Human and Non-Human Primate Coexistence in Argentina: Conflicts and Solutions
Abstract
:Simple Summary
Abstract
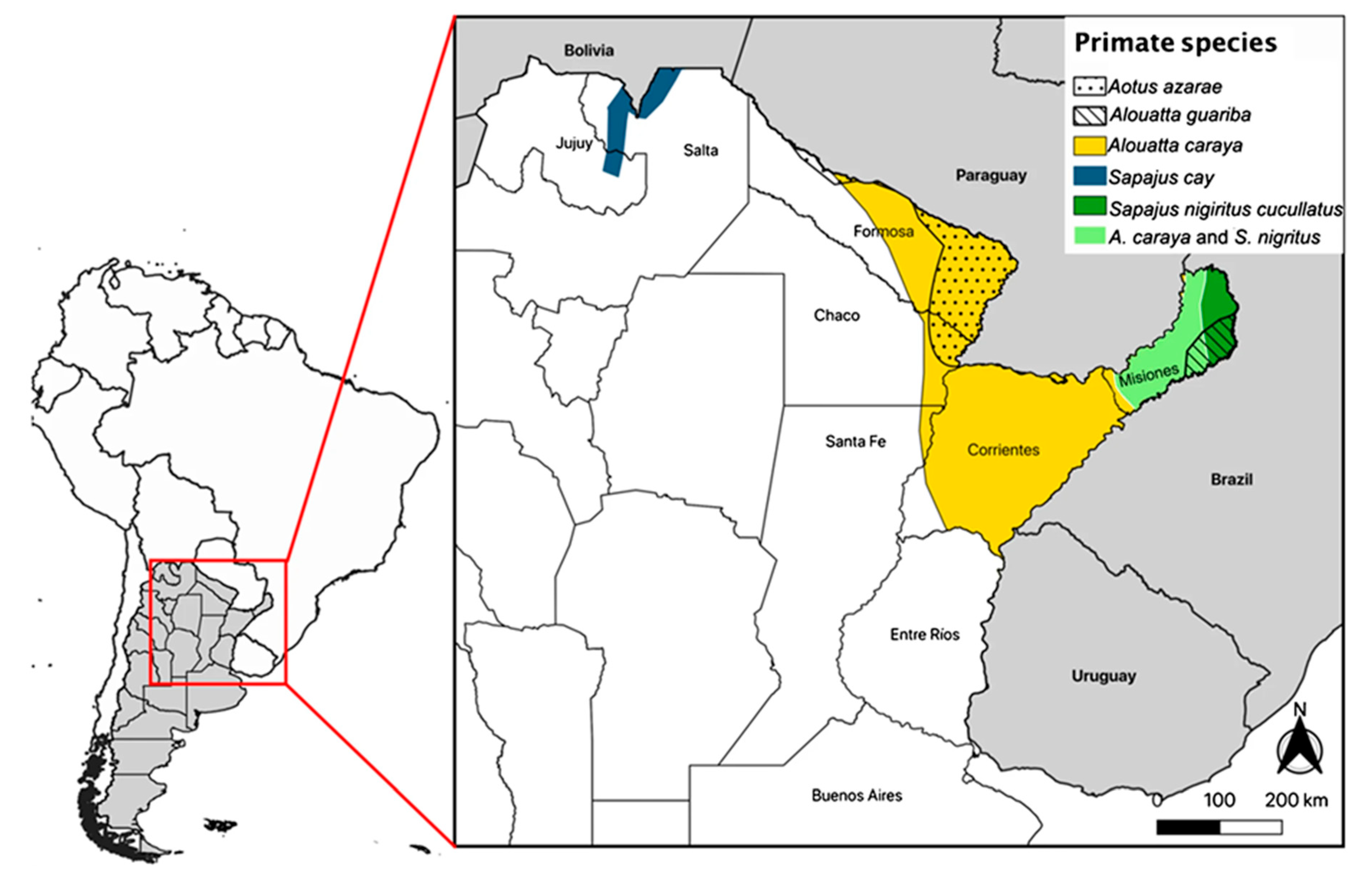
1. Primate Species in Argentina
2. Defining Human–Primate Conflict
3. Habitat Loss
3.1. Anthropogenically Induced Fires
3.2. “Urbanized” Monkeys
3.3. Power Lines and Electrocutions
3.4. Roadkills
3.5. Crop Foraging
4. Human Misconceptions about Primate Biology and Behavioral Ecology
4.1. Transmission of Infectious Diseases
4.2. Food Provisioning
4.3. Illegal Pet Trade
4.4. “Rescue” Centers
5. Regional and Local Approaches to Mitigate Human–Primate Conflict in Argentina
5.1. A National Primate Conservation Plan
5.2. Priority Areas for Conservation
5.3. Population Management for the Conservation of the Brown Howler Monkey
5.4. Provincial Legislation: Natural Monuments
5.5. Citizen Science and Environmental Education in Argentina
5.6. Primate Research Groups in Argentina
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juárez, C.P.; Baldovino, C.; Kowalewski, M.; Fernandez-Duque, E. Los Primates de Argentina: Ecología y Conservación. In Manejo de Fauna en la Argentina: Acciones para la Conservación de Especies Amenazadas; Fundación de Historia Natural “Felix de Azara”: Buenos Aires, Argentina, 2012. [Google Scholar]
- Oklander, L.I.; Baigorria, J.; Kowalewski, M. Current perspectives in wild primate research in Argentina. In Primatology in Argentina; Kowalewski, M., Oklander, L., Eds.; Sociedad Argentina para el Estudio de los Mamíferos Series A. Mammalogical Research; SAREM: Buenos Aires, Argentina, 2017; Volume 2, pp. 273–283. [Google Scholar]
- Kowalewski, M.; Oklander, L.I. Primatology in Argentina; Sociedad Argentina para el Estudio de los Mamíferos Series A. Mammalogical Research; SAREM: Buenos Aires, Argentina, 2017; Volume 2. [Google Scholar]
- Illia, G.A.; Bay Jouliá, R.; Citon, L.; Oklander, L.I.; Kowalewski, M. Parasites and Other Infectious Agents in Non-human Primates of Argentina. Curr. Trop. Med. Rep. 2022, 9, 267–277. [Google Scholar] [CrossRef]
- Abba, A.M.; Varela, D.; Cirignoli, S.; Pereira, J.A.; Bolkovic, M.L.; Peker, S.; Porini, G.; de Bustos, S.; Degrati, M.; Denuncio, P.E.; et al. Categorización de los mamíferos de Argentina 2019: Resumen y análisis de las amenazas. Mastozool. Neotrop. 2022, 29, e0657. [Google Scholar] [CrossRef]
- Estrada, A.; Garber, P.A.; Rylands, A.B.; Roos, C.; Fernandez-Duque, E.; Fiore, A.D.; Nekaris, K.A.-I.; Nijman, V.; Heymann, E.W.; Lambert, J.E.; et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 2017, 3, e1600946. [Google Scholar] [CrossRef]
- Colchero, F.; Aburto, J.M.; Archie, E.A.; Boesch, C.; Breuer, T.; Campos, F.A.; Collins, A.; Conde, D.A.; Cords, M.; Crockford, C.; et al. The long lives of primates and the ‘invariant rate of ageing’ hypothesis. Nat. Commun. 2021, 12, 3666. [Google Scholar] [CrossRef]
- Hurn, S. Human–animal relations (HAR). In The International Encyclopedia of Primatology; Fuentes, A., Ed.; Wiley-Blackwell: New York, NY, USA, 2017; pp. 1–7. [Google Scholar]
- Manfredo, M.J.; Dayer, A.A. Concepts for Exploring the Social Aspects of Human–Wildlife Conflict in a Global Context. Hum. Dimens. Wildl. 2004, 9, 1–20. [Google Scholar] [CrossRef]
- Radhakrishna, S.; Sengupta, A. What does human-animal studies have to offer ethology? Acta Ethol. 2020, 23, 193–199. [Google Scholar] [CrossRef]
- Birkhead, T.R.; Monaghan, P. Ingenious Ideas: The History of Behavioral Ecology. In Evolutionary Behavioral Ecology; Westneat, D.F., Fox, C.W., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 3–15. [Google Scholar]
- Bhatia, S.; Redpath, S.M.; Suryawanshi, K.; Mishra, C. Beyond conflict: Exploring the spectrum of human–wildlife interactions and their underlying mechanisms. Oryx 2020, 54, 621–628. [Google Scholar] [CrossRef]
- Frank, B.; Glikman, J.A.; Marchini, S. Human–Wildlife Interactions: Turning Conflict into Coexistence; Cambridge University Press: Cambridge, UK, 2019; Volume 23. [Google Scholar]
- Pooley, S.; Bhatia, S.; Vasava, A. Rethinking the study of human–wildlife coexistence. Conserv. Biol. 2021, 35, 784–793. [Google Scholar] [CrossRef]
- Hockings, K.J.; Humle, T. Best Practice Guide-Lines for the Prevention and Mitigation of Conflict between Humans and Great Apes; IUCN/SSC Primate Specialist Group (PSG): Gland, Switzerland, 2009. [Google Scholar]
- Humle, T.; Hill, C.M. People-primate interactions: Implications for primate conservation. In An Introduction to Primate Conservation; Wich, S.A., Marshall, A., Eds.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Madden, F. Creating coexistence between humans and wildlife: Global perspectives on local efforts to address human–wildlife conflict. Hum. Dimens. Wildl. 2004, 9, 247–257. [Google Scholar] [CrossRef]
- Inskip, C.; Zimmermann, A. Human-felid conflict: A review of patterns and priorities worldwide. Oryx 2009, 43, 18–34. [Google Scholar] [CrossRef]
