Simple Summary
Protein in the diet is essential for the growth and health of ruminants and other animal species. Protein is also one of the most expensive components in animals’ diets, and finding good protein sources can sometimes be challenging. Urea supplementation, as a non-protein nitrogen source, is often recommended for goats fed low-quality forages. In the rumen, microorganisms transform urea into microbial protein that can be assimilated by the animal. In this study, we investigated nitrogen utilization in goats fed low-quality hay supplemented with molasses blocks containing various levels of urea. The findings and discussion in this paper contribute to a better understanding of nitrogen utilization in goats using urea as a non-protein nitrogen source.
Abstract
The use of goats for meat production faces challenges from environmental and nutritional factors. Urea is an affordable non-protein nitrogen source commonly utilized in ruminant nutrition. The objective of this study was to investigate nitrogen utilization in goats fed low-quality hay supplemented with molasses blocks containing urea. Twenty Anglo-Nubian doelings were individually housed in metabolic cages and provided with chopped Buffelgrass (Cenchrus ciliaris) hay ad libitum. Goats were randomly assigned to four urea levels (0, 2, 4, and 6%; n = 5 per treatment) in molasses blocks for a duration of 30 days. A negative nitrogen balance (−2.458 g/day) was observed in doelings consuming blocks without urea, compared with a positive balance (0.895 g/d) for those consuming the 6% urea blocks. Block nitrogen intake significantly increased with urea level, but urea supplementation did not affect dry matter (DM) or neutral detergent fiber (NDFom) intake or digestibility. A minimum crude protein (CP) requirement of 8% for maintenance in doelings consuming low-quality forage with a urea-based supplement was determined through regression analysis between CP intake (% of DM) and N balance (r2 = 0.479; p < 0.002). The value of 8% of CP obtained in this study is similar to several previous studies reported in the literature, but in this case, the increments in CP came exclusively from urea. In this study, increasing the urea content of molasses blocks up to 6% significantly increased nitrogen intake, retention, and balance in goats. These results contribute to a better understanding of nitrogen utilization in goats fed low-quality hay with urea supplementation.
1. Introduction
Goats (Capra aegagrus hircus) are small ruminants capable of producing meat, milk, and other valuable products (cashmere and mohair) for humans. Most breeds are characterized by their ability to thrive in environmentally adverse conditions [1]. The global goat population exceeds one billion and continues to grow, particularly in Asia and Africa [2]. In some countries, especially those in semiarid regions, goats play a significant role in local economies and the livelihoods of smallholders [3]. However, the potential of goats to grow, reproduce, and yield useful products is challenged by environmental factors (e.g., weather) and nutritional factors (e.g., diet composition) [4].
In certain regions, goats are primarily fed with locally available grass and crop residues, but these feeds often fall short of providing the necessary energy, protein, minerals, and vitamins. Forage availability and quality are limiting factors. This is a concern because undernourished animals cannot fully realize their full potential to maintain health, reproduce, and simultaneously produce meat and milk [5]. Nutrient supplementation for animals consuming low-quality forages can have a positive impact, but it should be specific and take into consideration the protein and mineral concentrations of forages in different regions [1,6].
Dietary crude protein and nitrogen supply to the animal are vital for maintaining health, as amino acids and nitrogen are involved in the production of essential molecules such as antibodies, enzymes, and neurotransmitters [7]. However, nitrogen metabolism is not well understood because it involves different pathways for absorption and excretion, depending on the type and quantity of the dietary protein, as well as the protein synthesis rates in different organs [8,9]. Urea is a common ingredient in ruminant nutrition as a non-protein nitrogen source and has been used for over a century [10]. Urea is metabolized by ruminal microbes, which in turn produce protein that the host can assimilate [11,12]. Molasses blocks are an intriguing alternative for supplying urea as non-protein nitrogen source because the efficacy of urea microbial degradation depends on the presence of fermentable carbohydrates [13,14]. Additional advantages of molasses-urea blocks include easier transportation and more consistent consumption among animals.
Buffelgrass (Cenchrus ciliaris) is the predominant warm season perennial grass in northeastern Mexico and is also common in other semi-arid regions worldwide. It is highly productive and tolerant to the periodic droughts that occur in these regions [15]. The objective of this study was to investigate the effect of supplementing molasses blocks with various levels of urea on nitrogen utilization in Anglo-Nubian female doelings fed Buffelgrass hay.
2. Materials and Methods
2.1. Animals, Supplements, and Feeding Management
This study was approved by the Joint Graduate Program of the Faculties of Agronomy and Veterinary Science of the University of Nuevo León and registered under the code 36397-001290684 in the masters’ exam certificate and code 4768 in the digital collection of the masters’ degree thesis. Twenty crossbred Anglo-Nubian doelings, aged 6 to 8 months, with average body weights of 18.2 kg, were randomly assigned to one of the four treatments in a completely randomized-designed experiment. The treatments consisted of molasses blocks containing incremental urea levels of 0, 2, 4, and 6%, which were supplemented to doelings fed Buffelgrass hay ad libitum. These levels of urea were selected to provide enough protein for ruminal activity without the risk of intoxication [16].
The animals were housed in individual metabolic cages (0.9 m × 1.2 m) equipped with water and feed troughs. Daily water intake of doelings was measured. The study spanned 30 days, with the first 21 days dedicated to adapting the doelings to metabolic cages and Buffelgrass hay, as well as block consumption. The subsequent 9 days were allocated for data collection, including body weights, feed intake, and feces and urine excretion.
