Growth Performance and Disease Resistance against Vibrio parahaemolyticus of Whiteleg Shrimp (Litopenaeus vannamei) Fed Essential Oil Blend (Phyto AquaBiotic)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Tested Blend of EOs
2.2. Shrimp and Experimental Conditions
2.3. Calculation of Growth Parameters
- ○
- Daily weight gain (DWG, g/day) = (Wf − Wi)/42 days where Wi represents the initial weight of the shrimp (in grams) determined before the experiment and Wf represents the final weight of the shrimp (in grams) determined after 42 days;
- ○
- Specific growth rate (SGR, %/day) = (Ln(Wf) − Ln(Wi)) * 100/42 days;
- ○
- Feed conversion ratio (FCR) = consumed feed/weight gain where consumed feed is the total amount of feed consumed by the shrimp during the experiment and weight gain refers to the difference between the final weight and the initial weight of the shrimp;
- ○
- Survival rate (SR, %) = (final number of shrimp/initial number of shrimp) * 100 where the final number of shrimp represents the count of surviving shrimp at the end of the feeding trial while the initial number of shrimp represents the count of shrimp stocked in each tank at the beginning of the experiment;
- ○
- Biomass (kg m−3) = mean weight of shrimp x survival rate.
2.4. Challenge Experiment
2.4.1. Vibrio parahaemolyticus Strain
2.4.2. Experimental Challenge
2.5. Total Vibrio Counts
2.6. PCR Method for Detection of V. parahaemolyticus
2.7. Statistical Analysis
3. Results
3.1. Effects of PAB Supplement on Growth Performance of Whiteleg Shrimp
3.2. Effects of PAB Supplements on Disease Resistance of Whiteleg Shrimp
3.2.1. Vibrio parahaemolyticus Challenge
Confirmation of Bacterial Infection
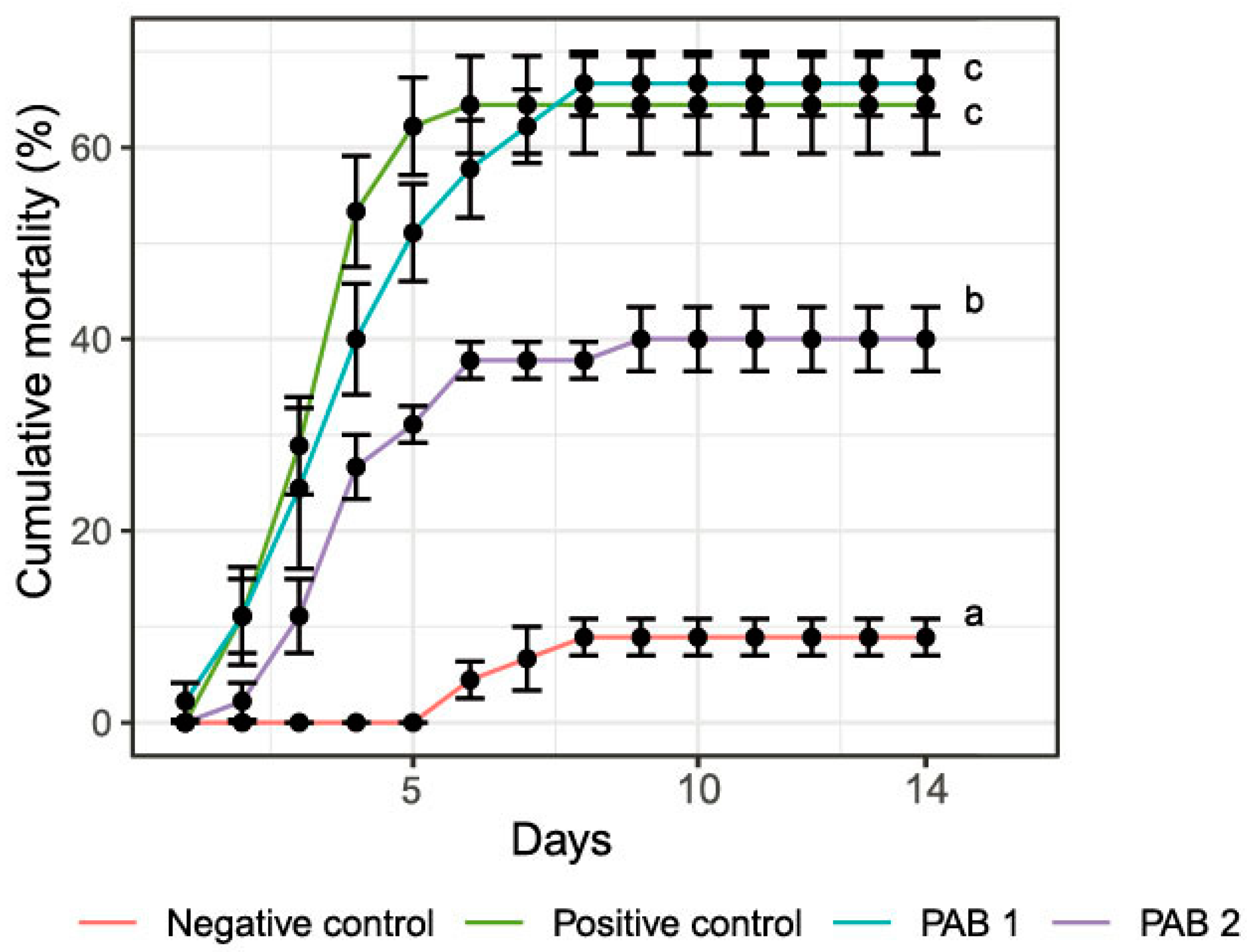
Cumulative Mortality of Shrimps Challenged with V. parahaemolyticus
3.2.2. Bacterial Density from Shrimp Hepatopancreas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azra, M.N.; Okomoda, V.T.; Tabatabaei, M.; Hassan, M.; Ikhwanuddin, M. The Contributions of Shellfish Aquaculture to Global Food Security: Assessing Its Characteristics from a Future Food Perspective. Front. Mar. Sci. 2021, 8, 654897. [Google Scholar] [CrossRef]