- Mateo-Tomas, P.; Olea, P.P.; Sanchez-Barbudo, I.S.; Mateo, R. Alleviating humane wildlife conflicts: Identifying the causes and mapping the risk of illegal poisoning of wild fauna. J. Appl. Ecol. 2012, 49, 376–385. [Google Scholar] [CrossRef]
- Michalski, F.; Boulhosa, R.L.P.; Faria, A.; Peres, C. Human-wildlife conflicts in a fragmented Amazonian forest landscape: Determinants of large felid depredation on livestock. Anim. Conserv. 2006, 9, 179–188. [Google Scholar] [CrossRef]
- Woodroffe, R.; Thirgood, S.; Rabinowitz, A. (Eds.) The impact of human-wildlife conflict on natural systems. In People and Wildlife: Conflict and Coexistence; Cambridge University Press: New York, NY, USA, 2005; pp. 1–12. [Google Scholar]
- Hockings, K.J. Mitigating Human-Nonhuman Primate Conflict. In The International Encyclopedia of Primatology; Bezanson, M., MacKinnon, K.C., Riley, E., Campbell, C.J., Nekaris, A., Estrada, A., Di Fiore, A., Ross, S., Jones-Engel, L.E., Thierry, B., et al., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–2. [Google Scholar]
- Radhakrishna, S.; Jamieson, D. Liberating primatology. J. Biosci. 2018, 43, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A. Ethnoprimatology and the anthropology of the human-primate interface. Annu. Rev. Anthropol. 2012, 41, 101–117. [Google Scholar] [CrossRef]
- Setchell, J.M.; Fairet, E.; Schutt, K.; Waters, S.; Bell, S. Biosocial Conservation: Integrating Biological and Ethnographic Methods to Study Human–Primate Interactions. Int. J. Primatol. 2017, 38, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Sponsel, L. The Human Niche in Amazonia: Explorations in Ethnoprimatology. In New World Primates: Ecology, Evolution, and Behavior; Kinzey, W.G., Ed.; Aldine de Gruyter: New York, NY, USA, 1997. [Google Scholar]
- Malone, N.; Wade, A.H.; Fuentes, A.; Riley, E.P.; Remis, M.; Robinson, C.J. Ethnoprimatology: Critical interdisciplinarity and multispecies approaches in anthropology. Crit. Anthropol. 2014, 34, 8–29. [Google Scholar] [CrossRef]
- Fuentes, A.; Hockings, K.J. The ethnoprimatological approach in primatology. Am. J. Primatol. 2010, 72, 841–847. [Google Scholar] [CrossRef]
- Hofner, A.N.; Jost Robinson, C.A.; Nekaris, K.A. Preserving Preuss’s Red Colobus (Piliocolobus preussi): An Ethnographic Analysis of Hunting, Conservation, and Changing Perceptions of Primates in Ikenge-Bakoko, Cameroon. Int. J. Primatol. 2018, 39, 895–917. [Google Scholar] [CrossRef]
- Riley, E. The maturation of ethnoprimatology: Theoretical and methodological pluralism. Int. J. Primatol. 2018, 39, 705–729. [Google Scholar] [CrossRef]
- Riley, E. The human–macaque interface: Conservation implications of current and future overlap and conflict in Lore Lindu National Park, Sulawesi, Indonesia. Am. Anthropol. 2007, 109, 473–484. [Google Scholar] [CrossRef]
- Hill, C. Perspectives of ‘conflict’ at the wildlife-agriculture boundary: 10 years on. Hum. Dimens. Wildl. 2015, 20, 296–301. [Google Scholar] [CrossRef]
- Redpath, S.; Young, J.; Evely, A.; Adams, W.; Sutherland, W.; Whitehouse, A.; Amar, A.; Lambert, R.; Linnell, J.; Watt, A.; et al. Understanding and managing conservation conflicts. Trends Ecol. Evol. 2013, 28, 100–109. [Google Scholar] [CrossRef] [PubMed]
- McLennan, M.R.; Spagnoletti, N.; Hockings, K.J. The Implications of Primate Behavioral Flexibility for Sustainable Human–Primate Coexistence in Anthropogenic Habitats. Int. J. Primatol. 2017, 38, 105–121. [Google Scholar] [CrossRef]
- Parathian, H.E.; McLennan, M.R.; Hill, C.M.; Frazão-Moreira, A.; Hockings, K.J. Breaking Through Disciplinary Barriers: Human-Wildlife Interactions and Multispecies Ethnography. Int. J. Primatol. 2018, 39, 749–775. [Google Scholar] [CrossRef]
- Dore, K.M.; Riley, E.; Fuentes, A. Ethnoprimatology: A Practical Guide to Research at the Human–Primate Interface; Cambridge University Press: New York, NY, USA, 2017. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. 2023. Available online: https://www.iucnredlist.org (accessed on 13 February 2023).
- Estrada, A.; Garber, P.A.; Chaudhary, A. Current and future trends in socio-economic, demographic and governance factors affecting global primate conservation. PeerJ 2020, 8, e9816. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A. Socioeconomic Contexts of Primate Conservation: Population, Poverty, Global Economic Demands, and Sustainable Land Use. Am. J. Primatol. 2012, 75, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Pauchard, A.; Barbosa, O. Regional Assessment of Latin America: Rapid Urban Development and Social Economic Inequity Threaten Biodiversity Hotspots. In Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities; Elmqvist, T., Fragkias, M., Goodness, J., Guneralp, B., Marcotullio, P.J., McDonald, R.I., Parnell, S., Schewenius, M., Sendstad, M., Seto, K.C., et al., Eds.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Instituto Nacional de Estadística y Censos (INDEC). Incidencia de la Pobreza y la Indigencia en 31 Aglomerados Urbanos. Available online: https://www.indec.gob.ar/uploads/informesdeprensa/eph_pobreza_09_2326FC0901C2.pdf (accessed on 16 October 2023).