The ingredients and chemical composition of the block supplements are presented in Table 1. These block supplements were manufactured using a mechanical block press, modified with a hydraulic jack [17]. The ingredients used in the formulation of the supplements included soybean hulls, cracked corn, urea, salt, calcium oxide, and a mineral premix with vitamin A. The four treatment block supplements contained increasing levels of urea, replacing cracked corn.

Table 1.
Ingredient and nutrient composition of molasses blocks with various urea levels.
Animals were provided with ad libitum access to chopped Buffelgrass (Cenchrus ciliaris) and their respective molasses block. The chemical composition of Buffelgrass was as follows: crude protein, 6.47%; crude fat, 0.94%; NDFom, 69.2%; ADF, 48.1%; ADL, 7.79%; and ash, 9.36%. Block intake was calculated as the difference between daily morning block weights. Total hay was offered in two portions during the day (09:00 and 16:00 h). Rejected Buffelgrass hay was weighted and recorded in the morning. At the end of the experimental period, time dedicated to eating, ruminating, or engaging in other activities was recorded every 5 min over a 24 h period [18].
2.2. Feed Sample Collection and Analysis
Offered and rejected hay samples were frozen for further analysis. Hay and ort samples were dried in an air-draft oven at 55 °C and ground through a 1 mm screen in a Wiley Mill before analysis. Dry matter content was determined at 105 °C [19]. Ash content was determined after sample combustion in a muffle furnace at 600 °C for 3 h, and ether extract was determined using the Ankom Technology XT10 Extractor. Nitrogen content of feed was determined using the micro-Kjeldahl procedure [19]. Crude protein (CP) was calculated as N × 6.25. Neutral detergent fiber (NDF) analysis was determined using the Ankom Technology model A200 fiber analyzer with filtration bags [20], and ash-free NDF (NDFom) was calculated [21]. Metabolizable energy was calculated using values reported for ingredients by the National Research Council [22]. Non-fibrous carbohydrates (NFC) content of the rations were estimated using the formula:
NFC (%) = DM − (CP + EE + ash + NDFom).
2.3. Feces and Urine Collection and Analysis
Daily samples of total fresh feces were collected from each doelings and then frozen. The nine fecal samples of each doeling were further thawed and mixed into a composite sample. Composite fecal samples were dried in an air-draft oven at 55 °C and ground through a 1 mm screen in a Wiley Mill. Urine was collected and weighed daily, and a daily 10% sample was accumulated in plastic containers for 9 days and immediately frozen at a temperature below −20 °C to prevent N loss. Urine was thawed and filtered through a fiber glass layer. Nitrogen in feces and urine was determined using the micro-Kjeldahl method [19] and used to calculate nitrogen balance and nitrogen retention:
N balance (g/d) = N consumed − (N in feces + N in urine)
Retained N (%) = (N balance/N intake) (100)
Retained N (%) = (N balance/N intake) (100)
2.4. Blood Analysis
Blood samples were obtained from each doeling at the end of the experiment (day 30). All samples were thawed for 30 min at ambient temperature and centrifuged at 1000× g for 15 min. Serum was separated and frozen at −72 °C until analysis to determine plasma urea nitrogen concentrations. Blood urea was determined using the Berthelot colorimetric method (Idexx Laboratories Inc., Westbrook, ME, USA).
2.5. Rumen Fluid Collection and pH Analysis
To obtain rumen fluid samples from the doelings, an esophageal probe was inserted orally. The rumen fluid was collected in 50 mL conical tubes, and pH was immediately measured using a Beckman pH meter.
2.6. Statistical Analysis
All data were analyzed using an analysis of variance for a completely randomized design using Statistics 9 Analytical Software (Tallahassee, FL, United States). The model included treatments, and all possible interactions, with the animal as experimental unit. Animal and the error term were considered random in the model. The statistical model used was as follows:
where yij is the response variable for the ith treatment (where i = 1, 2, 3, 4) and the jth observation treatment group (where j = 1, 2, …, ni); µ is the overall population mean (the average response across all treatments); Ti is the effect of the ith treatment level (incremental urea level), which represents the difference between the mean response for treatment i and the overall population mean. The treatments (urea levels) are fixed effect which represent the main factor being study.
yij = µ + Ti + µij
All variables were analyzed for lineal and quadratic responses to urea levels using orthogonal contrasts. The Tukey multiple comparison test was used to determine differences among means. Mean p-values were considered statistically significant at p < 0.05. The initial weight of the does was considered as a covariate. Correlations coefficients were obtained between block intake, water consumption, urine excretion, fecal excretion, and dry matter digestibility. A lineal regression analysis was performed to determine the maintenance crude protein requirement of adult doelings using diet crude protein and nitrogen balance data.
3. Results
3.1. Chemical Composition of Blocks
The crude protein content increased from 4.1% for blocks without urea to 21.7% for blocks containing 6% urea (Table 1). In contrast, NDFom decreased from 19.9% to 13.4% as urea replaced corn in the supplement. Blocks, with the inclusion of calcium oxide, salt, and minerals, exhibited high ash contents (ranging from 32.3 to 34.9%), and low energy density (varying from 1.933 to 2.095 Mcal ME/kg DM).
3.2. Dry Matter and NDFom Intake and Digestibility
No significant difference (p > 0.05) was observed in dry matter intake (DM) or digestibility among treatments (Table 2). Forage intake varied between 542 and 571 g/day, while block intake ranged from 121 to 168 g/day. Total DM intake varied from 663 and 736 g/day among treatments. Hay rejection was high, the percent of orts being 30.5, 34.5, 33.9, and 32.6% of Buffelgrass offered, for 0, 2, 4, and 6% of urea treatments, respectively.

Table 2.