- Nguyen, T.A.T.; Nguyen, K.A.T.; Jolly, C. Is Super-Intensification the Solution to Shrimp Production and Export Sustainability? Sustainability 2019, 11, 5277. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, C.; Xie, J.; Xu, C.; Zhao, Q.; Qin, J.G.; Chen, L.; Li, E. Intestinal bacterial signatures of the “cotton shrimp-like” disease explain the change of growth performance and immune responses in Pacific white shrimp (L. vannamei). Fish Shellfish Immunol. 2019, 92, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Millard, R.S.; Ellis, R.P.; Bateman, K.S.; Bickley, L.K.; Tyler, C.R.; Aerle, R.; Santos, E.M. How do abiotic environmental conditions influence shrimp susceptibility to disease? A critical analysis focussed on White Spot Disease. J. Invertebr. Pathol. 2021, 186, 107369. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K.; Babikian, Y.H.; Babikian, H.Y.; Khoa, L.V.; Wisoyo, D.; Srisombat, S.; Jiaravanon, B. Efficacy of natural herbal formulation against acute hepatopancreatic necrosis disease (AHPND) causing Vibrio parahaemolyticus in Penaeus vannamei. Vet. Med. Open J. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2023, 15, 1421–1451. [Google Scholar] [CrossRef]
- Fagnon, M.S.; Thorin, C.; Calvez, S. Meta-analysis of dietary supplementation effect of turmeric and curcumin on growth performance in fish. Rev. Aquac. 2020, 12, 2268–2283. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Deng, Z.; Ma, C.; Wang, N.; Fu, C.; Lambert, H.; Yan, F. Antibiotic governance and use on commercial and smallholder farms in eastern China. Front. Vet. Sci. 2023, 10, 1128707. [Google Scholar] [CrossRef]
- Da Silva, R.A.; Arenas, N.E.; Luiza, V.L.; Bermudez, J.A.Z.; Clarke, S.E. Regulations on the Use of Antibiotics in Livestock Production in South America: A Comparative Literature Analysis. Antibiotics 2023, 12, 1303. [Google Scholar] [CrossRef]
- Yeshi, K.; Wangchuk, P. Essential oils and their bioactive molecules in healthcare. In Herbal Biomolecules in Healthcare Applications; Mandal, S.C., Nayak, A.K., Dhara, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 215–237. [Google Scholar] [CrossRef]
- Park, J.W.; Wendt, M.; Heo, G.J. Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria. Lab. Anim. Res. 2016, 32, 87–90. [Google Scholar] [CrossRef]
- Van Doan, H.; Soltani, M.; Leitão, A.; Shafiei, S.; Asadi, S.; Lymbery, A.J.; Ringø, E. Streptococcosis a Re-Emerging Disease in Aquaculture: Significance and Phytotherapy. Animals 2022, 12, 2443. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.L.; YWTan, J.Y.W.; Liu, H.Y.; Zhou, X.H.; Xiang, X.; Wang, K.Y. Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 2009, 292, 214–218. [Google Scholar] [CrossRef]
