglets. Nutrients 2020, 12, 1125. [Google Scholar] [CrossRef]
- Zhao, J.; Harper, A.F.; Estienne, M.J.; Webb, K.E., Jr.; McElroy, A.P.; Denbow, D.M. Growth performance and intestinal morphology responses in early weaned pigs to supplementation of antibiotic-free diets with an organic copper complex and spray-dried plasma protein in sanitary and nonsanitary environments. J. Anim. Sci. 2007, 85, 1302–1310. [Google Scholar] [CrossRef]
- Torrallardona, D.; Conde, R.; Esteve-Garca, E.; Brufau, J. Use of spray dried animal plasma as an alternative to antimicrobial medication in weanling pigs. Anim. Feed. Sci. Technol. 2002, 99, 119–129. [Google Scholar] [CrossRef]
- Tran, H.; Bundy, J.W.; Li, Y.S.; Carney-Hinkle, E.E.; Miller, P.S.; Burkey, T.E. Effects of spray-dried porcine plasma on growth performance, immune response, total antioxidant capacity, and gut morphology of nursery pigs. J. Anim. Sci. 2014, 92, 4494–4504. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.D.; Wolfe, T.M. Effects of Spray-Dried Animal Plasma in Calf Milk Replacer on Health and Growth of Dairy Calves. J. Dairy Sci. 2003, 86, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, M.; Azhar, K.; Zulkifli, I.; Bejo, M.H.; Kamyab, A. Effects of non-antibiotic feed additives on performance, immunity and intestinal morphology of broilers fed different levels of protein. South Afr. J. Anim. Sci. 2012, 42, 22–32. [Google Scholar] [CrossRef]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef]
- Segawa, S.; Fujiya, M.; Konishi, H.; Ueno, N.; Kobayashi, N.; Shigyo, T.; Kohgo, Y. Probiotic-Derived Polyphosphate Enhances the Epithelial Barrier Function and Maintains Intestinal Homeostasis through Integrin–p38 MAPK Pathway. PLoS ONE 2011, 6, e23278–e23279. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Hu, L.; Zhou, Q.; Peng, X.; Liu, Y.; Luo, Y.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Microbial insight into dietary protein source affects intestinal function of pigs with intrauterine growth retardation. Eur. J. Nutr. 2020, 59, 327–344. [Google Scholar] [CrossRef]
- Amdi, C.; Pedersen, M.L.M.; Klaaborg, J.; Myhill, L.J.; Engelsmann, M.N.; Williams, A.R.; Thymann, T. Pre-weaning adaptation responses in piglets fed milk replacer with gradually increasing amounts of wheat. Br. J. Nutr. 2021, 126, 375–382. [Google Scholar] [CrossRef]
- Jensen, A.R.; Elnif, J.; Burrin, D.G.; Sangild, P.T. Development of Intestinal Immunoglobulin Absorption and Enzyme Activities in Neonatal Pigs Is Diet Dependent. J. Nutr. 2001, 131, 3259–3265. [Google Scholar] [CrossRef]
- Krone, J.E.C.; Agyekum, A.K.; Borgh, M.T.; Hamonic, K. Characterization of urea transport mechanisms in the intestinal tract of growing pigs. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G839–G844. [Google Scholar] [CrossRef]