Effects of urea content of multinutrient blocks supplemented to doelings consuming Buffelgrass hay on dry matter and neutral detergent fiber intakes and digestibility.
Due to the high inorganic matter content of the blocks, DM digestibility was lower than NDFom digestibility. Although the NDFom content in Buffelgrass was high (69.2%), due to doelings’ dietary selection, the forage consumed contained less NDFom (58.8, 59.7, 56.2, and 59.7% for 0, 2, 4, and 6% urea treatments, respectively). The NDFom in orts was 87.0, 86.5, 85.5, and 85.4%, respectively. The Fecal NDFom was 52.9%, 50.8%, 49.4%, and 52.1%, respectively. These low fecal NDFom values suggest that forage consumed was low in NDFom content.
3.3. Time Dedicated to Eating and Ruminate
The time dedicated to eating was not affected (p > 0.05) by urea block level, ranging from 412 to 471 min/day. In contrast, rumination time showed a quadratic response (p = 0.013) to an increased urea level in the blocks (Table 3). The total chewing time (sum of eating and rumination times) was not affected (p > 0.05) by block urea level. The ruminal pH of the doelings changed quadratically (p = 0.012) with more urea in the blocks (Table 3).

Table 3.
Eating, rumination, and total chewing times of doelings consuming Buffelgrass and supplemented multinutrient blocks with various urea levels.
3.4. Nitrogen Intake and Retention
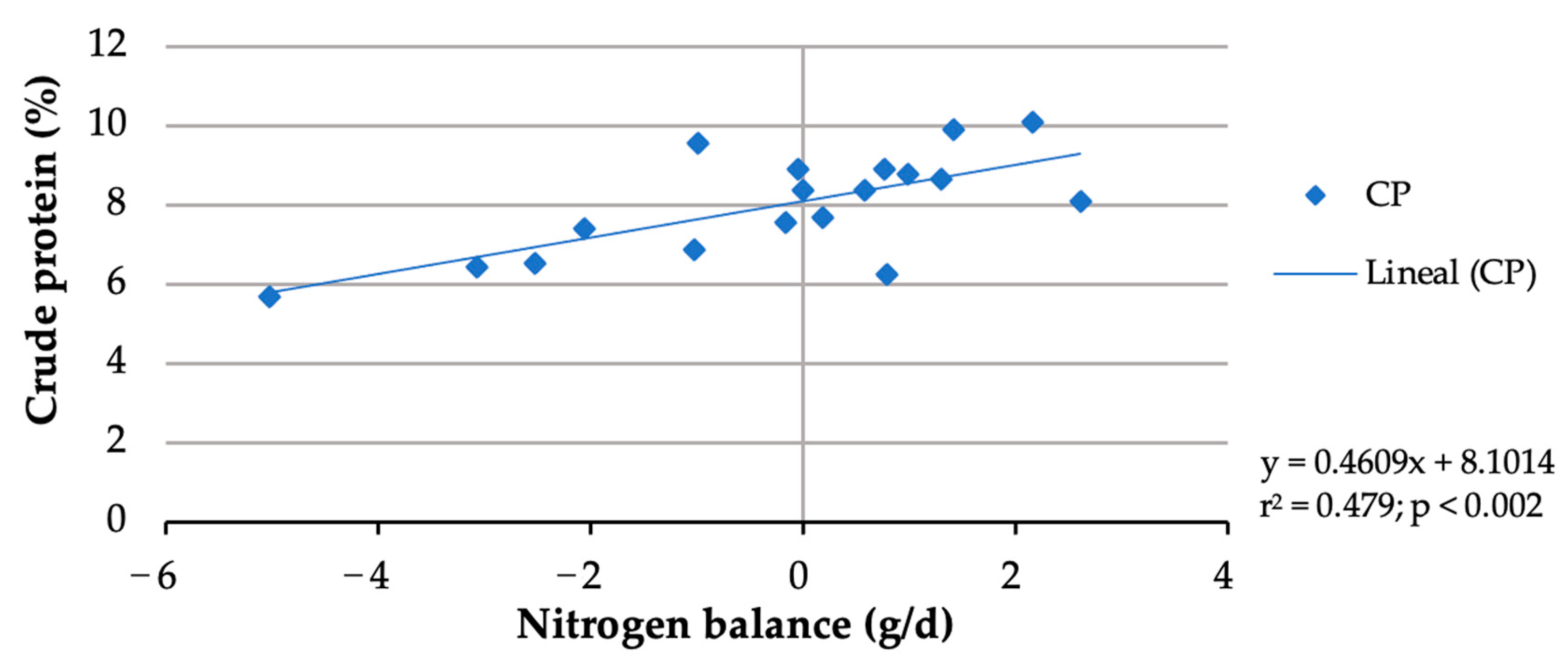
In this study, although buffelgrass contained 6.47%, due to forage selection, the crude protein of rejected forage ranged from 4.98 to 5.19%. The nitrogen balance data from two doelings were discarded due to urine lost from two metabolic cages during the 9-day urine-collection period. Whereas forage nitrogen intake did not differ (p > 0.05) among the treatments, block nitrogen intake increased (p < 0.001) from 1.065 g/d with the block without urea to 4.6 g/d with the block with 6% urea (Table 4), resulting in an increasing total nitrogen intake from 7.180 g/day to 10.605 g/day. No significant (p > 0.05) increase in fecal or urine N excretions was observed with an increasing block urea inclusion level (Table 4). With more urea in blocks, nitrogen retention (p = 0.005) and N balance (p = 0.010) linearly increased. Nitrogen balances shifted from negative (−2.458 g/day) for doelings consuming blocks without urea to positive (0.895 g/d) for those consuming the 6% urea blocks. Doe nitrogen retention shifted from negative (−35.93%) for blocks without urea to positive (10.06%) for the 6% urea block. The nitrogen balances of the doelings increased with more crude protein consumed (Figure 1). Considering a zero N balance, the maintenance CP requirement of the doelings was calculated to be 8%.