- Ramsay, M.S.; Mercado Malabet, F.; Klass, K.; Ahmed, T.; Muzaffar, S. Consequences of Habitat Loss and Fragmentation for Primate Behavioral Ecology. In Primates in Anthropogenic Landscapes. Developments in Primatology: Progress and Prospects; McKinney, T., Waters, S., Rodrigues, M.A., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Chapman, C.; Peres, C.A. Primate conservation in the new millennium: The role of scientists. Evol. Anthropol. 2001, 10, 16–33. [Google Scholar] [CrossRef]
- Oates, J.F. Primate conservation: Unmet challenges and the role of the International Primatological Society. Int. J. Primatol. 2013, 34, 235–245. [Google Scholar] [CrossRef]
- Grossberg, R.; Treves, A.; Naughton-Treves, L. The incidental ecotourist: Measuring visitor impacts on endangered howler monkeys at a Belizian archaeological site. Environ. Conserv. 2003, 30, 40–51. [Google Scholar] [CrossRef]
- McCarthy, M.S.; Matheson, M.; Lester, J.; Sheeran, L.K.; Li, J.H.; Wagner, R.S. Sequences of Tibetan macaque (Macaca thibetana) and tourist behaviors and Mt. Huangshan, China. Primate Conserv. 2009, 24, 145–151. [Google Scholar] [CrossRef]
- Piquer-Rodríguez, M.; Torella, S.; Gavier-Pizarro, G.; Volante, J.; Somma, D.; Ginzburg, R.; Kuemmerle, T. Effects of past and future land conversions on forest connectivity in the Argentine Chaco. Landsc. Ecol. 2015, 30, 817–833. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- de la Sancha, N.U.; Boyle, S.A.; McIntyre, N.E.; Brooks, D.M.; Yanosky, A.; Cuellar Soto, E.; Mereles, F.; Camino, M.; Stevens, R.D. The disappearing Dry Chaco, one of the last dry forest systems on earth. Landsc. Ecol. 2021, 36, 2997–3012. [Google Scholar] [CrossRef]
- MAyDS. Monitoreo de los Bosques Nativos. Available online: https://www.argentina.gob.ar/ambiente/bosques/umsef (accessed on 20 October 2023).
- Bicca-Marques, J.C.; Chaves, O.M.; Hass, G.P. Howler monkey tolerance to habitat shrinking: Lifetime warranty or death sentence? Am. J. Primatol. 2020, 82, e23089. [Google Scholar] [CrossRef] [PubMed]
- UMSEF. Monitoreo de la Superficie de Bosque Nativo de la República Argentina: Regiones Forestales Parque Chaqueño, Yungas, Selva Paranaense y Espinal; Ministerio de Ambiente y Desarrollo Sustentable de la Nación Argentina: Buenos Aires, Argentina, 2017. [Google Scholar]
- Oliveira Grande, T.; Alencar, R.M.; Ribeiro, P.P.; Melo, F.R. Fragment shape and size, landscape permeability and fragmentation level as predictors of primate occupancy in a region of Brazilian Cerrado. Acta Sci. Biol. Sci. 2020, 42, e48339. [Google Scholar] [CrossRef]
- Benchimol, M.; Peres, C. Predicting primate local extinctions within “real-world” forest fragments: A pan-neotropical analysis. Am. J. Primatol. 2013, 76, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Cantarelli, V.I.; Perez-Rueda, M.A.; Kowalewski, M.M.; Mastromonaco, G.F.; Ponzio, M.F. Validation of an enzyme immunoassay and comparison of fecal cortisol metabolite levels in black and gold howler monkeys (Alouatta caraya) inhabiting fragmented and continuous areas of the humid Chaco region, Argentina. Am. J. Primatol. 2017, 79, e22625. [Google Scholar] [CrossRef]
- Chaves, Ó.M.; CÉSar Bicca-Marques, J. Dietary Flexibility of the Brown Howler Monkey Throughout Its Geographic Distribution. Am. J. Primatol. 2013, 75, 16–29. [Google Scholar] [CrossRef]
- Zunino, G.E.; Kowalewski, M.; Oklander, L.I.; Gonzalez, V. Habitat fragmentation and population trends of the black and gold howler monkey (Alouatta caraya) in a semideciduous forest in northern Argentina. Am. J. Primatol. 2007, 69, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Oklander, L.I.; Kowalewski, M.M.; Corach, D. Genetic Consequences of Habitat Fragmentation in Black-and-Gold Howler (Alouatta caraya) Populations from Northern Argentina. Int. J. Primatol. 2010, 31, 813–832. [Google Scholar] [CrossRef]
- Babb, P.L.; Fernandez-Duque, E.; Baiduc, C.; Gagneux, P.; Evans, S.; Schurr, T.G. mtDNA Diversity in Azara’s owl monkeys (Aotus azarai azarai) of the Argentinean Chaco. Am. J. Phys. Anthropol. 2011, 146, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Juárez, C.P. Demografía e Historia de Vida del Mono Mirikiná (Aotus a. azarai) en el Chaco Húmedo Formoseño. Ph.D. Thesis, Universidad Nacional de Tucumán, Tucumán, Argentina, 2012. [Google Scholar]
- Graesser, J.; Ramankutty, N.; Coomes, O.T. Increasing expansion of large-scale crop production onto deforested land in sub-Andean South America. Environ. Res. Lett. 2018, 13, 084021. [Google Scholar] [CrossRef]
- Di Bitetti, M.S. Home-range use by tufted capuchin monkeys (Cebus apella nigritus) in a subtropical rainforest of Argentina. J. Zool. 2001, 253, 33–45. [Google Scholar] [CrossRef]
- Agostini, I.; Pizzio, E.; De Angelo, C.; Di Bitetti, M.S. Population status of primates in the Atlantic Forest of Argentina. Int. J. Primatol. 2015, 36, 244–258. [Google Scholar] [CrossRef]
- Brown, A.D.; Malizia, L.R. Las Selvas Pedemontanas de las Yungas: En el umbral de la extinción. Cienc. Hoy 2004, 14, 62–63. [Google Scholar]
- Boletta, P.E.; Ravelo, A.C.; Planchuelo, A.M.; Grilli, M. Assessing deforestation in the Argentine Chaco. For. Ecol. Manag. 2006, 228, 108–114. [Google Scholar] [CrossRef]
- Irigoin, N. Estadísticas de Incendios Forestales Año 2017; Secretaría de Agroindustria: Buenos Aires, Argentina, 2018; 76p. [Google Scholar]
- Smichowski, H.; Montiel, M.d.R.; Romero, V.; Kowalewski, M.; Contreras, F.I. EvaluaciÓn De Incendios En Áreas Periurbanas De La Ciudad De Corrientes (Argentina) Durante El AÑo 2020. Papeles Geogr. 2022, 67, 151–167. [Google Scholar] [CrossRef]
- Kowalewski, M. Avaliação e mitigação dos incêndios em um sítio de estudo de longo prazo no norte da Argentina. Simposio: A ameaça de incêndios florestais para populações de primatas. In Proceedings of the XIX Congresso Brasileiro de Primatologia, Sinop, Brasil, 27–31 August 2022. [Google Scholar]
- Brown, A.; Grau, H.R.; Malizia, L.R.; Grau, A. Argentina. In Bosques Nublados del Neotrópico; Kappelle, M., Brown, A., Eds.; Instituto Nacional de Biodiversidad: San José, Costa Rica, 2001. [Google Scholar]
- Lizárraga, L. Caracterización Espacial y Temporal de la Situación de Incendios en las Provincias de Salta y Jujuy a Partir de Focos de Calor MODIS (2003–2013). Universidad Nacional de Salta: Salta, Argentina, 2015. [Google Scholar]
- Chaves, Ó.M.; Júnior, J.C.S.; Buss, G.; Hirano, Z.M.; Jardim, M.M.A.; Amaral, E.L.S.; Godoy, J.C.; Peruchi, A.R.; Michel, T.; Bicca-Marques, J.C. Wildlife is imperiled in peri-urban landscapes: Threats to arboreal mammals. Sci. Total Environ. 2022, 821, 152883. [Google Scholar] [CrossRef]
- Nowak, K.; Lee, P.C. “Specialist” primates can be flexible in response to habitat alteration. In Primates in Fragments: Developments in Primatology: Progress and Prospects; Marsh, L., Chapman, C., Eds.; Springer: New York, NY, USA, 2013; pp. 199–211. [Google Scholar]