- Ishii, Y.; Schuessler, R.B.; Gaynor, S.L.; Hames, K. Postoperative atrial fibrillation: The role of the inflammatory response. J. Thorac. Cardiovasc. Surg. 2017, 153, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hooper, W.C.; Phillips, D.J.; Talkington, D.F. Regulation of Proinflammatory Cytokines in Human Lung Epithelial Cells Infected with Mycoplasma pneumoniae. Infect. Immun. 2002, 70, 3649–3655. [Google Scholar] [CrossRef]
- Takakuwa, A.; Nakamura, K.; Kikuchi, M.; Sugimoto, R. Butyric Acid and Leucine Induce α-Defensin Secretion from Small Intestinal Paneth Cells. Nutrients 2019, 11, 2817. [Google Scholar] [CrossRef] [PubMed]
- Heinritz, S.N.; Weiss, E.; Eklund, M.; Aumiller, T.; Louis, S.; Rings, A.; Messner, S.; Camarinha-Silva, A.; Seifert, J.; Bischoff, S.C.; et al. Intestinal Microbiota and Microbial Metabolites Are Changed in a Pig Model Fed a High-Fat/Low-Fiber or a Low-Fat/High-Fiber Diet. PLoS ONE 2016, 11, e0154329–e0154330. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Lim, J.Y.; Kim, B.S.; Cho, S.J.; Kim, N.Y.; Kim, O.B.; Kim, Y. Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr. Res. Pract. 2015, 9, 242–248. [Google Scholar] [CrossRef]
- Sim, W.H.; Wagner, J.; Cameron, D.J.; Catto-Smith, A.G.; Bishop, R.F.; Kirkwood, C.D. Novel Burkholderiales 23S rRNA genes identified in ileal biopsy samples from children: Preliminary evidence that a subtype is associated with perianal Crohn’s disease. J. Clin. Microbiol. 2010, 48, 1939–1942. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Ku, W.C.; Liu, C.Y.; Cheng, Y.C. Supplementation of Probiotic Butyricicoccus pullicaecorum Mediates Anticancer Effect on Bladder Urothelial Cells by Regulating Butyrate-Responsive Molecular Signatures. Diagnostics 2021, 11, 2270. [Google Scholar] [CrossRef]
- Devriese, S.; Eeckhaut, V.; Geirnaert, A.; Bossche, L.V.D. Reduced Mucosa-associated Butyricicoccus Activity in Patients with Ulcerative Colitis Correlates with Aberrant Claudin-1 Expression. J. Crohn’s Colitis 2017, 11, 229–236. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Yu, T.; Zhao, X.; Zhou, Z.; Yu, Y.; Xiong, L.; Yang, H.; Bilotta, A.J.; Yao, S.; et al. Glucose promotes regulatory T cell differentiation to maintain intestinal homeostasis. iScience 2022, 25, 105004–105005. [Google Scholar] [CrossRef]
- Xu, F.; Cheng, R.; Miao, S.; Zhu, Y.; Sun, Z.; Qiu, L.; Yang, J.; Zhou, L. Prior Toxoplasma Gondii Infection Ameliorates Liver Fibrosis Induced by Schistosoma Japonicum through Inhibiting Th2 Response and Improving Balance of Intestinal Flora in Mice. Int. J. Mol. Sci. 2020, 21, 2711. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Cheon, S.; Park, C.; Lee, J.H.; Lee, S.W.; Lee, S.A. Correction: Transcriptome profiling analysis of senescent gingival fibroblasts in response to Fusobacterium nucleatum infection. PLoS ONE 2018, 13, e0191209–e0191210. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ghosh, S.K.; Shokeen, B.; Eapan, B.; Lux, R.; Kiselar, J.; Nithianantham, S.; Young, A.; Pandiyan, P.; McCormick, T.S.; et al. FAD-I, a Fusobacterium nucleatum Cell Wall-Associated Diacylated Lipoprotein that Mediates Human Beta Defensin 2 Induction through Toll-Like Receptor-1/2 (TLR-1/2) and TLR-2/6. Infect. Immun. 2016, 84, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dörffel, Y.; Baucke, V.L.; Theissig, F.; Rückert, J.C.; Ismail, M.; Rau, W.A.; Gaschler, D.; Weizenegger, M.; Kühn, S.; et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 2011, 60, 34–40. [Google Scholar] [CrossRef] [PubMed]