Table 4.
Nitrogen balance of doelings consuming Buffelgrass hay and supplemented multinutrient blocks with various urea levels.
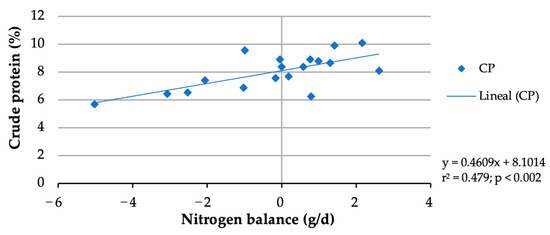
Figure 1.
Crude protein requirement estimation for maintenance of doelings consuming Buffelgrass hay and multinutrient blocks with various urea levels.
3.5. Blood Urea Nitrogen
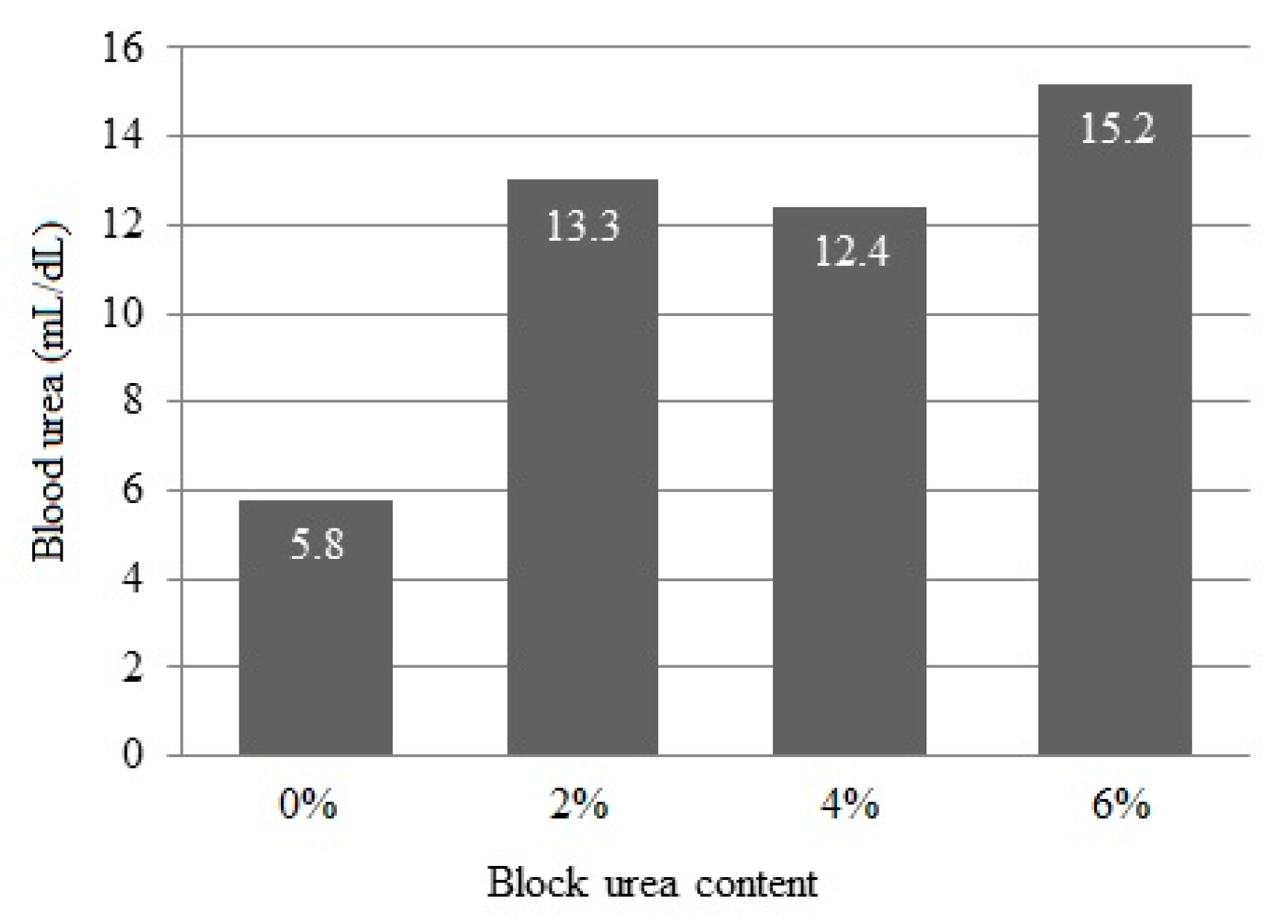
Blood urea concentrations linearly increased (p < 0.001) from 5.8 to 15.2 mg/dL as the urea inclusion in blocks increased (Figure 2).
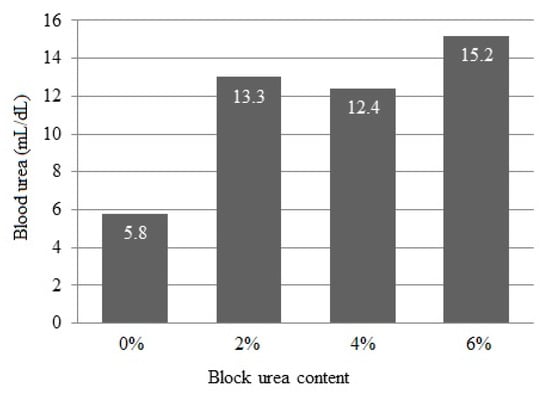
Figure 2.