- Hafsan, H.; Saleh, M.M.; Zabibah, R.S.; Obaid, R.F.; Jabbar, H.S.; Mustafa, Y.F.; Sultan, M.Q.; Gabr, G.A.; Ramírez-Coronel, A.A.; Khodadadi, M.; et al. Dietary Thymol Improved Growth, Body Composition, Digestive Enzyme Activities, Hematology, Immunity, Antioxidant Defense, and Resistance to Streptococcus iniae in the Rainbow Trout (Oncorhynchus mykiss). Aquac. Nutr. 2022, 2022, 3288139. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; El Triantafillou, S.S.; Mavridis, S.; Steinere, T.; Karagouni, E. Assessment of dietary supplementation with carvacrol or thymol containing feed additives on performance, intestinal microbiota and antioxidant status of rainbow trout (Oncorhynchus mykiss). Aquaculture 2012, 350–353, 26–32. [Google Scholar] [CrossRef]
- Amer, S.A.; Metwally, A.E.; Ahmed, S.A.A. The influence of dietary supplementation of cinnamaldehyde and thymol on the growth performance, immunity and antioxidant status of monosex Nile tilapia fingerlings (Oreochromis niloticus). Egypt. J. Aquat. Res. 2018, 3, 251–256. [Google Scholar] [CrossRef]
- Hernández-Cabanyero, C.; Carrascosa, E.; Jiménez, S.; Fouz, B. Exploring the Effect of Functional Diets Containing Phytobiotic Compounds in Whiteleg Shrimp Health: Resistance to Acute Hepatopancreatic Necrotic Disease Caused by Vibrio parahaemolyticus. Animals 2023, 13, 1354. [Google Scholar] [CrossRef]
- Cunha, J.A.; Heinzmann, B.M.; Baldisserotto, B. The effects of essential oils and their major compounds on fish bacterial pathogens—A review. J. Appl. Microbiol. 2018, 125, 328–344. [Google Scholar] [CrossRef]
- Fagnon, M.S.; Fournel, C.; Chabrillat, T.; Kerros, S.; Calvez, S. In vitro assessment of antibacterial activities of some essential oils against infectious fish pathogens. In Proceedings of the AFRAQ 2021, Alexandria, Egypt, 25–28 March 2022. [Google Scholar]
- Sritunyalucksana, S.; Dangtip, P.; Sanguanrut, R.; Sirikharin, S.; Thitamadee, S.; Taengchaiphum, R.; Mavichak, P.; Proespraiwong, T.W.F. A Two-Tube, Nested PCR Detection Method for AHPND Bacteria; Network of Aquaculture Centres in Aisa and the Pacific: Bangkok, Thailand, 2014. [Google Scholar]
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar] [CrossRef]
- Bandeira Junior, G.; Bianchini, A.E.; de Freitas Souza, C.; Descovi, S.N.; da Silva Fernandes, L.; de Lima Silva, L.; Cargnelutti, J.F.; Baldisserotto, B. The Use of Cinnamon Essential Oils in Aquaculture: Antibacterial, Anesthetic, Growth-Promoting, and Antioxidant Effects. Fishes 2022, 7, 133. [Google Scholar] [CrossRef]
- Yousefi, M.; Adineh, H.; Ghadamkheir, M.; Hashemianfar, S.A.M.; Yilmaz, S. Effects of dietary Pennyroyal essential oil on growth performance, digestive enzymes’ activity, and stress responses of common carp, Cyprinus carpio. Aquac. Rep. 2023, 30, 101574. [Google Scholar] [CrossRef]