| WPC | SDPP | SPI | |
|---|---|---|---|
| Ingredients | % | % | % |
| Whey powder | 12.17 | 11.18 | 12.95 |
| Whole milk powder | 10.00 | 10.00 | 10.00 |
| Skim milk powder | 24.00 | 24.00 | 24.00 |
| Whey protein concentrate, 73% CP 1 | 17.70 | 11.92 | 11.05 |
| Plasma protein powder, 72% CP 2 | - | 6.00 | - |
| Soy protein isolate, 83% CP 3 | - | - | 5.13 |
| Oil powder | 25.00 | 25.70 | 25.00 |
| Sucrose | 5.00 | 5.00 | 5.00 |
| L-lysine hydrochloride | - | 0.10 | 0.36 |
| DL-methionine | 0.34 | 0.37 | 0.50 |
| L-threonine | - | - | 0.15 |
| L-tryptophan | - | 0.02 | 0.05 |
| Calcium hydrogen phosphate | 1.94 | 1.48 | 1.84 |
| Calcium formate | 0.20 | 0.58 | 0.32 |
| Choline chloride, AR | 0.08 | 0.08 | 0.08 |
| Vitamin premix 4 | 0.03 | 0.03 | 0.03 |
| Mineral premix 5 | 0.02 | 0.02 | 0.02 |
| Antioxidants | 0.02 | 0.02 | 0.02 |
| Citric acid | 3.50 | 3.50 | 3.50 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient levels, calculated | |||
| Digestible energy, Mcal/kg | 4.26 | 4.26 | 4.26 |
| Crude protein, % | 25.02 | 25.03 | 25.03 |
| Ether extract, % | 16.13 | 16.42 | 16.07 |
| Lactose, % | 28.02 | 27.03 | 28.19 |
| Calcium, % | 1.00 | 1.00 | 1.00 |
| Total phosphorus, % | 0.71 | 0.71 | 0.71 |
| Total lysine, % | 2.56 | 2.56 | 2.56 |
| Total methionine, % | 0.95 | 0.91 | 1.03 |
| Total methionine + cystine, % | 1.42 | 1.42 | 1.42 |
| Total threonine, % | 1.57 | 1.58 | 1.57 |
| Total tryptophan, % | 0.46 | 0.46 | 0.46 |
| Genes | Primer Sequence (5′–3′) | Product (bp) | GenBank Accession |
|---|---|---|---|
| IL–6 | F: TGCAGTCACAGAACGAGTGG | 116 | NM_214399.1 |
| R: CAGGTGCCCCAGCTACATTAT | |||
| IL–10 | F: AATCTGCTCCAAGGTTCCCG | 224 | NM_214041.1 |
| R: TGAACACCATAGGGCACACC | |||
| IL–1β | F: TCTGCCCTGTACCCCAACTG | 64 | NM_214055.1 |
| R: CCAGGAAGACGGGCTTTTG | |||
| TNF–a | F: CCACGTTGTAGCCAATGTCA | 395 | NM_214022.1 |
| R: CAGCAAAGTCCAGATAGTCG | |||
| Occludin | F: CAGCAGCAGTGGTAACTTGG | 110 | NM_001163647.2 |
| R: CCGTCGTGTAGTCTGTCTCG | |||
| ZO–1 | F: CCGCCTCCTGAGTTTGATAG | 189 | XM_013993251.1 |
| R: CAGCTTTAGGCACTGTGCTG | |||
| Claudin–1 | F: ACTGGCTGGGCTGCTGCTTCTCT | 101 | NM_001244539.1 |
| R: GGATAGGGCCTTGGTGTTGGGTAA | |||
| β–actin | F: GGCGCCCAGCACGAT | 66 | XM_021086047.1 |
| R: CCGATCCACACGGAGTACTTG |
| Diets | SEM | p-Value | |||
|---|---|---|---|---|---|
| WPC | SDPP | SPI | |||
| Initial BW (kg) | 1.54 | 1.56 | 1.56 | 0.08 | 0.98 |
| Final BW (kg) | 3.39 | 4.37 | 3.04 | 0.37 | 0.05 |
| ADG (g/d) | 103 | 157 | 83 | 20.86 | 0.05 |
| ADFI (g/d) | 130 | 168 | 140 | 13.04 | 0.12 |
| F/G | 1.72 | 1.16 | 2.27 | 0.36 | 0.07 |
| Diets | SEM | p-Value | |||
|---|---|---|---|---|---|
| WPC | SDPP | SPI | |||