Blood serum urea concentration of doelings consuming Buffelgrass hay and supplemented with multinutrient blocks with various urea levels.
3.6. Block and Urea Consumption on Water Intake and Urine Excretion
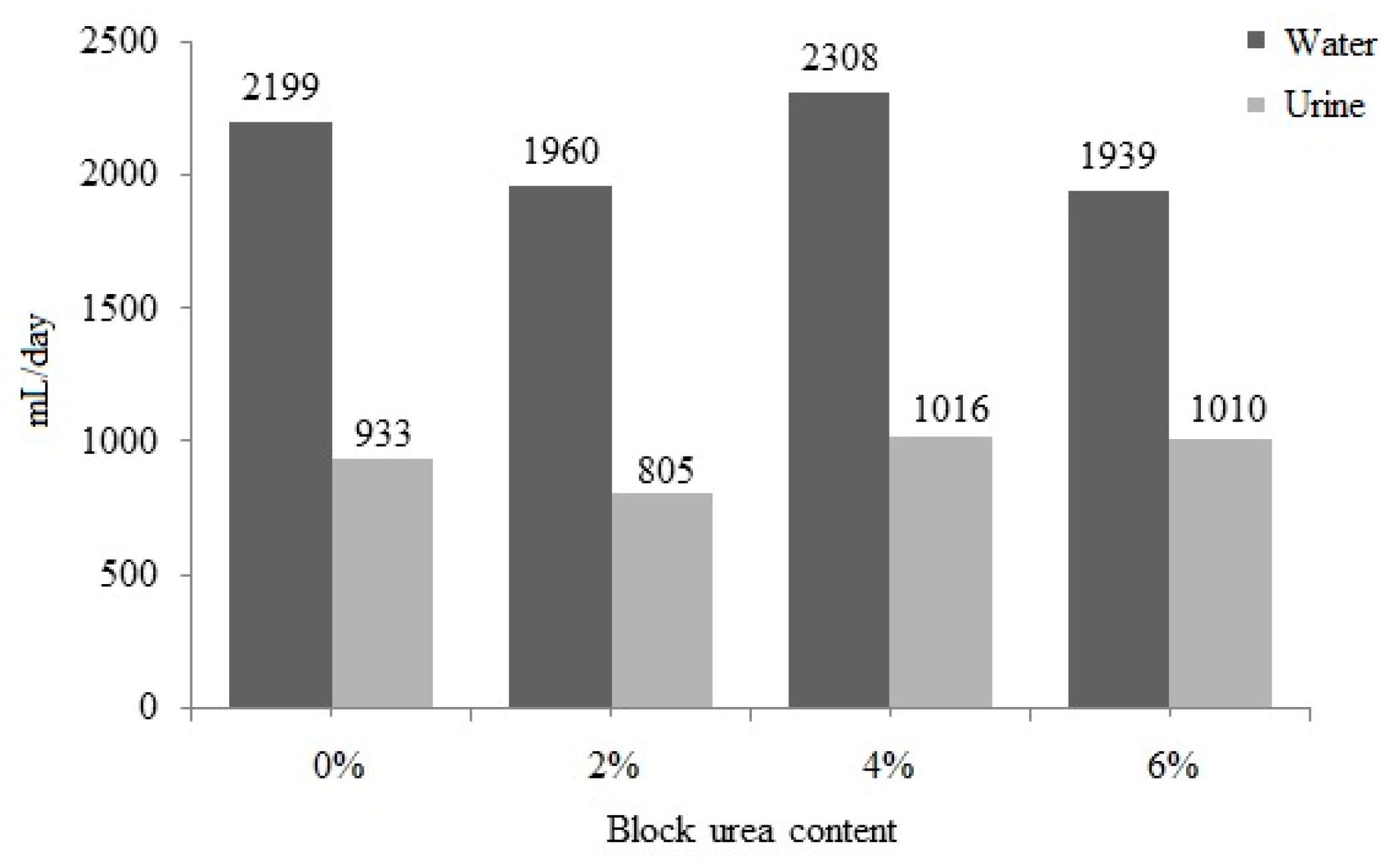
No significant difference (p > 0.05) was observed between water intake and urine excretion with an increase in the urea level in the blocks (Figure 3). Water intake ranged from 1939 to 2308 mL/day, and urine excretion ranged from 805 to 1016 mL/day. With a higher block intake, increases in water consumption (r = 0.442; p = 0.051) and urinary excretion (r = 0.441; p = 0.051) were observed. These data also suggest that as block intake increased, fecal excretion increased (r = 0.540; p = 0.014) and DM digestibility was reduced (r = −0.525; p = 0.018).
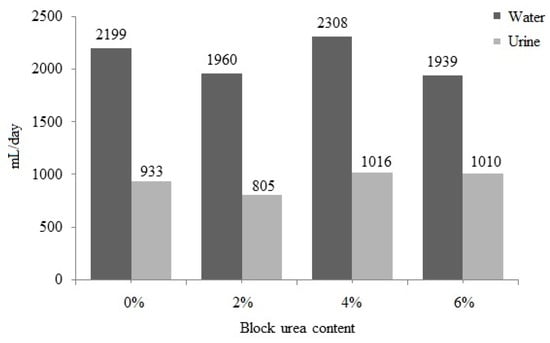
Figure 3.
Water intake and urinary excretion of doelings consuming Buffelgrass hay and supplemented molasses blocks with various urea levels.