- Kim, J.D.; Nhut, T.M.; Hai, T.N.; Ra, C.S. Effect of Dietary Essential Oils on Growth, Feed Utilization and Meat Yields of White Leg Shrimp L. vannamei. Asian-Aust. J. Anim. Sci. 2011, 24, 1136–1141. [Google Scholar] [CrossRef]
- Aktaş, M.; Genç, M.A.; Bircan Yıldırım, Y.; Kaya, D.; Narin, Ö.Ç.; Genç, E. Effects of thyme and thyme oil on the growth of white shrimp, Litopenaeus vannamei. Acta Aquat. Turc. 2022, 18, 81–92. [Google Scholar] [CrossRef]
- Chen, Z.; Jing, F.; Lu, M.; Su, C.; Tong, R.; Pan, L. Effects of dietary trans-cinnamaldehyde on growth performance, lipid metabolism, immune response and intestinal microbiota of Litopenaeus vannamei. Fish Shellfish Immunol. 2022, 131, 908–917. [Google Scholar] [CrossRef] [PubMed]
- El-Hamid, M.I.A.; Ibrahim, S.M.; Eldemery, F.; El-Mandrawy, S.A.M.; Metwally, A.S.; Khalifa, E.; Elnahriry, S.S.; Ibrahim, D. Dietary cinnamaldehyde nanoemulsion boosts growth and transcriptomes of antioxidant and immune related genes to fight Streptococcus agalactiae infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 113, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Khalil, R.H. Evaluation of two phytobiotics, Spirulina platensis and Origanum vulgare extract on growth, serum antioxidant activities and resistance of Nile tilapia (Oreochromis niloticus) to pathogenic Vibrio alginolyticus. Int. J. Fish. Aquat. Stud. 2014, 1, 250–255. [Google Scholar]
- Diler, O.; Gormez, O.; Diler, I.; Metin, S. Effect of oregano (Origanum onites L.) essential oil on growth, lysozyme and antioxidant activity and resistance against Lactococcus garvieae in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Nutr. 2016, 83, 844–851. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Saravana Bhavan, P.; Seenivasan, C.; Muralisankar, T.; Shanthi, R. Effects of native medicinal herbs (Alternantherasessilis, Eclipta alba and Cissus quadrangularis) on growth performance, digestive enzymes and biochemical constituents of the monsoon river prawn Macrobrachium malcolmsonii. Aquacult. Nutr. 2015, 21, 496–506. [Google Scholar] [CrossRef]
- Brackman, G.; Celen, S.; Hillaert, U.; Van Calenbergh, S.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Structure-Activity Relationship of Cinnamaldehyde Analogs as Inhibitors of AI-2 Based Quorum Sensing and Their Effect on Virulence of Vibrio spp. PLoS ONE 2011, 6, e16084. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Zheng, X.; Feyaerts, A.F.; Van Dijck, P.; Bossier, P. Inhibitory Activity of Essential Oils against Vibrio campbellii and Vibrio parahaemolyticus. Microorganisms 2020, 8, 1946. [Google Scholar] [CrossRef]