| C3, mg/L | 0.09 | 0.09 | 0.09 | 0.01 | 0.81 |
| IgG, g/L | 1.86 | 1.83 | 1.88 | 0.06 | 0.81 |
| Urea, mmol/L | 15.71 | 13.21 | 24.64 | 3.61 | 0.08 |
| GLU, mmol/L | 2.80 | 2.44 | 2.89 | 0.83 | 0.93 |
| TC, mmol/L | 3.18 | 3.40 | 3.62 | 0.29 | 0.56 |
| LDL–C, mmol/L | 1.36 | 1.49 | 1.71 | 0.18 | 0.37 |
| HDL–C, mmol/L | 0.81 | 0.74 | 0.67 | 0.06 | 0.25 |
| NEFA, µmol/L | 386.12 | 320.08 | 341.02 | 76.63 | 0.82 |
| TG, mmol/L | 0.76 | 0.88 | 0.91 | 0.16 | 0.72 |
| Diets | SEM | p-Value | |||
|---|---|---|---|---|---|
| WPC | SDPP | SPI | |||
| Shannon | 5.29 | 4.98 | 5.17 | 0.18 | 0.52 |
| Simpson | 0.90 | 0.89 | 0.89 | 0.02 | 0.46 |
| Dominance | 0.10 | 0.11 | 0.11 | 0.02 | 0.46 |
| Pielou_e | 0.59 | 0.56 | 0.57 | 0.02 | 0.62 |
| Chao1 | 534.32 | 492.19 | 541.98 | 29.13 | 0.44 |
| Diets | SEM | p-Value | |||
|---|---|---|---|---|---|
| WPC | SDPP | SPI | |||
| Acetic acid, μmoL/g | 31.48 | 29.75 | 31.33 | 2.12 | 0.81 |
| Propionic acid, μmoL/g | 11.61 | 12.17 | 11.29 | 0.50 | 0.47 |
| Isobutyric acid, μmoL/g | 1.06 | 0.99 | 1.07 | 0.07 | 0.69 |
| Butyric acid, μmoL/g | 3.33 | 4.32 | 2.86 | 0.45 | 0.08 |
| Isovaleric acid, μmoL/g | 2.21 | 2.05 | 2.23 | 0.19 | 0.77 |
| Valeric acid, μmoL/g | 0.49 | 0.47 | 0.51 | 0.07 | 0.92 |
| Total SCFAs, μmoL/g | 50.17 | 49.76 | 49.11 | 2.27 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, Q.; Liu, S.; Quan, X.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Zhuo, Y.; Wu, D.; et al. Partial Substitution of Whey Protein Concentrate with Spray–Dried Porcine Plasma or Soy Protein Isolate in Milk Replacer Differentially Modulates Ileal Morphology, Nutrient Digestion, Immunity and Intestinal Microbiota of Neonatal Piglets. Animals 2023, 13, 3308. https://doi.org/10.3390/ani13213308
Zhang Y, Zhou Q, Liu S, Quan X, Fang Z, Lin Y, Xu S, Feng B, Zhuo Y, Wu D, et al. Partial Substitution of Whey Protein Concentrate with Spray–Dried Porcine Plasma or Soy Protein Isolate in Milk Replacer Differentially Modulates Ileal Morphology, Nutrient Digestion, Immunity and Intestinal Microbiota of Neonatal Piglets. Animals. 2023; 13(21):3308. https://doi.org/10.3390/ani13213308
Chicago/Turabian StyleZhang, Yuwei, Qiang Zhou, Shiya Liu, Xiang Quan, Zhengfeng Fang, Yan Lin, Shengyu Xu, Bin Feng, Yong Zhuo, De Wu, and et al. 2023. "Partial Substitution of Whey Protein Concentrate with Spray–Dried Porcine Plasma or Soy Protein Isolate in Milk Replacer Differentially Modulates Ileal Morphology, Nutrient Digestion, Immunity and Intestinal Microbiota of Neonatal Piglets" Animals 13, no. 21: 3308. https://doi.org/10.3390/ani13213308
APA StyleZhang, Y., Zhou, Q., Liu, S., Quan, X., Fang, Z., Lin, Y., Xu, S., Feng, B., Zhuo, Y., Wu, D., & Che, L. (2023). Partial Substitution of Whey Protein Concentrate with Spray–Dried Porcine Plasma or Soy Protein Isolate in Milk Replacer Differentially Modulates Ileal Morphology, Nutrient Digestion, Immunity and Intestinal Microbiota of Neonatal Piglets. Animals, 13(21), 3308. https://doi.org/10.3390/ani13213308