- Sinha, A.; Vijayakrishnan, S. Primates in urban settings. In The International Encyclopedia of Primatology; Bezanson, M., MacKinnon, K.C., Riley, E., Campbell, C.J., Nekaris, A., Estrada, A., Di Fiore, A., Ross, S., Jones-Engel, L.E., Thierry, B., et al., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–8. [Google Scholar]
- Lokschin, L.; Rodrigo, C.; Hallal Cabral, J.; Buss, G. Power lines and howler monkey conservation in Porto Alegre, Rio Grande do Sul, Brazil. Neotrop. Primates 2007, 14, 76–80. [Google Scholar] [CrossRef]
- Praill, L.C.; Eppley, T.; Shanee, S.; Cunneyworth, P.M.K.; Abra, F.D.; Allgas, N.; Al-Razi, H.; Campera, M.; Cheyne, S.M.; Collinson, W.; et al. Road Infrastructure and Primate Conservation: Introducing the Global Primate Roadkill Database. Animals 2023, 13, 1692. [Google Scholar] [CrossRef]
- Raño, M.; Palazzo, M.C.; Soliz, A.; Holzer, J.C.; Perez, D.A.; Sanchaez, E.M.; Romero, V.; Sanchez Gavier, F.; Kowalewski, M. Community participatory action to build a canopy bridge for wild black and gold howler monkeys (Alouatta caraya) in northern Argentina. Folia Primatol. 2022, 93, 453–463. [Google Scholar] [CrossRef]
- Azofeifa-Rojas, R.; Sánchez-Porras, R.; Daniele, S. Mortalidad por electrocución de monos congo (Alouatta palliata) debido a líneas eléctricas en Guanacaste, Costa Rica. Mesoamericana 2021, 25, 15–21. [Google Scholar]
- Corrêa, F.; Chaves, O.; Cambará Printes, R.; Piccoli Romanowski, H. Surviving in the urban-forest interface: Feeding and ranging behavior of brown howlers (Alouatta guariba clamitans) in an urban fragment in southern Brazil. Am. J. Primatol. 2018, 80, e22865. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, C.; Maciel, P.C.; de Oliveira Garcia, F. Rope bridges provide safe connectivity for the southern brown howler monkey (Alouatta guariba clamitans Cabrera, 1940) in an urban Atlantic Forest remnant. Folia Primatol. 2022, 93, 519–532. [Google Scholar] [CrossRef]
- Alesci, M.; Smith, R.L.; Ayala Santacruz, J.D.; Camperio Ciani, A. Attitudes towards urban howler monkeys (Alouatta caraya) in Paraguay. Primates 2022, 63, 161–171. [Google Scholar] [CrossRef]
- Fernandez, J.; Ponzio, M.F.; Cantarelli, V.I.; Clennon, J.A.; Gennuso, M.S.; Raño, M.; Kowalewski, M. Comparative Stress Response of Black and Gold Howler Monkey (Alouatta caraya) in Urban and Rural Environments of Northern Argentina. Folia Primatol. 2021, 92, 227–234. [Google Scholar] [CrossRef]
- Citon, L.; Gilles, D.; Bay Jouliá, R.; Contreras, F.; Raño, M.; Natalini, M.; Kowalewski, M. Presencia y distribución de monos aulladores negros y dorados (Alouatta caraya) en áreas urbanasy periurbanas de la Ciudad de Corrientes Capital, Argentina. In Proceedings of the XXXIII Jornadas Argentinas de Mastozoología, Puerto Iguazú, Misiones, Argentina, 7–11 November 2022. [Google Scholar]
- Goymann, W. On the use of non-invasive hormone research in uncontrolled, natural environments: The problem with sex, diet, metabolic rate and the individual. Methods Ecol. Evol. 2012, 3, 757–765. [Google Scholar] [CrossRef]
- Corley, M.; Perea-Rodriguez, J.P.; Valeggia, C.; Fernandez-Duque, E. Associations between fecal cortisol and biparental care in a pair-living primate. Am. J. Phys. Anthropol. 2021, 176, 295–307. [Google Scholar] [CrossRef]
- Overbeck, V.; Gonzalez, P.; Lau, W.M.; DesJardins, E.; Alesci, M.; Wellian, J.; Kane, J.; Henderson, M.; Blood, R.; Smith, R. Activity budgets of black-&-gold howler monkeys living in urban and natural habitats in southwest paraguay. Neotrop. Primates 2022, 28, 1–9. [Google Scholar]
- Wellian, J.; Smith, R.L. Risk awareness of black-and-gold howler monkeys living in an urban environment in south-west Paraguay. J. Urban Ecol. 2021, 7, juab010. [Google Scholar] [CrossRef]
- Prates, H.; Hass, G.; Bicca-Marques, J.C. Ranging behavior of black-and-gold howler monkeys (Alouatta caraya) in an anthropogenic habitat patch in southern Brazil. In La Primatología en Latinoamérica 2—A Primatologia na America Latina 2. Tomo I Argentina-Colombia; Urbani, B., Kowaleski, M., Cunha, R., de la Torre, S., Cortes-Ortiz, L., Eds.; Ediciones IVIC; Instituto Venezolano de Investigaciones Científicas (IVIC): Caracas, Venezuela, 2018; pp. 259–266. [Google Scholar]
- Link, A.; Muñoz-Delgado, J.; Montilla, S. Patterns of nocturnal activity by night monkeys (Aotus spp.) in the tropics. In Owl Monkeys: Biology, Adaptive Radiation, and Behavioral Ecology of the Only Nocturnal Primate in the Americas; Fernandez-Duque, E., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Bustamante-Manrique, S.; Botero-Henao, N.; Castaño, J.H.; Link, A. Activity budget, home range and diet of the Colombian night monkey (Aotus lemurinus) in peri-urban forest fragments. Primates 2021, 62, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wookey, O.A. Human-Wildlife Coexistence in the Urban Domain: Promoting Welfare Through Effective Management, Responsibility and the Recognition of Mutual Interest. In Human/Animal Relationships in Transformation; Vitale, A., Pollo, S., Eds.; Palgrave Macmillan: Cham, Switzerland, 2022. [Google Scholar]
- Izquierdo, A.E.; Grau, H.R.; Aide, T.M. Implications of Rural–Urban Migration for Conservation of the Atlantic Forest and Urban Growth in Misiones, Argentina (1970–2030). Ambio 2011, 40, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Ignatieva, M.; Nilon, C.H.; Werner, P.; Zipperer, W.C. Patterns and Trends in Urban Biodiversity and Landscape Design. In Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities: A Global Assessment; Elmqvist, T., Fragkias, M., Goodness, J., Güneralp, B., Marcotullio, P.J., McDonald, R.I., Parnell, S., Schewenius, M., Sendstad, M., Seto, K.C., et al., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 123–174. [Google Scholar]
- Pereira, A.A.B.G.; Dias, B.; Castro, S.I.; Landi, M.F.A.; Melo, C.B.; Wilson, T.M.; Costa, G.R.T.; Passos, P.H.O.; Romano, A.P.; Szabó, M.P.J.; et al. Electrocutions in free-living black-tufted marmosets (Callithrix penicillata) in anthropogenic environments in the Federal District and surrounding areas, Brazil. Primates 2020, 61, 321–329. [Google Scholar] [CrossRef]
- Pozo-Montuy, G.; Bonilla-Sánchez, Y.M. Population decline of an endangered primate resulting from the impact of a road in the Catazajá wetlands, Chiapas, México. Therya Notes 2022, 3, 75–81. [Google Scholar] [CrossRef]
- Sánchez-Murillo, F.; Arguedas, R. Blood analytes of electrocuted mantled howler monkeys (Alouatta palliata) in the Nicoya peninsula of Costa Rica. J. Med. Primatol. 2021, 50, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Ham, S.; Oktaviani, R.; Dewi, M.C.; Nur, M.; Mardiastut, A.; Choe, J.C. Cases of fatal electrocuton of the endangered Javan Gibbons (Mammalia: Primates: Hylobatdae) by power lines. J. Threat. Taxa 2022, 14, 20964–20969. [Google Scholar] [CrossRef]