4. Discussion
Goats are known for their selective feeding behavior since they are not as efficient at utilizing structural carbohydrates as cattle and sheep. Their selectivity is inversely related to their ability to retain and digest fiber in the rumen [3,6]. In this study, doe diet selection was evident, as the NDFom of the forage consumed was lower (ranging from 56.2 to 59.7%), while the NDFom of forage rejected was higher (ranging from 85.4 to 87.0%) than those of the Buffelgrass hay that was offered (69.2%).
When supplementing nutrients for goats consuming low-quality forages in range or confinement conditions, it is crucial to consider the synchronization of nitrogen and carbohydrate degradability in the rumen to optimize rumen fermentation [17]. In this study, DM digestibility (%) was lower than NDFom digestibility (%). With the increased consumption of ash, fecal DM excretion also increased, resulting in lower DM digestibility. Additionally, as block intake increased, more water was consumed, and more urine was excreted, primarily due to the consumption of salt.
Supplementing with CP has been shown to increase the intake and digestibility of hay in cattle, sheep, goats, and other ruminants [23,24,25]. However, in this study, urea supplementation did not affect the intake or digestibility of hay. A review of the literature revealed other studies that have also failed to demonstrate a response in this regard in wethers [26] and steers [27,28,29]. Currier et al. (2004b) [24] suggested that a possible explanation for the lack of a forage intake response could be the NDFom intake, based on the results from Mertens (1985, 1994) [30,31], which showed that DM intake is maximized when NDFom intake is approximately 12.5 g/kg BW/day. This is consistent with other studies indicating that intake is sensitive to forage NDF in small ruminants [30,31,32]. In this study, NDFom intake ranged from 361.8 to 421.5 g/d, which, considering a body weight of 18 kg, would imply an approximate intake of 22 g/kg BW/day.
This study also demonstrated that urea supplementation did not affect NDFom intake or digestibility. These results align with those reported by Chanjula and Ngampongsai (2008) [33], who used native Thailand-Anglo Nubian crossbred doelings with an average weight of 19 kg and fed them elephant grass and yucca-based diets with four urea levels (0, 1, 2, and 3%). The authors found no significant difference (p > 0.05) between treatments regarding digestion coefficients for DM, OM, CP, NDF, and ADF. Schacht et al. (1992) [34] did not observe a growth response with the supplementation of urea (5 g/day) or molasses (140 g/day) alone to goat kids grazing native vegetation (Caatinga) in Northeast Brazil. However, when both were supplemented, the average daily gain was doubled, suggesting the need for an energy source to enable urea utilization by rumen bacteria for synthesizing microbial protein.
Other factors, such as DM intake and its effect on rumination, influenced ruminal pH [35]. Urea supplementation of a low protein diet would increase fermentation, resulting in VFA production, which may explain the reduction in ruminal pH [36]. A higher ruminal pH could be attributed to a buffering effect of ammonia nitrogen resulting from urea breakdown in the rumen [37,38]. The significant variation in the ammonia concentration found by Smith et al. (1980) [39] in fibrous diets containing urea was because urea is 100% soluble, rapidly increasing rumen ammonia concentrations after ingestion, as rumen microorganisms may not have sufficient energy available to fully metabolize it.
Diet digestibility and the rate of passage are reduced if the nutrient requirements of rumen bacteria are not met [38]. The nitrogen requirements for maximum ruminal microbial growth are primarily dependent on digestible dry matter intake [39]. The solubility and degradability of dietary protein play a role in protein availability to satisfy the nitrogen needs of microorganisms. Therefore, the required nitrogen level in the rumen to support a maximum rate of feed passage is expected to vary with carbohydrate digestibility in the rumen. The results from various studies with beef cattle, such as NRC (1987) [40], suggest that most diets satisfy the requirement of 6 to 8% CP for normal rumen function. The value of 8% CP that was estimated for adult doelings in this study aligns with values obtained in other studies reported for beef cattle [38] and goats [41].
Adamu et al. (1989) [42] observed that, in animals fed corn stover silage-based diets and supplemented with a protein concentrate containing various urea levels, the maximum microbial growth, measured through bacterial nitrogen reaching the duodenum, occurred when rumen ammonia levels reached 4.9 mg/dL. For animals fed four times a day, the optimum ammonia level to maximize dry matter intake and digestibility was approximately 13.3 mg/dL. In our study, this value was achieved with a block containing 2% urea. These authors concluded that to maintain the rumen ammonia levels in animals fed once per day, a value exceeding 18.2 mg/dL at two hours was required for maximum feed intake.
5. Conclusions
Urea supplementation is primarily recommended for goats fed low-quality forages. Buffelgrass generally contains less crude protein than what is required for normal rumen function or for maintaining goats. In this study, increasing the urea content of molasses blocks by up to 6% significantly increased nitrogen intake, retention, and balance in goats. To maintain a positive nitrogen balance, a minimum of 8% crude protein is required in urea-based diets.