- Yu, H.; Pei, J.; Qiu, W.; Mei, J.; Xie, J. The Antimicrobial Effect of Melissa officinalis L. Essential Oil on Vibrio parahaemolyticus: Insights Based on the Cell Membrane and External Structure. Front. Microbiol. 2022, 13, 2792. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.H.; Le, M.T.; Le, A.V.T.; Dang, D.M.T.; Dang, C.M.; Le, N.N. The Bactericidal Effect of Essential Oils in Vietnam to Vibrio parahaemolyticus Causing AHPND in Shrimp. J. Fish. Environ. 2023, 47, 11–24. [Google Scholar]
- Faleye, O.S.; Sathiyamoorthi, E.; Lee, J.-H.; Lee, J. Inhibitory Effects of Cinnamaldehyde Derivatives on Biofilm Formation and Virulence Factors in Vibrio Species. Pharmaceutics 2021, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamed, H.; Ozdemir, O.; Ibrahim, I.; Lawrence, M.; Karsi, A. Antibacterial activities of trans-cinnamaldehyde, caprylic acid, and β-resorcylic acid against catfish pathogens. Aquaculture 2019, 504, 334–344. [Google Scholar] [CrossRef]
- Shan, Z.; Wang, M.; Zhao, S.; Xie, X.; Yang, D.; Liu, W. Cinnamaldehyde exerts prophylactic and therapeutic effects against Vibrio anguillarum infection in Yesso scallop (Patinopecten yessoensis) by its direct antimicrobial activity and positive effect on the innate immunity. Aquaculture 2021, 538, 736588. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Balasundaram, C.; Doan, H.V.; Jaturasitha, S.; Saravanan, K.; Ringø, E. Impact of cinnamaldehyde on innate immunity and immune gene expression in Channa striatus against Aphanomyces invadans. Fish Shellfish Immunol. 2021, 117, 1–16. [Google Scholar] [CrossRef]
- Faikoh, E.N.; Hong, Y.H.; Hu, S.Y. Liposome-encapsulated cinnamaldehyde enhances zebrafish (Danio rerio) immunity and survival when challenged with Vibrio vulnificus and Streptococcus agalactiae. Fish Shellfish Immunol. 2014, 38, 15–24. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of plant essential oils and their components in animal agriculture—In vitro studies on antibacterial mode of action. Front. Vet. Sci. 2015, 2, 35. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2020, 13, 632–641. [Google Scholar] [CrossRef]