- Cunneyworth, P.M.K.; Slade, A.M. Impact of Electric Shock and Electrocution on Populations of Four Monkey Species in the Suburban Town of Diani, Kenya. Int. J. Primatol. 2021, 42, 171–186. [Google Scholar] [CrossRef]
- Schulze, C.; Peters, M.; Baumgärtner, W.; Wohlsein, P. Electrical injuries in animals: Causes, pathogenesis, and morphological findings. Vet. Pathol. 2016, 53, 1018–1029. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V. Seasonal electrocution fatalities in free-range rhesus macaques (Macaca mulatta) of Shivalik hills area in northern India. J. Med. Primatol. 2015, 44, 137–142. [Google Scholar] [CrossRef]
- Roscoe, C.J.; de Silva, M.; Hapuarachchi, N.; Krishantha, P. A new color morph of the southern purple-faced langur (Semnopithecus vetulus vetulus) from the rainforests of southwestern Sri Lanka. Primate Conserv. 2013, 26, 115–124. [Google Scholar] [CrossRef]
- Teixeira, F.; Printes, R.; Fagundes, J.; Alonso, A.; Kindel, A. Canopy bridges as road overpasses for wildlife in urban fragmented landscapes. Biota Neotrop. 2013, 13, 117–123. [Google Scholar] [CrossRef]
- Riley, C.; Koenig, B.; Gumert, M. Observations of a fatal dog attack on a juvenile long-tailed macaque in a human-modified environment in Singapore. Nat. Singap. 2015, 8, 57–63. [Google Scholar]
- Gregory, T.; Abra, F.; Linden, B.; Nekaris, K.; Soanes, K.; Teixeira, F.Z. A new window into canopy bridges as a mitigation strategy for arboreal mammals. Folia Primatol. 2022, 93, 197–203. [Google Scholar] [CrossRef]
- Azofeifa Rojas, I.; Gregory, T. Canopy bridges: Preventing and mitigating anthropogenic impacts on mantled howler monkeys (Alouatta palliata palliata) in Costa Rica. Folia Primatol. 2022, 93, 383–395. [Google Scholar] [CrossRef]
- Alamgir, M.; Campbell, M.J.; Sloan, S.; Goosem, M.; Clements, G.R.; Mahmoud, M.I.; Laurance, W.F. Economic, Socio-Political and Environmental Risks of Road Development in the Tropics. Curr. Biol. 2017, 27, R1130–R1140. [Google Scholar] [CrossRef]
- Gokula, V.; Lavanya, R. Impact of Road Traffic on Mysore Slender Loris Loris lydekkerianus lydekkerianus in Keelaveliyur, Tamil Nadu, India. Uttar Pradesh J. Zool. 2023, 25–34. [Google Scholar] [CrossRef]
- Pinto, F.A.S.; Cirino, D.W.; Cerqueira, R.C.; Rosa, C.; Freitas, S.R. How Many Mammals Are Killed on Brazilian Roads? Assessing Impacts and Conservation Implications. Diversity 2022, 14, 835. [Google Scholar] [CrossRef]
- Hill, C.M. Primate Crop Feeding Behavior, Crop Protection, and Conservation. Int. J. Primatol. 2017, 38, 385–400. [Google Scholar] [CrossRef]
- Bicca-Marques, J.C. Urbanization (and Primate Conservation). In The International Encyclopedia of Primatology; Bezanson, M., MacKinnon, K.C., Riley, E., Campbell, C.J., Nekaris, A., Estrada, A., Di Fiore, A., Ross, S., Jones-Engel, L.E., Thierry, B., et al., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–5. [Google Scholar]
- Di Bitetti, M.S. Primates bark-stripping trees in forest plantations—A review. For. Ecol. Manag. 2019, 449, 117482. [Google Scholar] [CrossRef]
- Chaves, Ó.M.; Bicca-Marques, J.C. Crop Feeding by Brown Howlers (Alouatta guariba clamitans) in Forest Fragments: The Conservation Value of Cultivated Species. Int. J. Primatol. 2016, 38, 263–281. [Google Scholar] [CrossRef]
- Freitas, C.H.; Setz, E.Z.F.; Araújo, A.R.B.; Gobbi, N. Agricultural crops in the diet of bearded capuchin monkeys, Cebus libidinosus spix (Primates: Cebidae), in forest fragments in southeast Brazil. Rev. Bras. De Zool. 2008, 25, 32–39. [Google Scholar] [CrossRef]
- Ludwig, G.; Aguiar, L.M.; Rocha, V.J. Comportamento de obtenção de Manihot esculenta Crantz (Euphorbiaceae), mandioca, por Cebus nigritus (Goldfuss, 1809) (Primates, Cebidae) como adaptação alimentar em períodos de escassez. Rev. Bras. Zool. 2006, 23, 888–890. [Google Scholar] [CrossRef]
- Smith, R.L.; Rebergen, K.; Payne, C.; Megapanos, E.; Lusseau, D. Dietary plasticity of a understudied primate (Sapajus cay) in a biodiversity hotspot: Applying ecological traits to habitat conservation in the Upper Paraná Atlantic Forest. Folia Primatol. 2022, 93, 53–68. [Google Scholar] [CrossRef]
- Morales, M.A.; Fabbri, C.M.; Zunino, G.E.; Kowalewski, M.M.; Luppo, V.C.; Enría, D.A.; Levis, S.C.; Calderón, G.E. Detection of the mosquito-borne flaviviruses, West Nile, Dengue, Saint Louis Encephalitis, Ilheus, Bussuquara, and Yellow Fever in free-ranging black howlers (Alouatta caraya) of Northeastern Argentina. PLOS Neglected Trop. Dis. 2017, 11, e0005351. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.; Salzer, J.S.; Deutsch, J.C.; Raño, M.; Kuhlenschmidt, M.S.; Gillespie, T.R. Black and gold howler monkeys (Alouatta caraya) as sentinels of ecosystem health: Patterns of zoonotic protozoa infection relative to degree of human-primate contact. Am. J. Primatol. 2011, 73, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, H.J.; Tajudeen, Y.A.; Sodiq Inaolaji, Y.; Oladunjoye, I.O.; Adebisi, Y.A.; Abdulkadir, M. Targeting the wrong enemies: Monkeys are not responsible for the current outbreak of monkeypox. Ann. Med. Surg. 2022, 81, 104520. [Google Scholar] [CrossRef]
- Bicca-Marques, J.C.; de Freitas, D.S. The role of monkeys, mosquitoes, and humans in the occurrence of a yellow fever outbreak in a fragmented landscape in south Brazil: Protecting howler monkeys is a matter of public health. Trop. Conserv. Sci. 2010, 3, 78–89. [Google Scholar] [CrossRef]
- Moreno, E.S.; Agostini, I.; Holzmann, I.; Di Bitetti, M.S.; Oklander, L.I.; Kowalewski, M.M.; Beldomenico, P.M.; Goenaga, S.; Martinez, M.; Lestani, E.; et al. Yellow fever impact on brown howler monkeys (Alouatta guariba clamitans) in Argentina: A metamodelling approach based on population viability analysis and epidemiological dynamics. Memórias Inst. Oswaldo Cruz 2015, 110, 865–876. [Google Scholar] [CrossRef]
- Oklander, L.I.; Mino, C.I.; Fernandez, G.; Caputo, M.; Corach, D. Genetic structure in the southernmost populations of black-and-gold howler monkeys (Alouatta caraya) and its conservation implications. PLoS ONE 2017, 12, e0185867. [Google Scholar] [CrossRef]
- Goenaga, S.; Fabbri, C.; Dueñas, J.C.R.; Gardenal, C.N.; Rossi, G.C.; Calderon, G.; Morales, M.A.; Garcia, J.B.; Enria, D.A.; Levis, S.; et al. Isolation of yellow fever virus from mosquitoes in Misiones Province. Argent. Vector-Borne Zoonotic Dis. 2012, 12, 986–993. [Google Scholar] [CrossRef]
- Holzmann, I.; Agostini, I.; Areta, J.I.; Ferreyra, H.; Beldomenico, P.; Di Bitetti, M.S. Impact of yellow fever outbreaks on two howler monkey species (Alouatta guariba clamitans and A. caraya) in Misiones, Argentina. Am. J. Primatol. 2010, 72, 475–480. [Google Scholar] [PubMed]