Author Contributions
Conceptualization, Z.T.-C., D.S.R.-C., G.M.-D. and J.R.K.; methodology, Z.T.-C., D.S.R.-C. and J.R.K.; software, Z.T.-C., D.S.R.-C., G.M.-Z., G.M.-D., S.P.H.-M., Y.R.-Z. and J.R.K.; validation, Z.T.-C., D.S.R.-C., G.M.-Z., S.P.H.-M., G.M.-D., Y.R.-Z. and J.R.K.; formal analysis, Z.T.-C., D.S.R.-C., S.P.H.-M., G.M.-Z., G.M.-D., Y.R.-Z. and J.R.K.; investigation, Z.T.-C., D.S.R.-C. and J.R.K.; resources, J.R.K.; data curation, S.P.H.-M., G.M.-Z. and J.R.K.; writing—original draft preparation, Z.T.-C., D.S.R.-C., Y.R.-Z., G.M.-Z., G.M.-D. and J.R.K.; writing—review and editing, Z.T.-C., D.S.R.-C., Y.R.-Z., G.M.-Z., S.P.H.-M., G.M.-D. and J.R.K.; visualization, Y.R.-Z., G.M.-D. and J.R.K.; supervision, J.R.K.; project administration, J.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was accepted by the Joint Graduate Program of the Faculties of Agronomy and Veterinary Science of the University of Nuevo León and registered under the code 36397-001290684 in the master’s exam certificate and code 4768 in the digital collection of the master’s degree thesis.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the personnel of MNA de México, AQUA Laboratorios, and UANL for their help during this research and other valuable resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alexandre, G.; Mandonnet, N. Goat meat production in harsh environments. Small Rumin. Res. 2005, 60, 53–66. [Google Scholar] [CrossRef]
- Miller, B.A.; Lu, C.D. Current status of global dairy goat production: An overview. Asian-Australas J. Anim. Sci. 2019, 32, 1219–1232. [Google Scholar] [CrossRef]
- Ogola, T.D.O.; Nguyo, W.K.; Kosgey, I.S. Economic contribution and viability of dairy goats: Implications for a breeding programme. Trop. Anim. Health Prod. 2010, 42, 875–885. [Google Scholar] [CrossRef]
- Huston, J.E.; Rector, B.S.; Ellis, W.C.; Allen, M.L. Dynamics of digestion in cattle, sheep, goats, and deer. J. Anim. Sci. 1986, 62, 208–215. [Google Scholar] [CrossRef]
- Ben Salem, H.; Smith, T. Feeding strategies to increase small ruminant production in dry environments. Small Rumin. Res. 2008, 77, 174–194. [Google Scholar] [CrossRef]
- Lu, C.D.; Kawas, J.R.; Mahgoub, O.G. Fibre digestion and utilization in goats. Small Rumin. Res. 2005, 60, 45–52. [Google Scholar] [CrossRef]
- van der Walt, J.G. Protein digestion in ruminants. S.-Afr. Tydskr. Veek 1988, 18, 30–41. [Google Scholar]
- Harmeyer, J.; Martens, H. Aspects of urea metabolism in ruminants with reference to the goat. J. Dairy Sci. 1980, 63, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. The State of the World’s Food and Agriculture; Food and Agriculture Organization: Rome, Italy, 1968. [Google Scholar]
- Kennedy, P.M.; Milligan, L.P. The degradation and utilization of endogenous urea in the gastrointestinal tract of ruminants: A review. Can. J. Anim. Sci. 1980, 60, 205–221. [Google Scholar] [CrossRef]
- Getahun, D.; Alemneh, T.; Akeberegn, D.; Getabalew, M.; Zewdie, D. Urea metabolism and recycling in ruminants. Biomed. J. Sci. Tech. Res. 2019, 20, 14790–14796. [Google Scholar]
- Sansoucy, R.G.; Aarts, G.; Leng, R.A. Molasses urea blocks. In Tropical Feeds; Gahl, B., Ed.; Food and Agricultural Organization of the United Nations: Rome, Italy, 1992; pp. 241–251. [Google Scholar]
- Kawas, J.R. Producción y utilización de bloques multinutrientes como complemento de forrajes de baja calidad para caprinos y ovinos: La experiencia en regiones semiáridas. Tecnol. Ciên. Agropec. 2008, 2, 63–69. [Google Scholar]
- Ramírez, R.G.; Huerta, J.; Kawas, J.R.; Alonso, D.S.; Mireles, E.; Gómez, M.V. Performance of lambs grazing in a buffelgrass (Cenchrus ciliaris) pasture and estimation of their maintenance and energy requirements for growth. Small Rumin. Res. 1995, 17, 117–121. [Google Scholar] [CrossRef]
- Patra, A.K. Urea/Ammonia Metabolism in the Rumen and Toxicity in Ruminants. In Rumen Microbiology: From Evolution to Revolution; Puniya, A., Singh, R., Kamra, D., Eds.; Springer: New Delhi, India, 2015. [Google Scholar]
- Kawas, J.R.; Andrade-Montemayor, H.; Lu, C.D. Strategic nutrient supplementation of free-ranging goats. Small Rumin. Res. 2010, 89, 234–243. [Google Scholar] [CrossRef]
- Fimbres, H.; Kawas, J.R.; Hernandez-Vidal, G.; Picón-Rubio, J.F.; Lu, C.D. Nutrient intake, digestibility, mastication, and ruminal fermentation of lambs fed finishing ration with various forages levels. Small Rumin. Res. 2002, 43, 275–281. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2016. [Google Scholar]
- AOCS. American Oil Chemists’ Society; Official Methods and Recommended Practices: Urbana, IL, USA, 2008. [Google Scholar]
- Udén, P.; Robinson, P.H.; Wiseman, J. Use of detergent system terminology and criteria for submission of manuscripts on new, or revised, analytical methods as well as descriptive information on feed analysis and/or variability. Anim. Feed. Sci. Technol. 2005, 118, 181–186. [Google Scholar] [CrossRef]
- DelCurto, T.; Cochran, R.C.; Harmon, D.L.; Beharka, A.A.; Jacques, K.A.; Towne, G.; Vanzant, E.S. Supplementation of dormant tallgrass-prairie forage: I. Influence of varying supplemental protein and(or) energy levels on forage utilization characteristics of beef steers in confinement. J. Anim. Sci. 1990, 68, 515–531. [Google Scholar] [CrossRef]