- Zargar, A.; Rahimi-Afzal, Z.; Soltani, E. Growth performance, immune response and disease resistance of rainbow trout (Oncorhynchus mykiss) fed Thymus vulgaris essential oils. Aquactic 2019, 50, 3097–3106. [Google Scholar]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Khafaga, A.F.; Dawood, M.A.O. Dietary oregano essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish Shellfish Immunol. 2020, 104, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gültepe, N.; Bilen, S.; Yılmaz, S.; Güuroy, D.; Aydın, S. Effects of herbs and spice on health status of tilapia (Oreochromis mossambicus) challenged with Streptococcus iniae. Acta Vet. Brno 2014, 3, 125–131. [Google Scholar] [CrossRef]
- Jùnior, O.T.; Kuhn, F.; Padilha, P.J.M.; Nesi, C.N.; Mestres, M.; Dal Magro, J. Effect of microencapsulated thyme essential oil on white spot virus-infected Litopenaeus vannamei. Aquac. Int. 2018, 26, 1459–1468. [Google Scholar] [CrossRef]
- Girard, C.; Fayolle, K.; Kerros, S.; Leriche, F. Flow cytometric assessment of the antimicrobial properties of an essential oil mixture against Escherichia coli. J. Anim. Feed Sci. 2019, 28, 187–198. [Google Scholar] [CrossRef]
- Yang, S.K.; Tan, N.P.; Wie, C.; Abushelaibi, A.; Lim, S.H.E.; Lai, K.S. The Missing Piece: Recent Approaches Investigation of the Antimicrobial Mode of Action of Essential Oils. Evol. Bioinform. 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelarr, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Oskuee, R.K.; Behravan, J.; Ramezani, M. Chemical composition, antimicrobial activity and antiviral activity of essential oil of Carum copticum from Iran. Avicenna J. Phytomed. 2011, 1, 83–90. [Google Scholar]
- Brackman, G.; Defoirdt, T.; Miyamoto, C. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. By decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008, 8, 149. [Google Scholar] [CrossRef]
- Michl, S.C.; Ratten, J.-M.; Beyer, M.; Hasler, M.; LaRoche, J.; Schulz, C. The malleable gut microbiome of juvenile rainbow trout (Oncorhynchus mykiss): Diet-dependent shifts of bacterial community structures. PLoS ONE 2017, 12, e0177735. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Liu, Y.; Qiao, F.; Chen, L.; Liu, W.T.; Du, Z.; Li, E. Response of gut microbiota to salinity change in two euryhaline aquatic animals with reverse salinity preference. Aquaculture 2016, 454, 72–80. [Google Scholar] [CrossRef]
- Ringø, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J.; Krogdahl, A.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquac. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Qin, J.G.; Chen, L. Gut Microbiota and its Modulation for Healthy Farming of Pacific White Shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Ramadhani, D.E.; Hendriana, A.; Wahjuningrum, D.; Mulya, M.A. Vibrio Dynamics and Health Status of Pacific White Shrimp Fed with Cinnamaldehyde-Containing Feed. J. Ilm. Perikan. Dan Kelaut. 2022, 14, 285–296. [Google Scholar] [CrossRef]