- Bicca Marques, J.C.; Calegaro Marques, C.; Rylands, A.; Strier, K.B.; Mittermeier, R.; De Almeida, M.A.; De Castro, P.H.; Chaves, O.M.; Ferraz, L.P.; Fortes, V.B.; et al. Yellow fever threatens Atlantic Forest primates. Sci. Adv. 2017, 1–3. [Google Scholar]
- Scheffer, M.; Paiva, V.S.F.; Barberia, L.G.; Russo, G. Monkeypox in Brazil between stigma, politics, and structural shortcomings: Have we not been here before? Lancet Reg. Health-Am. 2022, 17, 100394. [Google Scholar] [PubMed]
- Lee, P.; Priston, N. Human attitudes to primates: Perceptions of pests, conflict and consequences for primate conservation. In Commensalism and Conflict: The human-Primate Interface; Paterson, J., Wallis, J., Eds.; American Society of Primatologists: Norman, OK, USA, 2005. [Google Scholar]
- Sengupta, A.; McConkey, K.; Radhakrishna, S. Primates, Provisioning and Plants: Impacts of Human Cultural Behaviours on Primate Ecological Functions. PLoS ONE 2015, 10, e0140961. [Google Scholar] [CrossRef]
- Brennan, E.; Else, J.G.; Altmann, J. Ecology and behaviour of a pest primate: Vervet monkeys in a tourist-lodge habitat. Afr. J. Ecol. 1985, 23, 35–44. [Google Scholar] [CrossRef]
- Sengupta, A.; Radhakrishna, S. Factors Predicting Provisioning of Macaques by Humans at Tourist Sites. Int. J. Primatol. 2020, 41, 471–485. [Google Scholar] [CrossRef]
- Maréchal, L.; Semple, S.; Majolo, B.; MacLarnon, A. Assessing the effects of tourist provisioning on the health of wild Barbary macaques in Morocco. PLoS ONE 2016, 11, e0155920. [Google Scholar] [CrossRef] [PubMed]
- Westin, J. Habituation to tourists: Protective or harmful? In Ethnoprimatology: A Practical Guide to Research at the Human-Nonhuman Primate Interface; Dore, K.M., Riley, E., Fuentes, A., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 15–28. [Google Scholar]
- Tujague, M.P.; Casco, M.P. ¿Por qué los monos hacen monadas? El conflicto entre humanos y primates no humanos en áreas protegidas. In Proceedings of the Problemáticas de la Interfase Primates no Humanos y Humanos: Convivir en un Mundo Globalizado. XXVII Reunión Argentina de Ecologia y XXLII Reunión de la Sociedad de Ecología de Chile, Puerto Iguazú, Argentina, 18–22 September 2016. [Google Scholar]
- Martins, W.P.; Izar, P.; Araujo, W.S.; Rodrigues, F.H.; Lynch, J.W. Diet, activity patterns, and home range use in forest and cultivated areas for one wild group of endangered crested capuchin monkeys (Sapajus robustus) in Reserva Natural Vale, Espírito Santo, Brazil. Am. J. Primatol. 2022, 84, e23413. [Google Scholar] [CrossRef]
- Tiddi, B.; Pfoh, R.; Agostini, I. The impact of food provisioning on parasite infection in wild black capuchin monkeys: A network approach. Primates 2019, 60, 297–306. [Google Scholar] [CrossRef]
- Torge, I. Evaluación del Efecto del Turismo en Comportamientos Indicadores de Ansiedad en un Grupo de Monos Caí Silvestres (Sapajus nigritus) del Parque Nacional Iguazú; Universidad Nacional de Cordoba: Cordoba, Argentina, 2022. [Google Scholar]
- Wolovich, C.K.; Shanee, S.; Maldonado, A.M.; Méndez-Carvajal, P.G.; Perea-Rodriguez, J.P.; Tabares, S.; Garcia de la Chica, A.; Evans, S. A call-to-action to assist in efforts to protect owl monkeys (Aotus spp.). Am. J. Primatol. 2023, e23501. [Google Scholar] [CrossRef]
- Shanee, S. Threats and Conservation of Owl Monkeys (Aotus spp.) in the Andes. In Owl Monkeys: Biology, Adaptive Radiation, and Behavioral Ecology of the Only Nocturnal Primate in the Americas; Fernandez-Duque, E., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 649–671. [Google Scholar]
- Maldonado, A.M.; Soto-Calderón, I.D.; Hinek, A.; Moreno-Sierra, A.M.; Lafon, T.; Londoño, D.; Peralta-Aguilar, A.; Inga-Díaz, G.; Sánchez, N.; Mendoza, P. Conservation Status of the Nancy Ma’s Owl Monkey (Aotus nancymaae, Hershkovitz, 1983) on the Colombian-Peruvian Amazon Border. In Owl Monkeys: Biology, Adaptive Radiation, and Behavioral Ecology of the Only Nocturnal Primate in the Americas; Fernandez-Duque, E., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 623–647. [Google Scholar]
- Maldonado, A.M.; Waters, S. Primate Trade (Neotropics). In The International Encyclopedia of Primatology; Bezanson, M., MacKinnon, K.C., Riley, E., Campbell, C.J., Nekaris, A., Estrada, A., Di Fiore, A., Ross, S., Jones-Engel, L.E., Thierry, B., et al., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–7. [Google Scholar]
- Bertonatti, C. El comercio de primates en la República Argentina. Neotrop. Primates 1995, 3, 35–37. [Google Scholar]
- Urbani, B. The Ethnoprimatology of Owl Monkeys (Aotus spp.): From Past to Present. In Owl Monkeys: Biology, Adaptive Radiation, and Behavioral Ecology of the Only Nocturnal Primate in the Americas; Fernandez-Duque, E., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 155–169. [Google Scholar]
- Kowalewski, M.M.; Raño, M. Sanctuaries, Mesoamerica/South America. In The International Encyclopedia of Primatology; Bezanson, M., MacKinnon, K.C., Riley, E., Campbell, C.J., Nekaris, A., Estrada, A., Di Fiore, A., Ross, S., Jones-Engel, L.E., Thierry, B., et al., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–3. [Google Scholar]
- Ferreira, R.G.; Ruiz-Miranda, C.; Sita, S.; Sánchez-López, S.; Pissinatti, A.; Corte, S.; Jerusalinsky, L.; Wagner, P.G.; Maas, C. Primates Under Human Care in Developing Countries: Examples From Latin America. In Nonhuman Primate Welfare; Robinson, L.M., Weiss, A., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- King, T.; Chamberlan, C.; Courage, A. Assessing initial reintroduction success in long-lived primates by quantifying survival, reproduction, and dispersal parameters: Western Lowland Gorillas (Gorilla gorilla gorilla) in Congo and Gabon. Int. J. Primatol. 2012, 33, 134–149. [Google Scholar] [CrossRef]
- Oklander, L.I.; Caputo, M.; Solari, A.; Corach, D. Genetic assignment of illegally trafficked neotropical primates and implications for reintroduction programs. Sci. Rep. 2020, 10, 3676. [Google Scholar] [CrossRef] [PubMed]
- Oklander, L.I.; Caputo, M.; Kowalewski, M.; Anfuso, J.; Corach, D. Use of genetic tools to assess predation on reintroduced howler monkeys (Alouatta caraya) in Northeastern Argentina. Primates 2021, 62, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.O.; Cheyne, S.M.; Rawson, B.M. Best Practice Guidelines for the Rehabilitation and Translocation of Gibbons; IUCN Species Survival Commision: Gland, Switzerland, 2015; Volume 51. [Google Scholar]
- Tujague, M.P.; Agostini, I.; Oklander, L.I.; Peker, S.M.; Pfoh, R.; Baldovino, C.; Nieves, M.; Apellaniz, M. Sapajus nigritus. Categorización 2019 de los Mamíferos de Argentina Según su Riesgo de Extinción. Lista Roja de los Mamíferos de Argentina. Versión Digital. 2019. Available online: http://cma.sarem.org.ar (accessed on 20 October 2023).