- National Research Council of the National Academies; Committee on the Nutrient Requirements of Small Ruminants; Board on Agriculture and Natural Resources; Division on Earth and Life Studies. Vitamins. In Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007; pp. 150–172. [Google Scholar]
- Köster, H.H.; Cochran, R.C.; Titgemeyer, E.C.; Vanzant, E.S.; Abdelgadir, I.; St-Jean, G. Effect of increasing degradable intake protein on intake and digestion of low-quality, tall-grass-prairie forage by beef cows. J. Anim. Sci. 1996, 74, 2473–2481. [Google Scholar] [CrossRef]
- Bandyk, C.A.; Cochran, R.C.; Wickersham, T.A.; Titgemeyer, E.C.; Farmer, C.G.; Higgins, J.J. Effects of ruminal vs postruminal administration of degradable protein on utilization of low-quality forage by beef steers. J. Anim. Sci. 2001, 79, 225–231. [Google Scholar] [CrossRef]
- Currier, T.A.; Bohnert, D.W.; Falck, S.J.; Bartle, S.J. Daily and alternative-day supplementation of urea or biuret to ruminants consuming low-quality forage: I. Effects on cow performance and efficiency of nitrogen use in wethers. J. Anim. Sci. 2004, 82, 1508–1517. [Google Scholar] [CrossRef]
- Currier, T.A.; Bohnert, D.W.; Falck, S.J.; Schauer, C.S.; Bartle, S.J. Daily and alternate-day supplementation of urea or biuret to ruminants consuming low-quality forage: II. Effects on site of digestion and microbial efficiency in steers. J. Anim. Sci. 2004, 82, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.G.; Woods, B.C.; Cochran, R.C.; Heldt, J.S.; Mathis, C.P.; Olson, K.C.; Titgemeyer, E.C.; Wickersham, T.A. Effect of supplementation frequency and supplemental urea level on dormant tallgrass-prairie hay intake and digestion by beef steers and prepartum performance of beef cows grazing dormant tallgrass-prairie. J. Anim. Sci. 2004, 82, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Köster, H.H.; Woods, B.C.; Cochran, R.C.; Vanzant, E.S.; Titgemeyer, E.C.; Grieger, D.M.; Olson, K.C.; Stokka, G. Effect of increasing proportion of supplemental N from urea in prepartum supplements on range beef cow performance and on forage intake and digestibility by steers fed low-quality forage. J. Anim. Sci. 2002, 80, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Mertens, D.R. Factors influencing feed intake in lactating cows: From theory to application using neutral detergent fiber. Proc. Georgia Nutr. Conf. Feed Ind. Univ. Georgia Athens 1985, 1–18. [Google Scholar]
- Mertens, D.R. Regulation of Forage Intake. In Forage Quality, Evaluation and Utilization; Fahey, E., Madison, G.G., Eds.; American Society Agrononomic, Inc.; Crop Science Society American, Soil Science Society American, Inc.: Madison, WI, USA, 1994; pp. 450–493. [Google Scholar]
- Baumont, R.; Prache, S.; Meuret, M.; Morand-Fehr, P. How forage characteristics influence behaviour and intake in small ruminants: A review. Livest. Prod. Sci. 2000, 64, 15–28. [Google Scholar] [CrossRef]
- Chanjula, P.; Ngampongsai, W. Effect of supplemental nitrogen from urea on digestibility, rumen fermentation pattern, microbial populations and nitrogen balance in growing goats. Songklanakarin J. Sci. Technol. 2008, 30, 571–578. [Google Scholar]
- Schacht, W.H.; Kawas, J.R.; Malechek, J.C. Effects of supplemental urea and molasses on dry season weight gains of goats in semiarid tropical woodland, Brazil. Small Rumin. Res. 1992, 7, 235–244. [Google Scholar] [CrossRef]
- Owens, P.N.; Goetsch, A.L. Digesta passage and microbial protein synthesis. In Control of Digestion and Metabolism in Ruminants; Milligan, L.P., Grovum, W.L., Dobson, A., Eds.; Prentice Hall: Hoboken, NJ, USA, 1986; pp. 196–223. [Google Scholar]
- Amos, H.E.; Evans, J. Supplementary protein for low quality Bermudagrass diets and microbial protein synthesis. J. Anim. Sci. 1976, 43, 861–868. [Google Scholar] [CrossRef]
- Counotte, G.H.M.; Van’t Klooster, A.; Van der Kuilen, J.; Prints, R. Analysis of the buffer system in the rumen of dairy cattle. J. Anim. Sci. 1979, 49, 1536–1544. [Google Scholar] [CrossRef]
- Galina, M.A.; Guerrero, M.; Puga, C.D.; Haenlein, G.F.W. Effects of slow-intake urea supplementation on goat kids pasturing natural Mexican rangeland. Small Rumin. Res. 2004, 55, 85–95. [Google Scholar] [CrossRef]
- Smith, T.; Broster, V.; Hill, R. A comparison of source of supplementary nitrogen for young cattle receiving fibre-rich diets. J. Agric. Sci. Camb. 1980, 95, 687–695. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA; London, UK, 1994. [Google Scholar]
- National Research Council of the National Academies; Committee on the Nutrient Requirements of Small Ruminants; Board on Agriculture and Natural Resources; Division on Earth and Life Studies. Vitamins. In Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 1987; pp. 150–172. [Google Scholar]
- Adamu, A.D.; Russell, J.R.; Gilliard, M.C.; Trenkle, A. Effects of added dietary urea on the utilization of maize-stover silage by growing beef cattle. Anim. Feed Sci. Technol. 1989, 22, 227–236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).