- Huyben, D.; Chiasson, M.; Lumsden, J.S.; Pham, P.H.; Chowdhury, M.A.K. Dietary Microencapsulated Blend of Organic Acids and Plant Essential Oils Affects Intestinal Morphology and Microbiome of Rainbow Trout (Oncorhynchus mykiss). Microorganisms 2021, 9, 2063. [Google Scholar] [CrossRef]
- Habiba, M.M.; Hussein, E.E.; Ashry, A.M.; El-Zayat, A.M.; Hassan, A.M.; El-Shehawi, A.M.; Sewilam, H.; Van Doan, H.; Dawood, M.A.O. Dietary Cinnamon Successfully Enhanced the Growth Performance, Growth Hormone, Antibacterial Capacity, and Immunity of European Sea Bass (Dicentrarchus labrax). Animals 2021, 11, 2128. [Google Scholar] [CrossRef]
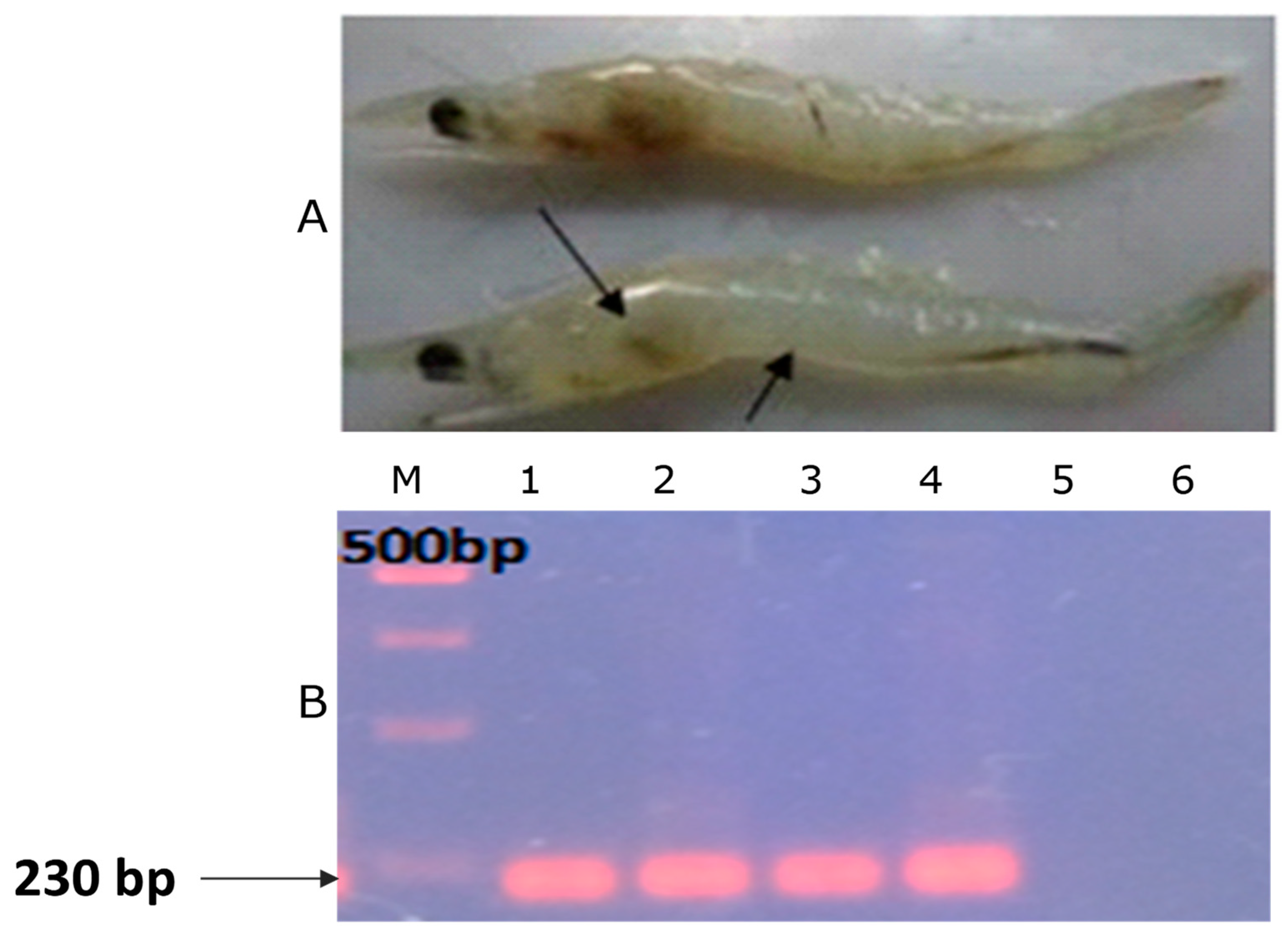
| Parameters | Treatments | ||
|---|---|---|---|
| Control | PAB-1 | PAB-2 | |
| Wi (g/ind.) | 1.02 ± 0.01 | 1.02 ± 0.01 | 1.04 ± 0.04 |
| Wf (g) | 8.19 ± 0.45 a | 8.70 ± 0.25 ab | 9.11 ± 0.12 b |
| DWG (g·d−1) | 0.17 ± 0.01 a | 0.18 ± 0.01 ab | 0.19 ± 0.00 b |
| SGR (%·d−1) | 4.98 ± 0.19 | 5.00 ± 0.09 | 5.21 ± 0.13 |
| FCR | 1.15 ± 0.10 | 1.14 ± 0.01 | 1.08 ± 0.10 |
| Biomass (kg·m−3) | 0.76 ± 0.04 a | 0.81 ± 0.01 b | 0.80 ± 0.01 b |
| Groups | Vibrio Count (×106 CFU/g) |
|---|---|
| Negative control | 4.49 ± 3.43 |
| Positive control | 8.06 ± 4.69 |
| PAB-1 | 5.77 ± 2.44 |
| PAB-2 | 5.28 ± 3.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoa, T.T.T.; Fagnon, M.S.; Thy, D.T.M.; Chabrillat, T.; Trung, N.B.; Kerros, S. Growth Performance and Disease Resistance against Vibrio parahaemolyticus of Whiteleg Shrimp (Litopenaeus vannamei) Fed Essential Oil Blend (Phyto AquaBiotic). Animals 2023, 13, 3320. https://doi.org/10.3390/ani13213320
Hoa TTT, Fagnon MS, Thy DTM, Chabrillat T, Trung NB, Kerros S. Growth Performance and Disease Resistance against Vibrio parahaemolyticus of Whiteleg Shrimp (Litopenaeus vannamei) Fed Essential Oil Blend (Phyto AquaBiotic). Animals. 2023; 13(21):3320. https://doi.org/10.3390/ani13213320
Chicago/Turabian StyleHoa, Tran Thi Tuyet, Mahougnon Siméon Fagnon, Dang Thuy Mai Thy, Thibaut Chabrillat, Nguyen Bao Trung, and Sylvain Kerros. 2023. "Growth Performance and Disease Resistance against Vibrio parahaemolyticus of Whiteleg Shrimp (Litopenaeus vannamei) Fed Essential Oil Blend (Phyto AquaBiotic)" Animals 13, no. 21: 3320. https://doi.org/10.3390/ani13213320