- Agostini, I.; Velazco, S.J.E.; Insaurralde, J.A.; Pavé, R.; Holzmann, I.; Fernández-Duque, E.; Tujague, M.P.; Peker, S.; Kowalewski, M.M.; Di Bitetti, M.S. Prioritizing Areas for Primate Conservation in Argentina. Diversity 2022, 14, 982. [Google Scholar] [CrossRef]
- Buss, G.; Oklander, L.I.; Bicca-Marques, J.C.; Hirano, Z.L.; Chaves, O.M.; Mendes, S.L.; Neves, L.G.; Melo, F.R.; Rylands, A.B.; Jerusalinsky, L. Brown howler monkey, Alouatta guariba Humboldt, 1812. In Primates in Peril: The World’s 25 Most Endangered Primates 2018–2020; Schwitzer, C., Mittermeier, R.A., Rylands, A.B., Chiozza, F., Williamson, E.A., Byler, D., Wich, S.A., Humle, T., Johnson, C., Mynott, H., et al., Eds.; IUCN SSC Primate Specialist Group, International Primatological Society, Global Wildlife Conservation, and Bristol Zoological Society: Washington, DC, USA, 2019; pp. 94–97. [Google Scholar]
- Oklander, L.I.; Peker, S.; Kowalewski, M.; Jerusalinsky, L.; Rocha, F.; Cordero, E. Evaluación del manejo poblacional para la conservación del mono aullador rojo (Alouatta guariba) en la provincia de Misiones, Argentina. In Proceedings of the VII Congreso Nacional de Conservación de la Biodiversidad, Puerto Iguazú, Argentina, 25–28 April 2023. [Google Scholar]
- Arroyo-Rodríguez, V.; Andresen, E.; Bravo, S.P.; Stevenson, P.R. Seed Dispersal by Howler Monkeys: Current Knowledge, Conservation Implications, and Future Directions. In Howler Monkeys: Behavior, Ecology, and Conservation; Kowalewski, M.M., Garber, P.A., Cortés-Ortiz, L., Urbani, B., Youlatos, D., Eds.; Springer: New York, NY, USA, 2015; pp. 111–139. [Google Scholar]
- Radhakrishna, S. Culture, conflict and conservation: Living with nonhuman primates in northeastern India. In Ethnoprimatology: A Practical Guide to Research at the Human-Nonhuman Primate Interface; Dore, K.M., Riley, E., Fuentes, A., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 271–283. [Google Scholar]
- Ley 1.582/2012, de 14 de Junio, Camára de Diputados de la Provincia de Formosa, Argentina. Available online: https://www.legislaturaformosa.gov.ar/informes.php?f=1576 (accessed on 20 October 2023).
- Svensson, M.S.; Shanee, S.; Shanee, N.; Bannister, F.B.; Cervera, L.; Donati, G.; Huck, M.; Jerusalinsky, L.; Juarez, C.P.; Maldonado, A.M.; et al. Disappearing in the Night: An Overview on Trade and Legislation of Night Monkeys in South and Central America. Folia Primatol. 2016, 87, 332–348. [Google Scholar] [CrossRef]
- Juárez, C.P.; Huck, M.; Fernandez-Duque, E. Azara’s owl monkey in the Humid Chaco: Primatological long-term studies in Argentina. Los monos mirikiná del Chaco Húmedo: 20 años de primatología en Argentina; In Primatology in Argentina; Kowalewski, M., Oklander, L., Eds.; Sociedad Argentina para el Estudio de los Mamíferos Series A. Mammalogical Research; SAREM: Buenos Aires, Argentina, 2017; Volume 2, pp. 255–272. [Google Scholar]
- Ley 6.590/2021, de 9 de Diciembre, Cámara de Diputados de la Provincia de Corrientes, Argentina. Available online: https://hcdcorrientes.gov.ar/wp-content/uploads/2021/11/Ley-6590-1.pdf (accessed on 20 October 2023).
- Ley XVI Nº154/2022, de 23 de Septiembre, Cámara de Representantes de la Provincia de Misiones, Argentina. Available online: https://www.boletindigital.misiones.gov.ar/boletines/15721.pdf (accessed on 20 October 2023).
- Monroe, M.; Plate, R.; Oxarart, A.; Bowers, A.; Chaves, W. Identifying effective climate change education strategies: A systematic review of the research. Environ. Educ. Res. 2017, 25, 791–812. [Google Scholar] [CrossRef]
- Ardoin, N.; Bowers, A.; Gaillard, E. Environmental education outcomes for conservation: A systematic review. Biol. Conserv. 2020, 241, 108224. [Google Scholar] [CrossRef]
- Abondano, L.A.; Webber, A.D.; Valencia, L.M.; Gómez-Posada, C.; Hending, D.; Cortes, F.A.; Fuentes, N. Community-Based Strategies to Promote Primate Conservation in Agricultural Landscapes: Lessons Learned from Case Studies in South America. In Primates in Anthropogenic Landscapes: Exploring Primate Behavioural Flexibility across Human Contexts; McKinney, T., Waters, S., Rodrigues, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 103–120. [Google Scholar]
- Rosales-Meda, M.; Hermes, M.S. Representation and Signification of Primates in Maya-Q’eqchi’ Cosmovision and Implications for Their Conservation in Northwestern Guatemala. In Neotropical Ethnoprimatology: Indigenous Peoples’ Perceptions of and Interactions with Nonhuman Primates; Urbani, B., Lizarralde, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 69–85. [Google Scholar]
- Bezanson, M.; Franquesa-Soler, M.; Kowalewski, M.; McNamara, A.; Oktaviani, R.; Rodrigues, M.A. Best practices are never best: Evaluating primate conservation education programs (PCEPs) with a decolonial perspective. Am. J. Primatol. 2023, 85, e23424. [Google Scholar] [CrossRef]
- Kowalewski, M.; Natalini, B.; Gennuso, M.; Fernández, J.; Agrelo, E.; González, J.; Díaz, J.; Kraemer, A.; Gualini, I.; Macarrein, T.; et al. Guardianes del carayá: Ciencia y educación como herramientas para la conservación de la biodiversidad. In Proceedings of the II Jornada Paraguaya de Mastozoología, II Jornada Paraguaya de Herpetología, Asunción, Paraguay, 29 November–1 December 2017. [Google Scholar]
- Romero, V.; Raño, M.; Natalini, M.; Godoy, A.; Quijano, F.; Sánchez, M.; Bay Joulia, R.; Pucheta, D.; Gilles, D.; Romero, B.; et al. Centinelas: Un proyecto de integración y acción ciudadana. Extensionismo Innov. Transf. Tecnol. Claves Desarro 2021, 7, 31–39. [Google Scholar] [CrossRef]
- Raño, M.; VL, R.; Kowalewski, M.; Natalini, M.; Godoy, A.; Quijano, F.; Sánchez, M.; Bay Jouliá, R.; Pucheta, D.; Alegre, R.; et al. Parabiólogos en acción: Redes de integración y ciencia ciudadana para la conservación de mamíferos silvestres en entornos urbanos y periurbanos de Corrientes. In Proceedings of the Jornada Científica y de Educación en Ciencias Biológicas, Corrientes, Argentina; 2019. [Google Scholar]
- Geffner, L.; Silvana, P.; Oklander, L.I.; Agostini, I.; Casas, N.; Kowalewski, M. Vigilancia de epizootias en primates no humanos por fiebre amarilla en Argentina: Trabajo multidisciplinario e intersectorial. Simposio: “UNA SALUD” y su enfoque integrador en el campo de la Zoología descriptiva. In In Proceedings of the I Congreso Paraguayo de Zoología, Asunción, Paraguay, 25–29 November 2019. [Google Scholar]
- Ramon, M.; McLennan, M.R.; Ruiz-Miranda, C.R.; Kalema-Zikusoka, G.; Bessa, J.; Bersacola, E.; Sanhá, A.; Jaló, M.; Barros, A.R.d.; Leendertz, F.H.; et al. Infectious Diseases in Primates in Human-Impacted Landscapes. In Primates in Anthropogenic Landscapes: Exploring Primate Behavioural Flexibility across Human Contexts; McKinney, T., Waters, S., Rodrigues, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 139–160. [Google Scholar]
- Ley 27.621/2021, de 3 de Junio, El Senado y Cámara de Diputados de la Nación Argentina. Available online: https://www.argentina.gob.ar/normativa/nacional/ley-27621-350594/texto (accessed on 20 October 2023).
- Corley, M.; Fernadez-Duque, E. Dispersal: A critical life-history stage influencing populations, social dynamics, and individual fitness. In Owl Monkeys: Biology, Adaptive Radiation, and Behavioral Ecology of the Only Nocturnal Primate in the Americas; Fernadez-Duque, E., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Garcia de la Chica, A.; Spence-Aizenberg, A.; Wolovich, C.; Evans, C.S.; Fernadez-Duque, E. The Social Life of Owl Monkeys. In Owl Monkeys: Biology, Adaptive Radiation, and Behavioral Ecology of the Only Nocturnal Primate in the Americas; Fernadez-Duque, E., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Huck, M.; Fernandez-Duque, E. The great unknown–The floating stage as a neglected aspect of social systems. In Owl Monkeys: Evolution, Behavioral Ecology and Conservation; Fernandez-Duque, E., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Fernandez-Duque, E.; Juarez, C.P.; Buss, G.; Jerusalinsky, L.; Defler, T.R. Distribution, systematics and taxonomy In Owl Monkeys: Evolution, Behavioral Ecology and Conservation; Fernandez-Duque, E., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- van der Heide, G.; Davalos, V.; Fernandez-Duque, E. Flexibility in the diet and feeding ecology of nocturnal and cathemeral Aotus. In Owl Monkeys: Biology, Adaptive Radiation, and Behavioral Ecology of the Only Nocturnal Primate in the Americas; Fernadez-Duque, E., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Fernandez-Duque, E.; Wallace, R.; Rylands, A. Aotus azarae. The IUCN Red List of Threatened Species 2008; IUCN: Gland, Switzerland, 2008; e.T41539A10494975; Available online: https://www.iucnredlist.org/species/41539/10494975 (accessed on 23 October 2023).
- Briggs, E.; Rotundo, M.; Dávalos, V.; García de la Chica, A.; Fernández-Duque, E. Aotus azarae. Categorización 2019 de los Mamíferos de Argentina Según su Riesgo de Extinción. 2019. Available online: http://cma.sarem.org.ar (accessed on 20 October 2023).
- Zunino, G.E.; Kowalewski, M.M. Primate Research and Conservation in Northern Argentina: The Field Station Corrientes (Estación Biológica de Usos Múltiples–EBCo). Trop. Conserv. Sci. 2008, 1, 140–150. [Google Scholar] [CrossRef]






| Species | Habitat/Ecoregion | IUCN Categorization (2023) | SAREM Categorization (2019) | Conservation Threats in Argentina |
|---|---|---|---|---|
| Alouatta caraya | Dry and Humid Chaco Forests and Paranaense Rainforest | Near Threatened | Vulnerable | Habitat Loss and Degradation, Diseases, Illegal pet trade |
| Alouatta guariba clamitans | Paranaense Rainforest | Vulnerable | Critically Endangered | Habitat Loss and Degradation, Diseases, Roadkills |
| Aotus azarae | Dry and Humid Chaco Forests | Least Concern | Vulnerable | Habitat Loss and Degradation, Illegal pet trade |
| Sapajus nigritus cucullatus | Paranaense Rainforest | Near Threatened | Vulnerable | Habitat Loss and Degradation, Illegal pet trade, Tourism |
| Sapajus cay | Yungas | Vulnerable | Vulnerable | Habitat Loss and Degradation, Illegal pet trade |
| Drivers | Context of Conflicts | Approaches to Mitigate Conflict |
|---|---|---|
| Habitat loss | (A) Anthropogenically induced Fires | Protected areas |
| (B) Urbanized monkeys | Natural Provincial Monuments, Educational Programs | |
| (C) Electrocutions | Canopy bridges | |
| (D) Roadkills | Canopy bridges | |
| (E) Crop foraging | Protected areas, Educational Programs | |
| Human misconceptions | (A) Infectious diseases | Protected areas, Educational Programs |
| (B) Food provisioning | Educational Programs | |
| (C) Reintroductions and relocations | Protected Areas, Research | |
| (D) Illegal Pet trade | Natural Provincial Monuments, Educational Programs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García de la Chica, A.; Oklander, L.I.; Kowalewski, M.M.; Fernandez-Duque, E. Human and Non-Human Primate Coexistence in Argentina: Conflicts and Solutions. Animals 2023, 13, 3331. https://doi.org/10.3390/ani13213331
García de la Chica A, Oklander LI, Kowalewski MM, Fernandez-Duque E. Human and Non-Human Primate Coexistence in Argentina: Conflicts and Solutions. Animals. 2023; 13(21):3331. https://doi.org/10.3390/ani13213331
Chicago/Turabian StyleGarcía de la Chica, Alba, Luciana I. Oklander, Martin M. Kowalewski, and Eduardo Fernandez-Duque. 2023. "Human and Non-Human Primate Coexistence in Argentina: Conflicts and Solutions" Animals 13, no. 21: 3331. https://doi.org/10.3390/ani13213331





